Abstract
Oxygenic photosynthesis evolved at least 2.4 Ga; all oxygenic organisms use the ribulose bisphosphate carboxylase-oxygenase (Rubisco)–photosynthetic carbon reduction cycle (PCRC) rather than one of the five other known pathways of autotrophic CO2 assimilation. The high CO2 and (initially) O2-free conditions permitted the use of a Rubisco with a high maximum specific reaction rate. As CO2 decreased and O2 increased, Rubisco oxygenase activity increased and 2-phosphoglycolate was produced, with the evolution of pathways recycling this inhibitory product to sugar phosphates. Changed atmospheric composition also selected for Rubiscos with higher CO2 affinity and CO2/O2 selectivity correlated with decreased CO2-saturated catalytic capacity and/or for CO2-concentrating mechanisms (CCMs). These changes increase the energy, nitrogen, phosphorus, iron, zinc and manganese cost of producing and operating Rubisco–PCRC, while biosphere oxygenation decreased the availability of nitrogen, phosphorus and iron. The majority of algae today have CCMs; the timing of their origins is unclear. If CCMs evolved in a low-CO2 episode followed by one or more lengthy high-CO2 episodes, CCM retention could involve a combination of environmental factors known to favour CCM retention in extant organisms that also occur in a warmer high-CO2 ocean. More investigations, including studies of genetic adaptation, are needed.
Keywords: inorganic carbon, mixing depth, photosynthetically active radiation, Rubisco, temperature, nutrients, UV radiation
1. Introduction
Algae are oxygenic photosynthetic organisms other than embryophytic plants, and by this definition include the cyanobacteria as well as a wide range of eukaryotic lineages. Cyanobacteria, as indicated by the occurrence of oxygenic photosynthesis, evolved at least 2.4 Ga, although fossil (including chemical biomarker) evidence for cyanobacteria does not go back beyond 2.1 Ga [1]. Eukaryotic algae have occurred since at least 1.2 Ga [2–4] and from freshwaters, and possibly lake margins, since 1.1 Ga [5] (table 1). Since 2.4 Ga, the biosphere has become increasingly oxygenated [1], reflecting the colonization of the oceans by cyanobacteria after their origin in freshwater habitats, with a corresponding increase in the capacity of these organisms to have global biogeochemical influence [15,16] (table 1). A significant increase in oxygen, with oxygenation of the deep ocean, occurred in the Neoproterozoic 0.54–1 Ga [32], with variations in the Phanaerozoic including the highest known level in the Permo-Carboniferous glaciation [33]. Carbon dioxide varied with a general downward trend, with minima generally related to glaciations [10,34]. CO2 was relatively constant at about 23 (range of estimates 10–100) times the present level between 2.5 and 1.8 Ga, with a very significant decrease between 1.8 and 1.1 Ga [35], further variations in the Neoproterozoic [36–38] and relatively well-established changes in the Phanaerozoic with minima in the Permo-Carboniferous and in the Pleistocene glaciations [33,39]. In this article, we consider how these environmental changes have influenced algal evolution, both through direct effects of the concentrations of CO2 and O2 on photosynthesis and related metabolism, and through indirect effects of changes in temperature (CO2) and the availability of combined nitrogen, phosphorus and iron (CO2 and O2).
Table 1.
Inorganic carbon acquisition characteristics of cyanobacteria and algae related to earliest known occurrence of the taxon. General references on earliest known occurrence of algae: [6–8]. References on presence or absence of CO2-concentrating mechanisms (CCMs): [9–14].
