Abstract
Savannahs are a mixture of trees and grasses often occurring as alternate states to closed forests. Savannah fires are frequent where grass productivity is high in the wet season. Fires help maintain grassy vegetation where the climate is suitable for woodlands or forests. Saplings in savannahs are particularly vulnerable to topkill of above-ground biomass. Larger trees are more fire-resistant and suffer little damage when burnt. Recruitment to large mature tree size classes depends on sapling growth rates to fire-resistant sizes and the time between fires. Carbon dioxide (CO2) can influence the growth rate of juvenile plants, thereby affecting tree recruitment and the conversion of open savannahs to woodlands. Trees have increased in many savannahs throughout the world, whereas some humid savannahs are being invaded by forests. CO2 has been implicated in this woody increase but attribution to global drivers has been controversial where changes in grazing and fire have also occurred. We report on diverse tests of the magnitude of CO2 effects on both ancient and modern ecosystems with a particular focus on African savannahs. Large increases in trees of mesic savannahs in the region cannot easily be explained by land use change but are consistent with experimental and simulation studies of CO2 effects. Changes in arid savannahs seem less obviously linked to CO2 effects and may be driven more by overgrazing. Large-scale shifts in the tree–grass balance in the past and the future need to be better understood. They not only have major impacts on the ecology of grassy ecosystems but also on Earth–atmosphere linkages and the global carbon cycle in ways that are still being discovered.
Keywords: vegetation fires, woody thickening, bush encroachment, C4 grass
1. Introduction
Concentrations of carbon dioxide (CO2) in the atmosphere have varied greatly over the history of terrestrial plants [1]. Major changes in CO2 in the geological record have been associated with large changes in vegetation. Over the last 65 million years (the Cenozoic) CO2 rose to greater than 1000 ppm in the Eocene (50 Ma) and forests spread to their greatest extent [2]. When CO2 fell in the Oligocene, forests retreated and grasslands began to spread. C4 grassland biomes became prominent from the late Miocene (approx. 8 Ma) [3] and their appearance has also been controversially associated with low CO2 [4–6]. During the Pleistocene, tropical grasslands expanded under low CO2 conditions of the glacials and contracted as forest expanded in the interglacials [4,7,8]. In historical times, anthropogenic increases in CO2 following industrialization have been proposed as a factor contributing to woody plant increases in grasslands (figure 1). Shifts between grasslands and forests are likely to have feedbacks on the atmosphere via diverse mechanisms [9,10]. Over geological time, changes in forested landcover may also have feedbacks to CO2 in the atmosphere by influencing rates of weathering, and therefore the terrestrial sink for CO2 [11]. The role of rising atmospheric CO2 in driving such shifts, and their global implications, are poorly studied in comparison with CO2 effects in temperate forest ecosystems. Here, we review studies on CO2 impacts on tree–grass interactions. CO2 effects cannot be studied in isolation from frequent disturbances by fire and grazing which are characteristic of savannahs. Consequently, we have selected studies where interactions between CO2 effects and the disturbance regime have been considered. Our main focus is on those grassy biomes where frequent fires are important in reducing tree cover.
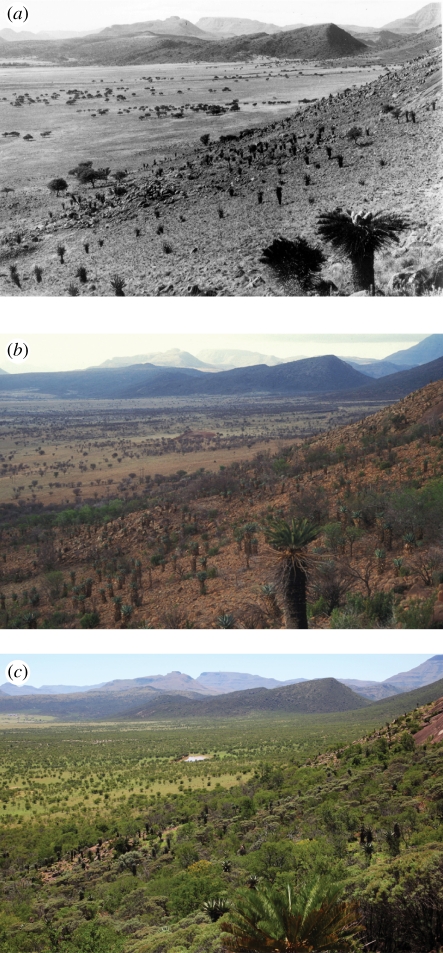
Figure 1.
Woody thickening over the last century near Queenstown, eastern Cape, South Africa. Acacia karroo is the most common tree species in this mesic savannah (approx. 750 mm mean annual precipitation). (a) 1925, (b) 1993, and (c) 2011. Note the large woody increase since the early 1990s. The original photograph was taken by the late IB Pole Evans (South African National Botanical Institute) and repeat photos courtesy of Timm Hoffman and James Puttick (Plant Conservation Unit, University of Cape Town).
2. Fire and the distribution of grassy biomes
In large regions of the world, direct climate control over vegetation is overridden by fire producing low biomass ecosystems where conditions are warm enough and wet enough to support forests [12]. C4 grasslands and savannahs cover greater than 15 per cent of the vegetated land surface, mostly at lower latitudes. These pyrophytic grassy biomes alternate with closed forests in seasonally wet tropical landscapes. They have been interpreted as alternative stable states which can switch on the order of decades from grassland to forest (or vice versa), depending on the interaction between plant growth rates and disturbance frequency and severity [13–15]. Because CO2 influences plant growth rates, changes in atmospheric CO2 could be a significant factor influencing the proportions of forests and grasslands in a landscape. Changing CO2 can also influence tree cover within savannahs. Savannahs are a mixed community of trees with C4 grasses usually dominant in the herbaceous layer. The trees are compositionally and functionally distinct from forest trees being more tolerant of competition with grasses and of the frequent fires characteristic of seasonally wet savannahs. Trees recovering from disturbance may be particularly sensitive to changes in CO2. After a burn, light, water and nutrients are least likely to limit growth thus facilitating maximum CO2 responsiveness. Top-killed plants have to re-build their stems so there is a strong carbon sink [16]. Furthermore, frequent surface fires in savannahs select for woody plants with underground storage organs, or clonally spreading root systems, which promote rapid post-burn recovery [17–19]. Post-burn resprouting of smaller size classes of savannah trees, whether using stored reserves or not, will produce large carbon sinks so that these plants should be particularly responsive to CO2 fertilization with less sink limitation of photosynthesis.
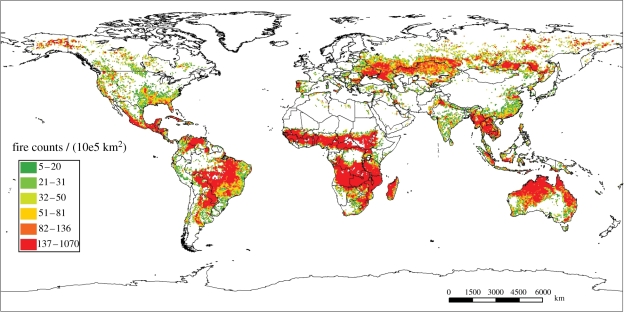
CO2 can influence plants by altering water use efficiency, photosynthetic rates, light and nutrient use efficiency, and the relative performance of C3 and C4 photosynthesis [20]. The latter effect has been invoked as an explanation for the timing of the evolution of the C4 photosynthetic pathway with a drop in CO2 [4,21,22]. However, C4 photosynthesis requires high-light environments and high temperatures for maximum photosynthetic performance [23]. The implication is that C4 grasses were restricted to open, well-lit ecosystems and that the Late Miocene–Pliocene expansion of the grassy biomes depended on the retreat of forests. Palaeoecologists often attribute the contraction of forests to increasing aridity at low latitudes (or cold at high elevation or latitudes). However, bunch grasses (whether C3 or C4) are highly flammable where decomposition is slow and, given sufficient water to produce continuous fuels, support very frequent fires. Fires are a major factor influencing the distribution of forests in the modern world, especially where C4 grasses predominate in low to mid latitudes, but also in flammable C3 grasslands in the steppes of Asia [14,24] (figure 2).