| taxon | occurrence of CCM in extant organisms | oldest known fossil | references |
|---|---|---|---|
| Cyanobacteria | CCM ubiquitous | 2.15 Ga (biomarkers); 2.45 Ga (O2) | [1,15–17] |
| Chlorophyta: Prasinophyceae | CCM ubiquitous | 1.3 Ga | [18,19] |
| Chlorophyta: Chlorophyceae | CCM present in all? | (450 Ma)32 | [19,20] |
| Chlorophyta: Trebouxiophyceae | CCM present or absent | 450 Ma | [19–23] |
| Chlorophyta: Ulvophyceae | CCM usually present; absent in some; C4 in one | 540 Ma | [6,19,20] |
| Streptophyta: Charophyceae | CCM present in all? | 450 Ma | [6,20] |
| Streptophyta: Embryophytes | CCM usually absent; pyrenoid-based CCM in some anthocerophytes, C4 or CAM in some freshwater tracheophytes, CCMs not involving C4 and CAM in some freshwater and all marine tracheophytes | 475 Ma | [6,24,25] |
| Rhodophyta: Bangiophyceae | CCM in all? | 1.2 Ga | [2] |
| Rhodophyta: Florideophyceae | CCM in many, absent from some marine, many freshwater | 600 Ma | [26,27] |
| Ochrista: Bacillariophyceae | CCM in all? | 120 Ma | [28,29] |
| Ochrista: Fucophyceae | CCM in all? | (570 Ma?) | [27,30] |
| Ochrista: Tribophyceae | CCM in all? | 600 Ma | [3,31] |
| Ochrista: Chrysophyceae and Synurophyceae | CCM absent in all | ? | [9] |
2. Autotrophic carboxylases
Six pathways of autotrophic CO2 fixation are known in extant organisms, including ribulose bisphosphate carboxylase-oxygenase carboxylase activity in the photosynthetic carbon reduction cycle (Rubisco–PCRC), using CO2 as the inorganic carbon species assimilated, which is at the core of inorganic carbon assimilation in extant oxygenic photosynthetic organisms [40–45]. These pathways are summarized in table 2 with respect to their stoichiometric requirement for reductant and ATP, their affinities for inorganic carbon expressed in terms of the half-saturation value for CO2 and the influence of oxygen on their functioning.
Table 2.
Energy (NADPH and ATP) stoichiometry, affinity for inorganic carbon expressed as the half-saturation concentration for CO2, competitive inhibition by O2 and damage by O2 for six autotrophic inorganic carbon assimilation pathways. Based on [40–45].
| pathway from inorganic carbon to carbohydrate | NAD(P)H per CO2 | ATP per CO2 | K1/2 CO2 mmol m−3 | O2 competitive inhibition | O2 damage to one or more enzymes |
|---|---|---|---|---|---|
| Rubisco–PCRC, saturating CO2 no O2 | 2 | 3 | ≥10 | yes | no |
| reverse TCAC | 2 | 1.67 | >1500 | no | yesa |
| 3--hydroxypropionate | 2 | 2 | 10 | no | nob |
| 3-hydroxypropionate–4-hydroxybutyrate | 2 | 3 | >2000 | no | O2-insensitive pathway in some organisms living in microaerobic habitats |
| dicarboxylate–4-hydroxybutyrate | 2 | 2.67 | >2000 | no | no; some organisms live in microaerophilic habitats |
| Wood–Ljungdahl pathway | 2 | 1 | 40 000c | no | yes |
aThe reverse tricarboxylic acid cycle (TCAC) can occur in thermophilic aerobic chemolithotrophs as a result of low O2 solubility and high respiratory rates maintaining a low O2 concentration inside the cells, and expression of an O2-insensitive version of the 2-oxoglutarate–ferredoxin oxidoreductase which has at least a fivefold lower specific activity than the O2-sensitive enzyme [43].
bThe most oxygen-sensitive enzyme, methylmalonyol-CoA mutase, can be assayed and even purified at atmospheric equilibrium O2 concentrations; it may not be sufficiently O2-tolerant to function in illuminated cells of oxygenic photosynthetic organisms [43].
cAlthough acetogens live in habitats with higher CO2 concentrations than correspond to atmospheric equilibrium [46], the in vitro K1/2 value cited does seem very high.