Figure 2.
Global fire activity from 2001 to 2006 from MODIS active fire counts. Tropical and sub-tropical grasslands and savannahs account for the largest burnt area over the period but grasslands in the steppes of Central Asia also burnt frequently. Reproduced with permission from Chuvieco et al. [24]. Copyright © Wiley.
Changing atmospheric CO2 can influence woody plant expansion into both arid grasslands and more humid flammable grasslands but by different hypothetical mechanisms. An increase in water use efficiency under elevated CO2 owing to reduced transpiration has been proposed as a key mechanism favouring woody plant thickening in semi-arid savannahs [25]. This has the indirect effect of increasing soil moisture supply which may favour tree seedling recruitment and subsequent growth in competition with grasses. CO2 enrichment experiments in grasslands have commonly shown an increase in soil moisture consistent with this mechanism [26,27].
In more humid grasslands, where fires are frequent, Bond & Midgley [28] suggested that direct CO2 effects on plant growth would favour an increase in tree cover. They noted that the rates of recovery of plants after a disturbance should vary inversely with the proportion of carbon allocated to support structures. This is because plant relative growth rates (RGRs) increase in proportion to leaf area ratio, the ratio of photosynthetic leaf area to total plant mass [29]. Woody plants which have to re-build large non-photosynthetic stems after a disturbance will have lower RGR than herbaceous plants such as grasses. Tree seedlings and saplings surviving fire and resprouting after a burn in a flammable grassland will soon face a repeated fire, as grasses accumulate fuel rapidly. Woody plants can increase the chance of escaping topkill by building below-ground reserves of carbon and nutrients that promote rapid regrowth. However, sprouting from underground storage organs comes at a cost of initially slower growth rates in seedlings that have to build below-ground reserves resulting in poorer establishment and reduced ability to colonize new areas [30]. Within savannahs, growth rates of trees to fire-resistant sizes may be particularly sensitive to CO2 as the replacement of stem tissue and investment in storage organs are easier to make under elevated CO2 [20]. Thus, regardless of whether grasses are C3 or C4, increasing CO2 should favour trees more than grasses in flammable (and other disturbed) ecosystems.
3. Tests of the fire–carbon dioxide hypothesis
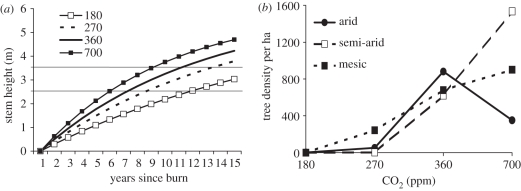
Savannah trees are a very useful model system for testing the effect of CO2 on plants recovering from disturbance. Seedlings and smaller plants not only compete with grasses but are also subjected to frequent grass-fuelled fires. Fires topkill the stems of smaller plants. Given sufficiently rapid stem growth rates, or an unusually long interval between fires, stems grow to fire-resistant sizes. Mortality from fires is very low in established plants and small plants can persist for decades suffering repeated topkill by fires [17,31,32]. Release from the flame zone (the ‘fire trap’) depends both on sapling growth rates and the rare periods between fires that are long enough for a small plant to grow to fire-resistant size. Unlike CO2 effects on closed forests, the impact of a change in growth rate on savannah trees has clearly quantifiable demographic effects on the transition to large trees. Demographic changes in large tree densities, in turn, have large ecosystem impacts on fluxes of water, nutrients, carbon and even, possibly, rates of weathering [11]. This chain of CO2 effects from increased growth rates to changes in tree cover has been simulated for South African savannahs [33]. A detailed demographic model of fire–savannah tree demography [31] showed great sensitivity of tree cover to changes in sapling growth rates. CO2 effects on sapling growth rates, simulated with a Dynamic Global Vegetation Model (DGVM) [34], and coupled to the demographic model, indicated a striking effect of changes in CO2 since the last glacial on tree cover in the region (figure 3). Simulations predicted that sapling growth rates would be so slow at Last Glacial Maximum (LGM) CO2 levels that trees would have been eliminated from flammable savannahs. The largest subsequent CO2-induced change to tree cover was predicted from pre-industrial to modern CO2 levels, especially over the twentieth century [33].
Figure 3.
Simulated change in tree growth and sapling response for African savannah trees. (a) Sapling growth rates to fire-resistant size (2.5–3.5 m) at varying CO2 levels simulated with a DGVM [34]. (b) Simulated median tree densities as a result of these growth rates simulated with a detailed demographic model of savannah trees burnt by fires [31]. Adapted from Bond et al. [33].
(a). Glasshouse experiments
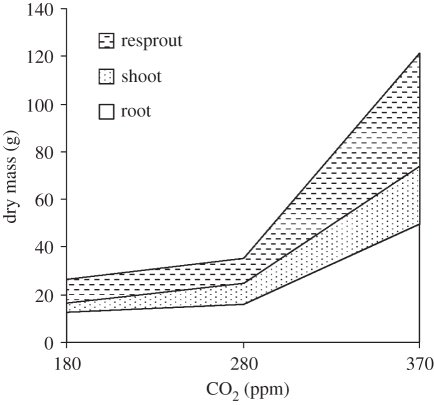
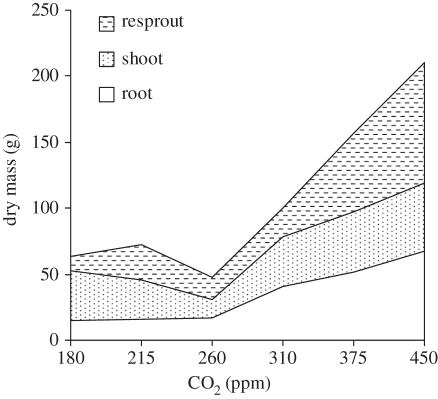
The simulated response to CO2 reported in [33] was tested for common savannah plants grown experimentally in open-top chambers in a greenhouse [35] (B. S. Kgope, G. F. Midgley & W. J. Bond 2011, unpublished data). Savannah tree seedlings were grown under well-watered conditions with nutrients supplied for a full growing season, after which they were cut to simulate fire in the subsequent dry season. After a second full growing season, the plants were harvested. Results were consistent with the DGVM simulations (figure 4) with very slow growth at 180 ppm (LGM), slightly greater at pre-industrial, and then very large increases at 360 ppm. Plants grown at elevated CO2 did not grow well probably because of pot size constraints [35]. In a second set of experiments, the soil volume was increased to 200 l and a broad-leaved savannah tree, Terminalia sericea, was included (figure 5) (B. S. Kgope, G. F. Midgley & W. J. Bond 2011, unpublished data). Kgope et al. [35] reported large changes in root size and starch concentration within the roots, especially from 260 ppm (pre-industrial) to 360 (‘ambient’) CO2 treatments (figure 6). The impact on sapling growth rates, scaled from total biomass differences across CO2 treatments, exceeds those used in the simulation study (figure 3). These very large effects imply that, during the last century, increasing CO2 has fuelled changes in savannah tree growth helping to create ‘super-seedlings’. Relative to a century ago, young trees have faster growth, massive root systems with larger starch reserves and a greatly enhanced capacity for resprouting after injury. In the Acacia seedlings, there is the added bonus of larger spines and higher tannin content in the leaves providing better protection against seedling herbivory.
Figure 4.
Acacia karroo growth response to CO2 treatments. Plants were grown for a year, cut to simulate fire (shoot) and harvested after a second growing season (resprout, root) [35].
Figure 5.
Terminalia sericea growth response to CO2 treatments. Plants were grown for a year, cut to simulate fire (shoot) and harvested after a second growing season (resprout, root). Adapted from B. S. Kgope, G. F. Midgley & W. J. Bond 2011, unpublished data.
Figure 6.
Acacia karroo root responses to a gradient of CO2 treatments. The scale on the left of each plant is a metre long. Plants were grown for a year, cut to simulate fire and harvested after a second growing season. Adapted from B. S. Kgope, G. F. Midgley & W. J. Bond 2011, unpublished data.