Converting one CO2 to the oxidation–reduction level of carbohydrates (CH2O) requires four reducing equivalents at, or lower than, the midpoint redox potential of the NADPH.NADP+ couple. The values given in table 2 indicate the minimal stoichiometry, assuming no redox side reactions or futile cycles. An example of a side reaction for the Rubisco–PCRC pathway is the Rubisco oxygenase activity in the photosynthetic carbon oxidation cycles(s) (Rubisco–PCOC), which occurs at a relatively low [CO2]/[O2] ratio. All of the autotrophic CO2 fixation pathways also require ATP (table 2), with a range of stoichiometries from one to three ATPs for CO2 converted to carbohydrate. The Rubisco–PCRC pathway has the equal highest energy cost of converting CO2 to carbohydrate, with the further energy cost of the side reaction of the Rubisco–PCOC at low [CO2]/[O2] ratios.
The Rubisco–PCRC pathway seems more appropriate to CO2 fixation at the present atmospheric CO2 level when the half-saturation value for CO2 is considered. The forms of Rubisco with the highest affinities for CO2 (Form IB from some algae and vascular plants relying on CO2 diffusion to Rubisco; Form ID from other algae) have half-saturation values for CO2 almost as low as those of two other pathways, while the values for the other three pathways are considerably higher (table 2). The final criterion in table 1 is the effect of oxygen on the pathways. While the Rubisco–PCRC pathway is competitively (with CO2) inhibited by O2, the enzymes of this pathway are not damaged by O2: some of the other pathways have one or more enzymes that are subject to irreversible inhibition by O2 (table 2). For lack of accurate information, the resource cost of synthesizing the enzymic machinery needed to fix one mole of CO2 per second from the present atmospheric CO2 concentration is not shown in table 2. This value is a function of the stoichiometry of the enzymic protein components in the pathway, with that of the carboxylase(s) set by their CO2 affinity, and the Mr (relative molecular mass) values of the enzymes [40,41]. Here, the relatively low-specific reaction rate and high Mr of Rubisco would tend to make the Rubisco–PCRC pathway expensive at present CO2 concentrations, even without the O2 effect, though some other pathways are probably at least as expensive as a result of the low CO2 affinity of their carboxylases and the consequent large quantity of carboxylase needed to fix one mole of CO2 per second from the present atmospheric CO2 concentration.
The occurrence of Rubisco–PCRC as the core carboxylase in oxygenic photosynthetic organisms can be related to opportunity and functionality. By opportunity is meant the occurrence of the pathway at the time (about 2.4 Ga) at which the earliest evidence for oxygenic photosynthetic organisms is known. The Rubisco–PCRC pathway originated before oxygenic photosynthesis evolved (see below). By functionality is meant the carboxylase CO2 affinity and carboxylase Mr values as determinants of the quantity of carboxylase (mass of protein) needed to fix one mole of CO2 per second from the current atmosphere, the extent to which O2 competitively inhibits or damages the enzymes of the pathway, and the ATP cost of the pathway per CO2 assimilated (table 2). The CO2 affinity criterion apparently rules out three of the five pathways (two of which are also very oxygen-sensitive), leaving the 3-hydroxypropionate pathway and the Rubisco–PCRC pathway. While not oxygen inhibited in the manner found for Rubisco, the 3-hydroxypropionate pathway may be sufficiently O2-sensitive to restrict its functionality in oxygenic organisms once O2 had begun to accumulate in the part of the biosphere occupied by oxygenic photosynthetic organisms (tables 1 and 2). In such ways can the role of the Rubisco–PCRC pathway in all known oxygenic photosynthetic organisms be rationalized.
3. Rubisco carboxylase activity and the photosynthetic carbon reduction cycle
Rubisco evolved before the origin of oxygenic photosynthesis [47–49]. The Rubisco gene family not only contains the forms I, II and III Rubiscos that catalyse the Rubisco carboxylase and Rubisco oxygenase reactions, but also the form IV Rubisco-like protein (RLP) which does not catalyse the typical Rubisco reactions and which is involved in methionine salvage in some bacteria [47–49]. Molecular phylogenetic analysis [47–49] suggests that an ancestral form III Rubisco arose in a methanogen (i.e. a member of the Archaea) and gave rise, by two vertical transmissions, to all other form III Rubiscos and to form IV. Horizontal gene transfer then moved form III and form IV Rubiscos to an ancestral bacterium, where form III gave rise to the ancestors of forms I and II, and hence by vertical transmission to the form IV RLP of bacilli and to forms I, II and IV of an ancestral proteobacterium.