Responses of savannah trees to CO2 have been studied from below to above ambient levels in North American Prosopis species [36,37], and to elevated CO2 for Australian [38,39] and South American woody species [40]. As has been found for plants from other ecosystems, responses vary among species with faster growing species tending to respond more strongly [37,39]. Hoffman et al. [40] also showed that CO2 responses were sensitive to nutrient supply which suggests that CO2 effects may be muted on nutrient poor soils.
(b). Field experiments
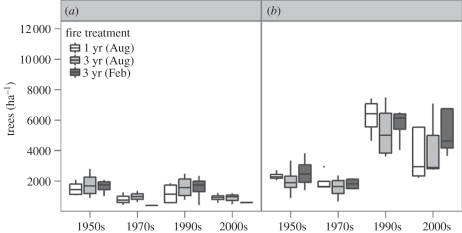
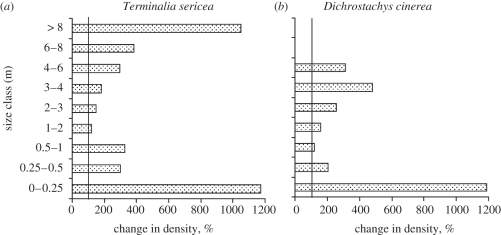
There are no field studies manipulating CO2 in tree–grass ecosystems in Africa. However, there are several long-term burning experiments in which the same fire treatments have been applied for several decades. If the CO2 effects on tree growth observed for African species were carried over to the field setting, then there should be CO2-enhanced seedling recruitment and more frequent transitions from smaller to larger tree size classes. During the twentieth century, CO2 levels rose from 296 ppm in 1900 to 310 ppm in 1950, 330 ppm in 1975 and 376 ppm by 2003 [41]. The physiological responses to CO2 are steepest from sub-ambient to ambient CO2 concentrations relative to above ambient [42]. If physiological responses fed into changes in the ecology of woody plants, then long-term burning experiments would be expected to show changes in tree responses in the early versus later years of the experiments. Buitenwerf et al. [43] have analysed the results of two long-term burning studies in South Africa testing for temporal trends. The longest running experiments are at Kruger National Park [44–46]. Figure 7 shows trends in densities for a semi-arid and mesic savannah for censuses conducted in the 1950s, 1970s, 1990s and 2000s. The 1970s and 2000s censuses used permanently marked grids providing spatially explicit records of changes in tree densities and sizes. Data for the most common tree and shrub species in the mesic savannah are shown in figure 8. There were negligible temporal trends in woody plant densities in the semi-arid savannah (figure 7). In the mesic savannah, there were negligible changes in the first two decades (1950s–1970s) but very large increases from the 1970s to the 2000s. Both the most common tree and shrub species increased in density and both showed an increase in recruitment into the larger size classes (figure 8; see also Higgins et al. [45]). Terminalia sericea, the tree species, responded strongly to CO2 in glasshouse experiments (figure 5) so that its increase in the field is consistent with CO2 effects.
Figure 7.
Changes in woody plant densities in a long-term burning experiment in the Kruger National Park, South Africa. Results from (a) a semi-arid savannah (mean annual precipitation (MAP) approx. 500 mm) and (b) a mesic savannah (MAP approx. 750 mm). Adapted from Buitenwerf et al. [43].
Figure 8.
Changes in density across size classes of the (a) most common tree and (b) shrub species in a mesic savannah from the 1970s to the 2000s. Data is from identical areas sampled 30 years apart and subjected to the same fire treatments since the 1950s in the experimental burn plots, Kruger National Park, South Africa. Adapted from Buitenwerf et al. [43].
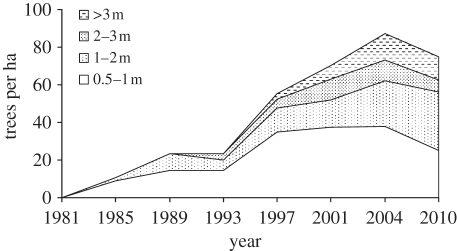
A second fire experiment has been maintained for three decades near the southern limit of savannahs in South Africa (latitude 32° S). Figure 9 shows changes in Acacia karroo, the most common savannah tree species in this system. Changes in tree densities were similar to the mesic savannah of Kruger National Park with a large increase in both densities and size over the second half of the experiment from the mid-1990s to 2010. Interpretation of these results is complicated by the removal of trees before the experiment started [43]. However, large increases in this tree species have been reported elsewhere in this region, especially in higher rainfall areas, over the last few decades (figure 1). Acacia karroo was very responsive to CO2 increases from pre-industrial to current values in glasshouse experiments (figures 4 and 6; [35]) so that the field observations are consistent with experimentally observed CO2 fertilization effects.
Figure 9.
Changes in Acacia karroo populations subjected to the same fire treatments for 30 years. Eastern Cape, South Africa. Adapted from Buitenwerf et al. [43].
There are a number of long-term burning experiments in Africa and elsewhere. Where treatments have been maintained and sufficient censuses are available, these would be worth exploring for temporal trends as predicted from tree responses to increasing CO2. For example, Briggs et al. [47] reported longitudinal trends of woody plant responses to long-maintained fire regimes in tall grass prairies in Kansas. In this system, different catchments have been burnt annually, at moderate frequency or left unburnt since 1980. Bison were introduced into some of the treatment areas in the 1990s. In the ungrazed treatments, two woody species showed large increases in the intermediate fire frequencies but no change under annual burning. The greatest increases were in catchments that were both grazed and burnt. Briggs et al. [47] noted that the increase in these woody species was paradoxical. Historical accounts reported the area to be tree-less. Fire frequencies and bison densities several centuries ago were thought to be similar to those applied over the experimental period. Thus, the increase in woody plants in all but the most extreme annual burning treatment is a departure from the historical condition. They attributed the woody increase to habitat fragmentation and changes in seed dispersal and did not consider CO2 fertilization as a possible factor.
4. Carbon dioxide and woody plant cover increase
Savannah ecosystems are likely to be particularly sensitive to CO2 effects because small increases in stem growth rates can have very large effects on the number and biomass of trees. The potential for change from an open to a wooded savannah is greatest in higher rainfall savannahs [48]. Once trees have escaped the firetrap and a woodland patch has developed, CO2 effects on further changes in woodland structure are likely to greatly diminish. Many studies from around the world have reported woody thickening in savannahs (see list in Archer et al. [49]). Attribution to global drivers has been controversial since changes in grazing and fire are a common and well-studied alternative explanation [12,45,46,50]. CO2 is unlikely to be implicated in all cases of woody thickening. We suggest that the most probable environmental settings for CO2 effects on woody increase are higher rainfall savannahs which burn frequently. These contain fast growing woody plants that depend on stored carbon for resprouting so that the potential for sink-driven increases in carbon gain are particularly strong. In more arid savannahs, CO2 effects may be small relative to the effects of other drivers [50] (but see Morgan et al. [51] for large increase in a shrub in short grass steppes under elevated CO2). CO2 effects are least likely where edaphic constraints, such as shallow rooting depth, limit tree growth [52]. Low soil nutrients may also reduce CO2 responses in nutrient poor savannahs. Despite these uncertainties, there is clear evidence for CO2 fertilization contributing to woody thickening over the past few decades in some savannahs. The implication is that land users will have to work much harder than in the past to maintain open grassy ecosystems.
5. Forest expansion into grasslands
Our discussion thus far has been on CO2 effects on tree cover within savannahs. There is growing evidence that elevated CO2 may also be promoting conversion of savannahs to closed forests. Forests are distinct from savannah woodlands in their species composition, structure and function [19]. They are too shady to support a C4 grass understorey so that grass-fuelled fires seldom spread beyond the forest edge. Forest trees are far more sensitive to fire than savannah trees so that the two ecosystems have been characterized as pyrophytic and pyrophobic alternative ecosystem states [13–15]. Tropical forests have changed in recent decades with increased net primary productivity, and increased tree growth, recruitment, mortality rates and forest biomass [53]. There has been much debate over the possible cause of these changes, but a growing consensus that increasing atmospheric CO2 is the most probable cause [53,54].