Vertical descent and further diversification gave rise to the forms IA, IC, ID and II, and the form IV RLP in extant Proteobacteria. Horizontal gene transfer from the Proteobacteria transferred form IA to cyanobacteria, where form IB evolved from form IA [47–50]. However, there is also evidence that extant α-cyanobacteria with form 1A Rubisco (α-cyanobacteria) acquired their form IA Rubisco (and other α-carboxysomal proteins) from a proteobacterium, displacing the form IB Rubisco and associated β-carboxysomal proteins [51–56]. Endosymbiosis of a heterocystous β-cyanobacterium [57] gave rise to the plastids of the Archaeoplastida (i.e. Plantae), where the form IB Rubisco of the endosymbiont was retained by glaucocystophyte and green algae and hence by vertical transfer to embryophytic (‘higher’) plants, and was moved by secondary endosymbiosis to the (rhizarian) chlorarachniophytes and the (excavate-discicristate) euglenoids. Horizontal gene transfer from a proteobacterium accounts for the presence of form ID Rubisco in red algae and in the chromistan algae (ochrista or heterokontophytes, cryptophytes and haptophytes) whose plastids arose by secondary endosymbiosis of a red alga. The peridinin-containing dinoflagellate and basal apicomplexans (represented today by the photosynthentically competent Chromera velia and an as yet un-named close relative) also obtained their plastids by secondary endosymbiosis from a red alga, but horizontal transfer of form II Rubisco from a proteobacterium replaced the form ID Rubisco [58]. Other dinoflagellates have other forms of Rubisco as a result of tertiary endosymbioses. Finally, a second primary endosymbiosis involving an α-cyanobacterium with form IA Rubisco accounts for the presence of plastids and form IA Rubisco in the (rhizarian) euglyphid amoeba Paulinella [54,55].
Some of the enzymes of the PCRC of vascular plants are derived from cyanobacteria in the primary endosymbiosis leading to the Archaeoplastida (i.e. Plantae), while others are of host origin [59]. This work has now been extended to include the glaucocystophyte and red algal members of the Archaeoplastida [60] and the diatoms whose plastids arose from secondary endosymbiosis of a red alga [61] following an earlier secondary endosymbiosis of a green alga [62]. There are also differences among algae in the regulation of enzymes of the PCRC [63]. Further work is needed to establish the relevance, if any, of atmospheric changes to these differences in the regulation of enzymes of the PCRC [63,64], and also the absence of Rubisco activase from algae with the form ID Rubisco in the few cases examined [61].
It is likely that the earliest Rubiscos in chemolithotrophic and anoxygenic photosynthetic Archaea and Bacteria were operating in a high-CO2 environment and, because there was no oxygenic photosynthesis, in the essential absence of O2. Such an environment would have provided little or no selective pressure for a high CO2 affinity or a high CO2/O2 selectivity relative to a high CO2-saturated maximum catalytic rate, using the mechanistic arguments of Tcherkez et al. [65] that a high maximum catalytic rate is incompatible with high CO2 affinity and higher CO2/O2 selectivity.
The early organisms using Rubisco as a carboxylase would, then, be able to use diffusive entry of CO2, with none of the resource costs associated with Rubisco operating at below the CO2-saturated rate in the presence of O2 when Rubisco oxygenase activity is expressed. The additional resource costs can be considered as capital (synthetic) costs and running costs. The capital costs are those of synthesizing additional Rubisco enzyme per cell if the per cell rate of CO2 assimilation is to be retained, as well as the costs of making the enzymes and transporters related to the operation of a PCOC and/or a CO2-concentrating mechanism (CCM), and include energy, carbon and nitrogen as well as phosphorus for any additional ribosomes that are required [66]. The running costs are in terms of energy, both for synthesizing 2-phosphoglycolate and metabolizing it back to sugar through a PCOC and for operating a CCM.