Tropical forests are also expanding into grassy biomes in many regions where there is no human deforestation. Expansion is the most conspicuous in landscapes with mosaics of grasslands and forests. Gallery forests (along riparian areas) and other forest patches have expanded into flammable grassy ecosystems in South America [55], North America [56,57], Australia [58,59] and different parts of Africa [60–63]. Forest expansion seems to be the most common in wetter climates [59]. In semi-arid central Australia, for example, mosaics of closed ‘mulga’ (Acacia aneura) woodland and open spinifex (Triodia spp.) grasslands have remained fairly stable for decades [59]. Thus, forest invasion of grassland may be similar to tree increases within savannahs, with the greatest changes in wetter climates and the least in more arid regions.
The causes of forest expansion are also a subject of vigorous debate over the relative importance of changes in fire regimes versus global drivers, especially CO2 increase. There are far fewer studies on forest edges than tropical forest interiors. Changes in the forest boundaries have mostly been studied by historical aerial photography and not from plot data. Despite the difficulties, studies of forest expansion into flammable grassy ecosystems provide key insights into the potential for major landcover changes. If savannahs were to be replaced by forests on large continental scales, the repercussions would be far greater than a shift from a less to a more wooded savannah.
Experimental and modelling studies have shown that large areas of savannahs could support forests in the absence of fire. Forest expansion into savannahs has commonly been attributed to reduced fire activity near forest margins. Global drivers are implicated where forests have expanded despite stable or even more frequent fires. In northern Australia, for example, Bowman et al. [59] noted that forest expansion has occurred from small rainforest patches even though there have been landscape-wide increases in the size and frequency of fires. Annual rainfall and the number of rain days have increased in the region but Bowman et al. [59] attribute the forest expansion primarily to the physiological effects of CO2 on forest trees exposed to fire at the forest edge. In South Africa, Wigley et al. [63] compared forest expansion over a near 70 year period in three adjacent areas with sharply contrasting fire and herbivory regimes. This study included a nature reserve, effectively a natural ‘control’, with an extant African megafauna, including elephants and other large browsers and grazers, and frequent fire. At the other extreme was a densely populated subsistence farming area where trees are used by people and livestock for building and fuels. Forest expansion was greatest in the nature reserve ‘control’ increasing from 14 per cent in 1937 to 58 per cent by 2004. The subsistence farming area showed the smallest increase (6–25%). However, the striking fact is that scrub forests increased in all land use types regardless of the intensity and types of disturbance. This strongly suggests a global driver tipping the balance towards trees. The most probably driver is increasing atmospheric CO2 because no significant trends in temperature and rainfall were identified in the region [63].
Recent studies suggest that processes operating at forest–savannah edges are similar to these within savannahs and are likely to be similarly sensitive to changing CO2 [15,64]. Working in Brazil, Hoffmann et al. [64] studied the dynamics of forest recovery after fire. On the rare occasions where fire spreads into a forest from an adjacent savannah, smaller forest trees are top-killed by fire while larger trees are much more fire-resistant. As in savannahs, most top-killed smaller trees resprout and mortality rates are low, at least after a single fire. The ability to repair the forest edge is a function of growth rates to fire-resistant size versus the fire return time. If growth rates are slow, then the forest edge will retreat and grasses will invade. If growth rates are fast, then the forest edge may expand into the grassland, despite the fires. Forest trees have denser canopies than savannah trees and can suppress fires by shading out the grasses [65]. Forest trees had thinner bark than savannah trees and were therefore more vulnerable to topkill for a given size. It is interesting to note that forest trees at this forest edge site had much thicker bark and were far more resilient to burning than trees in the interior of Amazon forests [64,66]. Because of their thinner bark, forest trees would need at least twice as long as savannah trees to reach fire-resistant sizes making them far more sensitive to burning.
This analysis of the mechanistic responses of forest trees to grassland fires is very similar to the fire trap models for savannah tree populations [15,31,64]. It suggests the same sensitivity of the process to atmospheric CO2. After a fire, resprouting forest trees would be growing in well-watered, high-light and high-nutrient environments and carbon gain will be strongly sink-driven as trees re-build their stems. CO2 effects are likely to be the most pronounced under these conditions. We know of neither any glasshouse experiments testing CO2 responsiveness of forest margin trees bordering on savannahs nor any field studies of CO2 responsiveness of resprouting tropical forest margins. The closest analogue seems to be a Florida scrub oak system exposed to elevated CO2 after the ecosystem was burnt. The scrub oak ecosystem showed a 67 per cent increase in above-ground stem biomass under elevated CO2, with the response sustained over the 11 years that the experiment has been maintained [67].
Forest margins have been avoided as study sites being neither fish nor fowl. However, local processes at these margins may determine large-scale patterns of forest–savannah distribution. A continent-wide study of tree cover in Africa, for example, found that patterns of forest versus savannah distribution over a rainfall gradient were invariant across scales implying that the distribution of the two vegetation types is determined by local processes [15]. Hoffmann et al. [64] make the same point. The implication is that changes in the processes operating at a forest–grassland boundary a few metres wide are the frontline for wholesale biome switches. If this is the case, then changes in atmospheric CO2, interacting with fire, could have major effects on the distribution of forest in the tropics with forest retreats in glacial periods and expansion in interglacial periods. Focused CO2 studies on boundary dynamics will go a long way in showing the sensitivity of forest boundaries to future shifts in CO2.
6. Palaeoecology of tree–grass interactions
Studies of CO2 and other drivers of tree–grass interactions in contemporary ecosystems are complicated by their short duration and multiple interacting factors. Current woody cover may reflect past events, such as the rinderpest epidemic in Africa [68] or severe droughts [69,70] rather than current factors [71]. It has also been argued that CO2 responses reflect short-term changes and that other factors, such as nitrogen supply, will become limiting as ecosystems equilibrate [72,73]. One way out of the morass is to explore the palaeorecord. The last glacial period was not only colder but also had much lower atmospheric CO2 (185–200 ppm) than interglacial periods (280–300 ppm). If CO2 effects were indeed trivial over longer periods of time, then the palaeorecord of biome shifts should be predictable from palaeoclimates alone. That this was not the case began to emerge from attempts to model the distribution of last glacial vegetation [74–76]. Prentice & Harrison [77,78] have recently summarized diverse lines of evidence showing that palaeoclimate is not a good predictor of biome shifts and that CO2 effects are fundamental to explaining vegetation change from glacial to interglacial periods.
Firstly, they note that changes in δ13C in the shells of marine sub-fossil foraminifera indicate a reduction in terrestrial C in the last glacial on the order of 300–750 Pg with recent estimates suggesting a loss of approximately 600 Pg of C. Reductions of terrestrial carbon of this magnitude cannot be predicted from palaeoclimate models. Indeed some models simulate a gain in carbon largely because low temperatures in glacial periods would have slowed decomposition of litter thereby causing an increase in C stored in soils. Only models that incorporate both climate change and the physiological effects of low CO2 produce vegetation change of the magnitude necessary to explain the estimated reduction in terrestrial C. Palaeo-reconstructions of biome distribution can also be used to test CO2 effects. Attempts to simulate biome distributions from General Circulation Model (GCM) reconstructions of last glacial climates have failed to produce forest reduction of the magnitude necessary to explain the pollen evidence, especially in the tropics where savannahs expanded and forests contracted. Biome models incorporating the physiological effects of CO2 simulate far greater forest reduction consistent with the palaeodata [77,78]. Finally, recent studies have used inverse modelling to test the kinds of climates needed to produce the biome distributions reconstructed from pollen data. Inverse modelling failed to reconstruct climates matching those from GCM modelling unless physiological CO2 effects were also incorporated [77].