The early Rubisco-containing organisms could still permit CO2 saturation of a high specific reaction rate Rubisco, with corresponding savings of resources in constructing the enzymic machinery able to assimilate one mole of CO2 per second. In other words, the conditions described would give the lowest possible energy, carbon and nitrogen costs of producing the amount of Rubisco capable of fixing one mole of CO2 per second, and also give the lowest possible energy cost of operating Rubisco, i.e. two NADPH and three ATP per CO2 converted to carbohydrate. A correlated saving associated with this minimal requirement for NADPH and ATP in autotrophic CO2 assimilation is in the quantity of redox and ATP synthesis machinery needed for NADPH and ATP production. Minimizing the protein requirements for autotrophy also decreases the phosphorus requirement for mRNA in ribosomes and in tRNA, and mRNA needed to produce the Rubisco and associated enzymes. These characteristics would minimize the nitrogen needed to produce the machinery associated with a given rate of CO2 fixation, and thus the phosphorus in RNA needed to synthesize the proteins [66]. Costs in energy, nitrogen, phosphorus, iron and manganese will be considered below in the context of decreasing CO2 and increasing O2 [66–74].
The mechanistically constrained covariation in Rubisco kinetics mentioned above, i.e. a high CO2-saturated specific reaction rate correlated with low CO2 affinity and CO2/O2 selectivity and vice versa [65] has parallels with the growth strategies of vascular plants [75] and of algae [76] according to the competitive–stress-tolerant–ruderal (CSR) paradigm, for which there are also mechanistic bases in terms of having high rates of metabolism and growth in ruderals, and lower rates but perhaps a more effective use of resources in stress-tolerators [77–79].
4. Oxygen accumulation, rubisco oxygenase and the metabolism of phosphoglycolate
The build-up of O2 has had many effects on algal evolution, permitting respiration and, via the occurrence of stratospheric ozone, a decreased UV-B flux, and production of reactive oxygen species from O2 rather than UV-B action on cell constituents [79,80]. The presence of O2 does not, however, inhibit O2 production by oxygenic photosynthetic organisms [81,82]. The accumulation of O2 in the habitats of oxygenic photosynthetic organisms using Rubisco as their autotrophic carboxylase permits Rubisco oxygenase activity to occur, provided the CO2 concentration is below saturation for Rubisco. Such decreased CO2 concentrations, combined with at least local O2 accumulation, probably occurred about 2 Ga, when there is evidence of an ice age extending to low palaeolatitudes [35,83].
The product of the Rubisco oxygenase activity is, as well as one 3-phosphoglycerate per O2 consumed, one 2-phosphoglycolate. In addition to sequestering the often limiting resource phosphorus if 2-phosphoglycolate continues to accumulate, 2-phosphoglycolate is also an inhibitor of some reactions involving phosphate esters, including some in the PCRC [84]. Accordingly, all organisms using Rubisco in the presence of O2, i.e. oxygenic photolithotrophs and some chemolithotrophs, have 2-phosphoglycolate phosphatase [84,85]. This enzyme would not have been needed to deal with 2-phosphoglycolate from Rubisco oxygenase activity in anoxygenic photosynthetic proteobacteria, or in any anaerobic chemolithtrophs, using the Rubisco–PRCR pathway before build-up of O2 in the biosphere. 2-Phosphoglycolate phosphatase also occurs in some non-autotrophic bacteria that have no Rubisco, where it is thought to be involved in some forms of DNA repair [86,87]. Since DNA damage and its repair must have occurred before oxygenic photosynthesis, the 2-phosphoglycolate phosphatase in oxygenic photosynthetic organisms could have been recruited from bacteria lacking autotrophic 2-phosphoglycolate synthesis. However, the 2-phosphoglycolate phosphatase from eukaryotes does not seem to have been derived from the 2-phospho-glycolate phosphatase of cyanobacteria [88].
The organic carbon product of 2-phosphoglycolate phosphatase is glycolate. This can be excreted, with loss from the organism of the energy and carbon used in its synthesis. Alternatively, glycolate can be salvaged by metabolism to 3-phosphoglycerate, and hence triose phosphate, which occur in the PCRC, albeit with the input of energy and the release of CO2 [85]. The cyanobacteria have two variants on pathways converting glycolate to 3-phosphoglycerate.