The evidence from the palaeodata clearly supports a major role for CO2 in large-scale biome shifts from low CO2 glacial periods to higher CO2 in the interglacials (though the role of fire–CO2 interactions over these timescales has been little studied [33]). Palaeoecologists have been aware of the potential importance of CO2 for some time but have been slow to interpret atmospheric CO2 as a contributor to past vegetation change. Wooller et al. [7] suggested that fire, together with low CO2, helped drive the retreat of forests and expansion of flammable grasslands in equatorial Africa. Damste et al. [8] used diverse proxies to test for low CO2 and fire synergies in their analysis of forest–savannah interactions in Africa. Studies of the palaeoecology of fire have also increased greatly over the past decade allowing comparisons of glacial and interglacial fire activity. Though data for tropical fire regimes (savannahs, C4 grasslands) are still rare compared with woody-fuelled ecosystems, long temporal record of fires is accumulating and is beginning to contribute major insights into the determinants of global fire regimes [79]. Coupled with increasing availability of data on past vegetation and past fires are new developments in global fire modelling [80] and DGVMs [78,81]. These new tools should help greatly in testing the relative importance of CO2, fire and climate on forest–savannah distributions over far longer periods than are feasible in contemporary experimental studies.
7. Biogeochemistry of savannahs versus forests
Changes in tree cover in savannahs or switches from savannah to forest could have wide-ranging consequences, including feedbacks to the Earth–atmosphere system [9]. Savannahs cover more than 17 million km2 and account for about 21–23% of global productivity [51]. With their large spatial extent, even small increases in woody biomass would represent a substantial carbon sink [49,82,83]. Savannahs differ from forests in their energy budget and hydrology [84]. They typically have a higher albedo than forests, partly because they retain dead, reflective leaves over the dry season. Trees generally transpire more water than grasses because of their more extensive root system, greater leaf area, and greater canopy roughness. Consequently, large-scale change in tree cover, or biome switches to forest, would have feedbacks to the regional climate [84]. Extensive grass-fuelled fires also have feedbacks to climate [10]. Black aerosols alter energy budgets and reduce the size of cloud droplets causing precipitation to be less frequent but more intense [85–87]. Savannah fires are also important sources of NOx which promote increased tropospheric ozone formation and transport reactive N to potentially N-limited ecosystems [88].
A recent estimate of global vegetation fire emissions from 1997 to 2009 (when MODIS satellite imagery became available) estimated average emissions of 2.0 Pg C per year [89]. Grasslands and savannahs accounted for 44 per cent of this, with (mostly tropical) woodlands an additional 16 per cent. Thus, the tropical grassy systems accounted for about 60 per cent of global fire emissions. African savannah fires alone contributed approximately 1 Pg C per year. Increases in tree cover could profoundly alter these global burning patterns. An analysis of fire spread in Africa has shown threshold effects with fire activity declining sharply once tree cover exceeds about 40 per cent [90]. If large-scale tree thickening occurs across the continent and fires cease to burn, then there are likely to be multiple effects on the Earth–atmosphere system [9,91].
Over longer timescales (million of years), Pagani et al. [11] have suggested that reductions in tree cover at low atmospheric CO2 may have limited further CO2 decline. These authors argue that silicate weathering of rock, the major sink for atmospheric CO2, would have decreased with loss of forest cover and their replacement by C4 grasslands and savannahs. This negative feedback mechanism opposing higher rates of silicate chemical weathering may explain why CO2 did not decline further during warmer periods (e.g. from the Early to Mid-Miocene) when high weathering activity should have promoted further CO2 reduction.
8. Conclusions
Savannahs span from climates too arid to support trees to climates so productive that they switch to forest within a decade or two of fire suppression. Within this diverse climate setting, the ecology of trees in savannahs is frustratingly complex with multiple factors interacting to determine changes in tree populations. For these reasons, it is difficult to predict the future of savannahs, or to identify the key drivers of changes in woody cover in the immediate past. There is enough evidence to indicate that elevated CO2 is increasingly tipping the balance in savannahs in favour of trees, especially in the more open, frequently burnt savannahs. But, there are still far too few data to predict which parts of this complex biome are most and which least likely to respond to increasing CO2.
Contemplation of the more distant past can help provide perspective. C4 grasses evolved in a low CO2 world in the Oligocene more than 25 million years ago and first assembled as a major biome, the world's youngest, in the low CO2 atmospheres from about eight million years ago. Within the next hundred years, savannah plant species are therefore likely to experience CO2 levels outside their entire evolutionary history. Given the decreasing physiological advantages of C4 grasses at high CO2, and the ecological and palaeoecological studies reviewed above, we suggest that it is not unreasonable to postulate that the rapid rise of the savannah biome from the Late Miocene may be matched by an abrupt decline owing to anthropogenic impacts on the carbon cycle. The likelihood of large-scale loss of savannahs (other than by land clearing), and the repercussions for local land users, biodiversity and Earth–atmosphere feedbacks deserve far more attention.
Acknowledgements
Many people have contributed to the studies reported here from simulation modellers and physiologists to field staff who maintained field experiments and burning treatments for decades. We particularly thank Ian Woodward, Steve Higgins, Winston Trollope, Barney Kgope, Ben Wigley and Rob Buitenwerf for their diverse contributions. For three decades, Andre Potgieter and his staff maintained the Kruger burn plots and Winston Trollope and Wellington Shabangu maintained the eastern Cape burn plots. We are very grateful to the Royal Society for hosting the Research Fellows International Scientific Seminar on Atmospheric CO2 and Green Evolution at the Kavli Centre and to David Beerling for inviting us to participate and inspiring us to take a broad perspective on CO2 and plant ecology and evolution.
References
- 1.Royer D. L., Berner R. A., Park J. 2007. Climate sensitivity constrained by CO2 concentrations over the past 420 million years. Nature 446, 530–532 10.1038/nature05699 (doi:10.1038/nature05699) [DOI] [PubMed] [Google Scholar]
- 2.Willis K. J., McElwain J. C. 2002. The evolution of plants. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Cerling T. E., Harris J. M., MacFadden B. J., Leakey M. G., Quade J., Eisenmann V., Ehleringer J. R. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158 10.1038/38229 (doi:10.1038/38229) [DOI] [Google Scholar]
- 4.Ehleringer J. R., Cerling T. E., Helliker B. R. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299 10.1007/s004420050311 (doi:10.1007/s004420050311) [DOI] [PubMed] [Google Scholar]
- 5.Osborne C. P. 2008. Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? J. Ecol. 96, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards E. J., Osborne C. P., Strömberg C. A. E., Smith S. A. & C4 Grasses Consortium 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591 10.1126/science.1177216 (doi:10.1126/science.1177216) [DOI] [PubMed] [Google Scholar]
- 7.Wooller M. J., Street-Perrott F. A., Agnew A. D. Q. 2000. Late Quaternary fires and grassland palaeoecology of Mount Kenya, East Africa: evidence from charred grass cuticles in lake sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 164, 207–230 10.1016/S0031-0182(00)00187-5 (doi:10.1016/S0031-0182(00)00187-5) [DOI] [Google Scholar]
- 8.Damste J. S. S., Verschuren D., Ossebaar J., Blokker J., van Houten R., van der Meer M. T. J., Plessen B., Schouten S. 2011. A 25,000-year record of climate-induced changes in lowland vegetation of eastern equatorial Africa revealed by the stable carbon-isotopic composition of fossil plant leaf waxes. Earth Plan. Sci. Lett. 302, 236–246 10.1016/j.epsl.2010.12.025 (doi:10.1016/j.epsl.2010.12.025) [DOI] [Google Scholar]
- 9.Beerling D. J., Osborne C. P. 2006. The origin of the savanna biome. Glob. Change Biol. 12, 2023–2031 10.1111/j.1365-2486.2006.01239.x (doi:10.1111/j.1365-2486.2006.01239.x) [DOI] [Google Scholar]
- 10.Hoffmann W. A., Schroeder W., Jackson R. B. 2002. Positive feedbacks of fire, climate, and vegetation and the conversion of tropical savannas. J. Geophys. Res. 108, 4721. 10.1029/2003JD003494 (doi:10.1029/2003JD003494) [DOI] [Google Scholar]
- 11.Pagani M., Caldeira K., Berner R., Beerling D. 2009. The role of terrestrial plants in limiting atmospheric CO2 decline over the past 24 million years. Nature 460, 85–89 10.1038/nature08133 (doi:10.1038/nature08133) [DOI] [PubMed] [Google Scholar]
- 12.Bond W. J., Woodward F. I., Midgley G. F. 2005. The global distribution of ecosystems in a world without fire. New Phytol. 165, 525–538 10.1111/j.1469-8137.2004.01252.x (doi:10.1111/j.1469-8137.2004.01252.x) [DOI] [PubMed] [Google Scholar]
- 13.Warman L., Moles A. T. 2009. Alternative stable states in Australia's wet tropics: a theoretical framework for the field data and a field-case for the theory. Landscape Ecol. 24, 1–13 10.1007/s10980-008-9285-9 (doi:10.1007/s10980-008-9285-9) [DOI] [Google Scholar]
- 14.Lehmann C. E. R., Archibald S. A., Hoffmann W. A., Bond W. J. 2011. Deciphering the distribution of the savanna biome. New Phytol. 190, 197–209 10.1111/j.1469-8137.2011.03689.x (doi:10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 15.Staver A. C., Archibald S., Levin S. 2011. Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology. 92, 1063–1072 10.1890/10-1684.1 (doi:10.1890/10-1684.1) [DOI] [PubMed] [Google Scholar]
- 16.Schutz A. E. N., Bond W. J., Cramer M. D. 2009. Juggling carbon allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 160, 235–246 10.1007/s00442-009-1293-1 (doi:10.1007/s00442-009-1293-1) [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann W. A. 1999. Fire frequency and population dynamics of woody plants in a neotropical savanna. Ecology 80, 1354–1369 10.1890/0012-9658(1999)080[1354:FAPDOW]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[1354:FAPDOW]2.0.CO;2) [DOI] [Google Scholar]
- 18.Simon M. F., Grether R., de Queiroz L. P., Skema C., Pennington R. T., Hughes C. E. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl Acad. Sci. USA 106, 20 359–20 364 10.1073/pnas.0903410106 (doi:10.1073/pnas.0903410106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratnam J., Bond W. J., Fensham R. J., Hoffmann W. A., Archibald S., Lehmann C. E. R., Anderson M. T., Higgins S. I., Sankaran M. 2011. When is a ‘forest’ a savanna, and why does it matter? Global Ecol. Biogeogr. 20, 653–660 10.1111/j.1466-8238.2010.00634.x (doi:10.1111/j.1466-8238.2010.00634.x) [DOI] [Google Scholar]
- 20.Drake B. G., Gonzalez-Meler M. A., Long S. P. 1997. More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant. Mol. Biol. 48, 609–639 10.1146/annurev.arplant.48.1.609 (doi:10.1146/annurev.arplant.48.1.609) [DOI] [PubMed] [Google Scholar]
- 21.Pagani M. 2002. The alkenone–CO2 proxy and ancient atmospheric carbon dioxide. Phil. Trans. R. Soc. Lond. A 360, 609–632 10.1098/rsta.2001.0959 (doi:10.1098/rsta.2001.0959) [DOI] [PubMed] [Google Scholar]
- 22.Christin P.-A., Besnard G., Samaritani E., Duvall M. R., Hodkinson T. R., Savolainen V., Salamin N. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr. Biol. 18, 37–43 10.1016/j.cub.2007.11.058 (doi:10.1016/j.cub.2007.11.058) [DOI] [PubMed] [Google Scholar]
- 23.Sage R. F. 2004. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 10.1111/j.1469-8137.2004.00974.x (doi:10.1111/j.1469-8137.2004.00974.x) [DOI] [PubMed] [Google Scholar]
- 24.Chuvieco E., Giglio L., Justice C. O. 2008. Global characterization of fire activity: toward defining fire regimes from Earth observation data. Glob. Change Biol. 14, 1488–1502 10.1111/j.1365-2486.2008.01585.x,2008 (doi:10.1111/j.1365-2486.2008.01585.x,2008) [DOI] [Google Scholar]
- 25.Polley H. W., Mayeux H. S., Johnson H. B., Tischler C. R. 1997. Viewpoint: atmospheric CO2, soil water, and shrub/grass ratios on rangelands. J. Range Manage. 50, 278–284 10.2307/4003730 (doi:10.2307/4003730) [DOI] [Google Scholar]
- 26.Morgan J. A., et al. 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140, 11–25 10.1007/s00442-004-1550-2 (doi:10.1007/s00442-004-1550-2) [DOI] [PubMed] [Google Scholar]
- 27.Koehler I. H., Poulton P. R., Auerswald K., Schnyder H. 2010. Intrinsic water-use efficiency of temperate seminatural grassland has increased since 1857: an analysis of carbon isotope discrimination of herbage from the Park Grass Experiment. Glob. Change Biol. 16, 1531–1541 10.1111/j.1365-2486.2009.02067.x (doi:10.1111/j.1365-2486.2009.02067.x) [DOI] [Google Scholar]
- 28.Bond W. J., Midgley G. F. 2000. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Change Biol. 6, 865–869 10.1046/j.1365-2486.2000.00365.x (doi:10.1046/j.1365-2486.2000.00365.x) [DOI] [Google Scholar]
- 29.Lambers H., Poorter H. 1992. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv. Ecol. Res. 23, 187–261 10.1016/S0065-2504(08)60148-8 (doi:10.1016/S0065-2504(08)60148-8) [DOI] [Google Scholar]
- 30.Bond W. J., Midgley J. M. 2001. The persistence niche: ecology of sprouting in woody plants. Trends Ecol. Evol. 16, 45–51 10.1016/S0169-5347(00)02033-4 (doi:10.1016/S0169-5347(00)02033-4) [DOI] [PubMed] [Google Scholar]
- 31.Higgins S. I., Bond W. J., Trollope W. S. W. 2000. Fire, resprouting and variability: a recipe for tree–grass coexistence in savanna. J. Ecol. 88, 213–229 10.1046/j.1365-2745.2000.00435.x (doi:10.1046/j.1365-2745.2000.00435.x) [DOI] [Google Scholar]
- 32.Prior L. D., Williams R. J., Bowman D. M. J. S. 2010. Experimental evidence that fire causes a tree recruitment bottleneck in an Australian tropical savanna. J. Trop. Ecol. 26, 595–603 10.1017/S0266467410000362 (doi:10.1017/S0266467410000362) [DOI] [Google Scholar]
- 33.Bond W. J., Midgley G. F., Woodward F. I. 2003. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Glob. Change Biol. 9, 973–982 10.1046/j.1365-2486.2003.00577.x (doi:10.1046/j.1365-2486.2003.00577.x) [DOI] [Google Scholar]