One means of converting glycolate to 3-phosphoglycerate is the pathway via glycine and serine, with recycling of ammonia, as in the classic PCOC of embryophytic plants and at least some eukaryotic algae [85,88–92]. The pathway through glycine and serine seems to have been gained by eukaryotic photosynthetic organisms during the primary endosymbiosis yielding the plastids of the Archaeplastida (i.e. Plantae), although some of the genes in eukaryotes came from α-proteobacteria rather than cyanobacteria [88]. The β-cyanobacterial plastid ancestor gave rise to the glycolate oxidase, glycerate kinase and hydroxypyruvate reductase of algae and embryophytes, while serine hydroxymethyl transferase and the L, P and T subunits of glycine decarboxylase came from α-proteobacteria by horizontal gene transfer [88]. The origin of the other eukaryotic PCOC genes, i.e. those encoding the H subunit of glycine decarboxylase, glutamate–glyoxylate aminotransferase and serine–glyoxylate aminotransferase, has not yet been established [88].
The other pathway from glycolate to 3-phosphoglycerate involves tartronic semialdehyde, and is called the tartronic semialdehyde pathway by phycologists; bacteriologists call it the glycerate pathway, even though glycerate is also an intermediate of the PCOC [85,88–90]. Parts of the PCOC (glycerate dehydrogenase, serine transminases and serine hydroxymethyltransferase) could have been recruited from core metabolism synthesizing serine and glycine from glycolytic intermediates, while others (glycolate dehydrogenase/oxidase, glycine decarboxylase and glycerate kinase) have no known roles other than in the metabolism of glycolate to PCRC intermediates [93,94].
It seems likely that at least one of the metabolic pathways from glycolate to 3-phosphoglycerate evolved in oxygenic photosynthetic organisms relying on diffusive CO2 entry before CCMs evolved. Not only is there at least a minimal flux through Rubisco oxygenase and thence to intermediates of a glycolate metabolism pathway despite high levels of expression of CCMs [85,89,90,92,95], but elimination of the pathways of glycolate metabolism is fatal to the organism [85,90]. Previous misgivings [91,92] about the occurrence of the complete PCOC in diatoms have now been largely overcome, although there are still doubts as to the glycerate kinase step [61,88].
The changes to CO2 fixation in oxygenic photolithotrophs in relation to decreasing CO2 and, especially, increasing O2 is part of a wider range of resource cost increases as the biosphere becomes oxygenated. Falkowski & Godfrey [72] point out that not only is Rubisco impacted by increasing O2 with decreasing CO2, but that the potential for oxygen damage to nitrogenase becomes manifest, and the very source of the O2, the reaction centre of photosystem II, is itself subject to photodamage both directly through excitation energy transfer to the reaction centre, but also indirectly through the accumulated O2 forming reactive O2 species ([66]). As Raven [66,68–70] points out, the effects on Rubisco demand additional nitrogen in the enzyme itself and in related enzymes, more iron and manganese in additional thylakoid redox agents, and more phosphorus in the RNA needed to make the additional protein, if the rate of photosynthesis is to be maintained. More energy input as NADPH and ATP is also needed to run CO2 assimilation [66,69,70]. For oxygen damage to nitrogenase, there is generally synthesis of ‘reserve’ nitrogenase in addition to what is needed in the absence of oxygen damage to satisfy the combined nitrogen requirements of cell growth. Synthesis of the ‘reserve’ nitrogenase requires the nitrogen and energy needed for the synthesis of any protein, but also the iron and (in almost all cases) molybdenum used in the nitrogenase cofactors. When oxygen damage does occur and reserve nitrogenase is used catalytically, more energy (but not nitrogen, iron and molybdenum) is needed to synthesize nitrogenase to replace what is damaged. In both cases, the production of more nitrogenase than would be required in the absence of oxygen involves the use of more phosphorus for the RNA required for the additional protein synthesis. In the case of photoinhibition, more nitrogen and energy are needed to synthesize reserve photosystem II reaction centres, more energy is needed to synthesize replacement photosystem II reaction centres, and more phosphorus is needed for the RNAs needed for the extra protein synthesis. A further aspect of damage to proteins by O2 concerns the absence of any core autotrophic CO2 assimilation pathway other than Rubisco–PCRC from oxygenic photosynthetic organisms. In addition to the CO2 affinity problems outlined for some of the alternative pathways, there would also be the requirement for additional resources (photosynthetically active radiation (PAR), nitrogen and phosphorus) to make and use additional RNA needed for the additional resynthesis of O2-damaged protein (see [66] and above, for other cases).