- 34.Woodward F. I., Lomas M. R., Lee S. E. 2001. Predicting the future productivity and distribution of global vegetation. In Terrestrial global productivity (eds Jacques R., Saugier B., Mooney H.), pp. 521–541 New York, NY: Academic Press [Google Scholar]
- 35.Kgope B. S., Bond W. J., Midgley G. F. 2010. Growth responses of African savanna trees implicate atmospheric [CO2] as a driver of past and current changes in savanna tree cover. Aust. Ecol. 35, 451–463 10.1111/j.1442-9993.2009.02046.x (doi:10.1111/j.1442-9993.2009.02046.x) [DOI] [Google Scholar]
- 36.Polley H. W., Johnson H. B., Mayeux H. S. 1994. Increasing CO2: comparative responses of the C4 grass Schizachyrium and grassland invader Prosopis. Ecology 75, 976–988 10.2307/1939421 (doi:10.2307/1939421) [DOI] [Google Scholar]
- 37.Polley H. W., Tischler C. R., Johnson H. B. 2006. Elevated atmospheric CO2 magnifies intra-specific variation in seedling growth of honey mesquite: an assessment of relative growth rates. Rangeland Ecol. Manage. 59, 128–134 10.2111/05-073R1.1 (doi:10.2111/05-073R1.1) [DOI] [Google Scholar]
- 38.Duff G. A., Berryman C. A., Eamus D. 1994. Growth, biomass allocation and foliar nutrient contents of two Eucalyptus species of the wet-dry tropics of Australia grown under CO2 enrichment. Funct. Ecol. 8, 502–508 10.2307/2390075 (doi:10.2307/2390075) [DOI] [Google Scholar]
- 39.Atkin O. K., Schortemeyer M., McFarlane N., Evans J. R. 1999. The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia 120, 544–554 10.1007/s004420050889 (doi:10.1007/s004420050889) [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann W. A., Bazzaz F. A., Chatterton N. J., Harrison P. A., Jackson R. B. 2000. Elevated CO2 enhances resprouting of a tropical savanna tree. Oecologia 123, 312–317 10.1007/s004420051017 (doi:10.1007/s004420051017) [DOI] [PubMed] [Google Scholar]
- 41.Etheridge D. M., Steele L. P., Langenfelds R. L., Francey R. J., Barnola J.-M., Morgan V. I. 1996. Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. J. Geophys. Res. 101, 4115–4128 10.1029/95JD03410 (doi:10.1029/95JD03410) [DOI] [Google Scholar]
- 42.Lloyd J., Farquhar G. D. 1996. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct. Ecol. 10, 4–32 10.2307/2390258 (doi:10.2307/2390258) [DOI] [Google Scholar]
- 43.Buitenwerf R., Bond W. J., Stevens N., Trollope W. S. W. In press Increased tree densities in two South African savannas: >50 years of data suggests CO2 as a driver. Glob. Change Biol. (doi:10.1111/j.1365-2486.2011.02561.x) [Google Scholar]
- 44.Biggs R., Biggs H. C., Dunne T. T., Govender N., Potgieter A. L. F. 2003. Experimental burn plot trial in the Kruger National Park: history, experimental design and suggestions for data analysis. Koedoe 46, 1–15 [Google Scholar]
- 45.Higgins S. I., et al. 2007. Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology 88, 1119–1125 10.1890/06-1664 (doi:10.1890/06-1664) [DOI] [PubMed] [Google Scholar]
- 46.Smit I. P. J., Asner G. P., Govender N., Kennedy-Bowdoin T., Knapp D. E., Jacobson J. 2010. Effects of fire on woody vegetation structure in African savanna. Ecol. Appl. 20, 1865–1875 10.1890/09-0929.1 (doi:10.1890/09-0929.1) [DOI] [PubMed] [Google Scholar]
- 47.Briggs J. M., Knapp A. K., Brock B. L. 2002. Expansion of woody plants in a tallgrass prairie: a fifteen-year study of fire and fire–grazing interactions. Am. Midland Nat. 147, 287–294 10.1674/0003-0031(2002)147[0287:EOWPIT]2.0.CO;2 (doi:10.1674/0003-0031(2002)147[0287:EOWPIT]2.0.CO;2) [DOI] [Google Scholar]
- 48.Sankaran M., et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849 10.1038/nature04070 (doi:10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 49.Archer S., Boutton T. W., Hibbard K. A. 2001. Trees in grasslands: biogeochemical consequences of woody plant expansion. In Global biogeochemical cycles in the climate system (eds Schulze E.-D., Heimann M., Harrison S., Holland E., Lloyd J., Prentice I., Schimel D.), pp. 115–138 San Diego, CA: Academic Press [Google Scholar]
- 50.Van Auken O. W. 2009. Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manage. 90, 2931–2942 10.1016/j.jenvman.2009.04.023 (doi:10.1016/j.jenvman.2009.04.023) [DOI] [PubMed] [Google Scholar]
- 51.Morgan J. A., Milchunas D. G., Lecain D. R., West M., Mosier A. R. 2007. Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proc. Natl Acad. Sci. USA 104, 14 724–14 729 10.1073/pnas.0703427104 (doi:10.1073/pnas.0703427104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd J., Bird M. I., Vellen L., Miranda A. C., Veenendaal E. M., Djagbletey G., Miranda H. S., Cook G., Farquhar G. D. 2008. Contributions of woody and herbaceous vegetation to tropical savanna ecosystem productivity: a quasi-global estimate. Tree Physiol. 28, 451. 10.1093/treephys/28.3.451 (doi:10.1093/treephys/28.3.451) [DOI] [PubMed] [Google Scholar]
- 53.Lewis S. L., Lloyd J., Sitch S., Mitchard E. T. A., Laurance W. F. 2009. Changing ecology of tropical forests: evidence and drivers. Annu. Rev. Ecol. Evol. Syst. 40, 529–549 10.1146/annurev.ecolsys.39.110707.173345 (doi:10.1146/annurev.ecolsys.39.110707.173345) [DOI] [Google Scholar]
- 54.Lloyd J., Farquhar G. D. 2008. Effects of rising temperatures and CO2 on the physiology of tropical forest trees. Phil. Trans. R. Soc. B 363, 1811–1817 10.1098/rstb.2007.0032 (doi:10.1098/rstb.2007.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva L. C. R., Sternberg L., Haridasan M., Hoffmann W. A., Miralles-Wilhelm F., Franco A. C. 2008. Expansion of gallery forests into central Brazilian savannas. Glob. Change Biol. 14, 2108–2118 10.1111/j.1365-2486.2008.01637.x (doi:10.1111/j.1365-2486.2008.01637.x) [DOI] [Google Scholar]
- 56.Loehle C., Li B. L., Sundell R. C. 1996. Forest spread and phase transitions at forest-prarie ecotones in Kansas, USA. Landscape Ecol. 11, 225–235 10.1007/BF02071813 (doi:10.1007/BF02071813) [DOI] [Google Scholar]
- 57.Briggs J. M., Knapp A. K., Blair J. B., Heisler J. L., Hoch G. A. 2005. An ecosystem in transition: causes and consequences of the conversion of mesic grassland to shrubland. Bioscience 55, 243–254 10.1641/0006-3568(2005)055[0243:AEITCA]2.0.CO;2 (doi:10.1641/0006-3568(2005)055[0243:AEITCA]2.0.CO;2) [DOI] [Google Scholar]
- 58.Russell-Smith J., Stanton P. J., Edwards A. C., Whitehead P. J. 2004. Rain forest invasion of eucalypt-dominated woodland savanna, Iron Range, north-eastern Australia: II. Rates of landscape change. J. Biogeogr. 31, 1305–1316 10.1111/j.1365-2699.2004.01070.x (doi:10.1111/j.1365-2699.2004.01070.x) [DOI] [Google Scholar]
- 59.Bowman D. M. J. S., Murphy B. P., Banfai D. S. 2011. Has global environmental change caused monsoon rainforests to expand in the Australian monsoon tropics? Landscape Ecol. 25, 1247–1260 10.1007/s10980-010-9496-8 (doi:10.1007/s10980-010-9496-8) [DOI] [Google Scholar]
- 60.Favier C., de Namur C., Dubois M. A. 2004. Forest progression modes in littoral Congo, Central Atlantic Africa. J. Biogeogr. 31, 1445–1461 10.1111/j.1365-2699.2004.01094.x (doi:10.1111/j.1365-2699.2004.01094.x) [DOI] [Google Scholar]
- 61.Goetze D., Horsch B., Porembski S. 2006. Dynamics of forest–savanna mosaics in north-eastern Ivory Coast from 1954 to 2002. J. Biogeogr. 33, 653–664 10.1111/j.1365-2699.2005.01312.x (doi:10.1111/j.1365-2699.2005.01312.x) [DOI] [Google Scholar]
- 62.Mitchard E. T. A., Saatchi S. S., Gerard F. F., Lewis S. L., Meir P. 2009. Measuring woody encroachment along a forest–savanna boundary in Central Africa. Earth Interact. 13, 1–29 10.1175/2009EI278.1 (doi:10.1175/2009EI278.1) [DOI] [Google Scholar]