Compounding this need for additional nitrogen and phosphorus per unit CO2 or N2 assimilated and photons used in photochemistry is the effect of increased O2 on the availability of nitrogen and phosphorus. Falkowski & Godfrey [72] point out that oxygenation of the biosphere not only decreases the potential for diazotrophy, but allows nitrifying microbes to convert 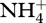 to
to  , which in hypoxic or anoxic micro-habitats can be denitrified to produce N2O and N2. This nitrification–denitrification sequence decreases the availability of combined nitrogen to non-diazotrophic primary producers. In the case of phosphorus, the availability of O2 converts Fe(II) to oxidized iron (Fe(III), which binds phosphate and thus decreases global phosphorus availability [96,97]. Phosphorus is one of the biogeochemical regulators of the O2 content of the atmosphere [96,97]. This topic will be returned to below in the context of ocean deoxygenation as a function of increases in CO2 and temperature.
, which in hypoxic or anoxic micro-habitats can be denitrified to produce N2O and N2. This nitrification–denitrification sequence decreases the availability of combined nitrogen to non-diazotrophic primary producers. In the case of phosphorus, the availability of O2 converts Fe(II) to oxidized iron (Fe(III), which binds phosphate and thus decreases global phosphorus availability [96,97]. Phosphorus is one of the biogeochemical regulators of the O2 content of the atmosphere [96,97]. This topic will be returned to below in the context of ocean deoxygenation as a function of increases in CO2 and temperature.
5. Introduction to co2-concentrating mechanisms
Diffusive entry of CO2 to Rubisco was presumably the ancestral mechanism of autotrophic CO2 assimilation in oxygenic photosynthetic organisms. Entry of CO2 to Rubisco by diffusion is found today in the majority, by species number and contribution to global primary productivity, of terrestrial oxygenic photosynthetic organisms, but in a minority of oxygenic photosynthetic organisms in aquatic environments where photolithotrophs with CCMs predominate [11–13,34,67,79,98–101] (table 1). The references just cited show that CCMs are very widely distributed among algae, both phylogenetically and geographically, although they seem to be absent from chrysophycean and synurophycean algae [9]. The mechanistic, including molecular, details of the CCMs of cyanobacteria are now known [51–53,56,102]. The CCMs of eukaryotic algae are less clearly understood at both the molecular and the mechanistic levels, although they are clearly polyphyletic [10,13,34,67,79,103,104].
As to the evolutionary origin of CCMs, the selective factors were presumably decreasing CO2 and increasing O2. The variability of other gases over the last 2.4 Gyr suggests that there were several periods at which CCMs could have been resource-effective (energy, nitrogen, phosphorus, iron, zinc and manganese) alternatives to diffusive CO2 entry to Rubisco with attendant high activity of Rubisco oxygenase and expression of high levels of enzymes converting 2-phosphoglycolate to sugar phosphate [10,67–71,74,105,106]. We shall return to the question of the timing(s) of the origin(s) of CCMs and how, if they originated early, they were retained through intervening high-CO2 episodes. First, we consider what the transition from diffusive CO2 entry to CCM-based delivery of CO2 to Rubisco involves.