- 63.Wigley B. J., Bond W. J., Hoffman M. T. 2010. Thicket expansion in a South African savanna under divergent land use: local versus global drivers? Glob. Change Biol. 16, 964–976 10.1111/j.1365-2486.2009.02030.x (doi:10.1111/j.1365-2486.2009.02030.x) [DOI] [Google Scholar]
- 64.Hoffmann W. A., Adasme R., Haridasan M., de Carvalho M. T., Geiger E. L., Pereira M. A. B., Gotsch S. G., Franco A. C. 2009. Tree topkill, not mortality, governs the dynamics of savanna–forest boundaries under frequent fire in central Brazil. Ecology 90, 1326–1337 10.1890/08-0741.1 (doi:10.1890/08-0741.1) [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann W. A., da Silva E. R., Machado G. C., Bucci S. J., Scholz F. G., Goldstein G., Meinzer F. C. 2005. Seasonal leaf dynamics across a tree density gradient in a Brazilian savanna. Oecologia 145, 307–316 [DOI] [PubMed] [Google Scholar]
- 66.Uhl C., Kauffman J. B. 1990. Deforestation, fire susceptibility and potential tree responses to fire in the eastern Amazon. Ecology 71, 437–449 10.2307/1940299 (doi:10.2307/1940299) [DOI] [Google Scholar]
- 67.Seiler T. J., et al. 2009. Disturbance, rainfall and contrasting species responses mediated aboveground biomass response to 11 years of CO2 enrichment in a Florida scrub-oak ecosystem. Glob. Change Biol. 15, 356–367 10.1111/j.1365-2486.2008.01740.x (doi:10.1111/j.1365-2486.2008.01740.x) [DOI] [Google Scholar]
- 68.Holdo R. M., Sinclair A. R. E., Dobson A. P., Metzger K. L., Bolker B. M., Ritchie M. E., Holt R. D. 2009. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C. PLoS Biol. 7, e1000210. 10.1371/journal.pbio.1000210 (doi:10.1371/journal.pbio.1000210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillson L. 2004. Evidence of hierarchical patch dynamics in an east African savanna? Landscape Ecol. 19, 883–894 10.1007/s10980-004-0248-5 (doi:10.1007/s10980-004-0248-5) [DOI] [Google Scholar]
- 70.Fensham R. J., Fairfax R. J., Ward D. P. 2009. Drought-induced tree death in savanna. Glob. Change Biol. 15, 380–387 10.1111/j.1365-2486.2008.01718.x (doi:10.1111/j.1365-2486.2008.01718.x) [DOI] [Google Scholar]
- 71.Staver A. C., Bond W. J., February E. C. 2011. History matters: tree establishment variability and species turnover in an African savanna. Ecosphere 2, 1–12 10.1890/ES11-00029.1 (doi:10.1890/ES11-00029.1) [DOI] [Google Scholar]
- 72.Korner C. 2006. Plant CO2 responses: an issue of definition, time and resource supply. New Phytol. 172, 393–411 10.1111/j.1469-8137.2006.01886.x (doi:10.1111/j.1469-8137.2006.01886.x) [DOI] [PubMed] [Google Scholar]
- 73.Luo Y., et al. 2004. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54, 731–739 10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;2) [DOI] [Google Scholar]
- 74.Cowling S. A., Sykes M. T. 1999. Physiological significance of low atmospheric CO2 for plant–climate interactions. Quat. Res. 52, 237–242 10.1006/qres.1999.2065 (doi:10.1006/qres.1999.2065) [DOI] [Google Scholar]
- 75.Beerling D. J., Woodward F. I. 2001. Vegetation and the terrestrial carbon cycle: modelling the first 400 million years. Cambridge, UK: Cambridge University Press [Google Scholar]
- 76.Harrison S. P., Prentice I. C. 2003. Climate and CO2 controls on global vegetation distribution at the last glacial maximum: analysis based on palaeovegetation data, biome modeling and palaeoclimate simulations. Glob. Change Biol. 9, 983–1004 10.1046/j.1365-2486.2003.00640.x (doi:10.1046/j.1365-2486.2003.00640.x) [DOI] [Google Scholar]
- 77.Prentice I. C., Harrison S. P. 2009. Ecosystem effects of CO2 concentration: evidence from past climates. Clim. Past 5, 297–307 10.5194/cp-5-297-2009 (doi:10.5194/cp-5-297-2009) [DOI] [Google Scholar]
- 78.Prentice I. C., Harrison S. P., Bartlein P. J. 2011. Global vegetation and terrestrial carbon cycle changes after the last ice age. New Phytol. 189, 988–998 10.1111/j.1469-8137.2010.03620.x (doi:10.1111/j.1469-8137.2010.03620.x) [DOI] [PubMed] [Google Scholar]
- 79.Whitlock C., Higuera P. E., McWethy D. B., Briles C. E. 2010. Paleoecological perspective on fire ecology: revisiting the fire regime concept. Open Ecol. J. 3, 6–23 10.2174/1874213001003020006 (doi:10.2174/1874213001003020006) [DOI] [Google Scholar]
- 80.Thonicke K., Spessa A., Prentice I. C., Harrison S. P., Dong L., Carmona-Moreno C. 2010. The influence of vegetation, fire spread and fire behaviour on global biomass burning and trace gas emissions: results from a process-based model. Biogeosciences 7, 1999–2011 10.5194/bg-7-1991-2010 (doi:10.5194/bg-7-1991-2010) [DOI] [Google Scholar]
- 81.Scheiter S., Higgins S. I. 2009. Impacts of climate change on the vegetation of Africa: an adaptive dynamic modeling approach. Glob. Change Biol. 15, 2224–2246 10.1111/j.1365-2486.2008.01838.x (doi:10.1111/j.1365-2486.2008.01838.x) [DOI] [Google Scholar]
- 82.Asner G. P., Archer S., Hughes R. F., Ansley R. J., Wessman C. A. 2003. Net changes in regional woody vegetation cover and carbon storage in Texas Drylands, 1937–1999. Glob. Change Biol. 9, 316–335 10.1046/j.1365-2486.2003.00594.x (doi:10.1046/j.1365-2486.2003.00594.x) [DOI] [Google Scholar]
- 83.Williams R. J., Carter J., Duff G. A., Woinarski J. C. Z., Cook G. D., Farrer S. L. 2005. Carbon stores, carbon accounting, land management, science and policy uncertainty in Australian savanna landscapes: introduction and overview. Aust. J. Bot. 53, 583–588 10.1071/BT05181 (doi:10.1071/BT05181) [DOI] [Google Scholar]
- 84.Hayden B. P. 1998. Ecosystem feedbacks on climate at the landscape scale. Phil. Trans. R. Soc. Lond. B 353, 5–18 10.1098/rstb.1998.0186 (doi:10.1098/rstb.1998.0186) [DOI] [Google Scholar]
- 85.Koren I., Kaufman Y. J., Remer L. A., Martins J. V. 2004. Measurement of the effect of Amazon smoke on inhibition of cloud formation. Science 303, 1342–1345 10.1126/science.1089424 (doi:10.1126/science.1089424) [DOI] [PubMed] [Google Scholar]
- 86.Andreae M. O., Rosenfield D., Artaxo P., Costa A. A., Frank G. P., Longo K. M., Silva-Dias M. A. F. 2004. Smoking rain clouds over the Amazon. Science 303, 1337–1342 10.1126/science.1092779 (doi:10.1126/science.1092779) [DOI] [PubMed] [Google Scholar]
- 87.Bevan S. L., North P. R. J., Grey W. M. F., Los S. O., Plummer S. E. 2009. Impact of atmospheric aerosol from biomass burning on Amazon dry-season drought. J. Geophys. Res. 114, 1–11 10.1029/2008JD011112 (doi:10.1029/2008JD011112) [DOI] [Google Scholar]
- 88.Hobbs P. V., Sinha P., Yokelson R. J., Christian T. J., Blake D. R., Gao S., Kirchstetter T. W., Novakov T., Pilewskie P. 2003. Evolution of gases and particles from a savanna fire in South Africa. J. Geophys. Res. 108, 8485. 10.1029/2002JD002352 (doi:10.1029/2002JD002352) [DOI] [Google Scholar]
- 89.van der Werf G. R., et al. 2010. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 10, 11 707–11 735 10.5194/acp-10-11707-2010,2010 (doi:10.5194/acp-10-11707-2010,2010) [DOI] [Google Scholar]
- 90.Archibald S., Roy D. P., van Wilgen B. W., Scholes R. J. 2009. What limits fire? An examination of drivers of burnt area in Southern Africa. Glob. Change Biol. 15, 613–630 10.1111/j.1365-2486.2008.01754.x (doi:10.1111/j.1365-2486.2008.01754.x) [DOI] [Google Scholar]
- 91.Bowman D. M. J. S., et al. 2009. Fire in the Earth system. Science 324, 481–484 10.1126/science.1163886 (doi:10.1126/science.1163886) [DOI] [PubMed] [Google Scholar]