6. The functioning of co2-concentrating mechanisms in comparison with the diffusive entry of co2
One factor in the evolution of a CCM in an organism previously relying on diffusive CO2 movement from the medium to Rubisco as CO2 availability decreases is the kinetics of the Rubisco used by the organism. A Rubisco with a high CO2-saturated catalytic capacity, and a correspondingly low CO2 affinity and CO2/O2 selectivity, such as occurs in the form IA and form IB Rubiscos of extant cyanobacteria, would be expected to present a stronger evolutionary case for a CCM at a given CO2 concentration and CO2/O2 ratio than would eukaryotic form IB and form ID Rubisco with higher CO2 affinity and CO2/O2 selectivity but a lower CO2-saturated maximum catalytic rate [65]. Young et al. [107] have used molecular phylogenetic evidence to show that there was positive selection of the form ID Rubiscos in some eukaryotes that correspond to low-CO2 and low-CO2/O2 episodes in the geological record, and that these episodes of positive selection could have corresponded to the time of evolution of CCMs. To be effective, the CCM must maintain a higher CO2 concentration at the site of Rubisco than would be possible by CO2 diffusion alone [10].
The essential component of a CCM is the accumulation of CO2 in the compartment containing Rubisco to a higher steady-state concentration than occurs in the growth medium, and hence even higher than the steady-state concentration near Rubisco which could occur with diffusive CO2 entry. For algae, one mechanism of accumulation could involve C4-like photosynthetic metabolism with an ATP-dependent (C3 + C1) carboxylation in the cytosol, using  obtained directly or indirectly (via CO2 entry from the medium followed by carbonic anhydrase catalysis) from the medium, followed by a (C4–C1) decarboxylation in the chloroplast [104]. The alternative mechanisms do not involve the inorganic carbon transferred from the medium to Rubisco forming organic intermediates. These alternative mechanisms involve transmembrane-active transport mechanisms which move an inorganic carbon species (CO2 or
obtained directly or indirectly (via CO2 entry from the medium followed by carbonic anhydrase catalysis) from the medium, followed by a (C4–C1) decarboxylation in the chloroplast [104]. The alternative mechanisms do not involve the inorganic carbon transferred from the medium to Rubisco forming organic intermediates. These alternative mechanisms involve transmembrane-active transport mechanisms which move an inorganic carbon species (CO2 or  ), or H+, against a free-energy gradient. Such transporters could be (and have been) derived from transporter gene families by change of specificity of the transported substrate (to CO2 or
), or H+, against a free-energy gradient. Such transporters could be (and have been) derived from transporter gene families by change of specificity of the transported substrate (to CO2 or 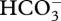 ), with changes in regulation and, perhaps, changes in intracellular targeting [51–53,56]. An exception to the need for active transport across a membrane is CO2 use in the cyanobacterial CCMs, where diffusive CO2 entry across the plasmalemma is followed by energized conversion to
), with changes in regulation and, perhaps, changes in intracellular targeting [51–53,56]. An exception to the need for active transport across a membrane is CO2 use in the cyanobacterial CCMs, where diffusive CO2 entry across the plasmalemma is followed by energized conversion to 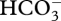 by the NAD(P)H—PQ oxidoreductase of the thylakoid membrane [51–53,56]. Here, a high CO2 permeability of the plasmalemma is required. Such a high membrane permeability to CO2 is needed for diffusive CO2 entry all the way to Rubisco in organisms lacking a CCM, and (as noted above) in CO2 ‘active transport’ in cyanobacteria. A high CO2 permeability is also necessary in organisms with a CCM mechanism involving CO2 production from
by the NAD(P)H—PQ oxidoreductase of the thylakoid membrane [51–53,56]. Here, a high CO2 permeability of the plasmalemma is required. Such a high membrane permeability to CO2 is needed for diffusive CO2 entry all the way to Rubisco in organisms lacking a CCM, and (as noted above) in CO2 ‘active transport’ in cyanobacteria. A high CO2 permeability is also necessary in organisms with a CCM mechanism involving CO2 production from 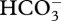 in a compartment acidified by a H+ pump followed by transmembrane movement of CO2 into the compartment containing Rubisco [13]. The energized conversion of CO2 to
in a compartment acidified by a H+ pump followed by transmembrane movement of CO2 into the compartment containing Rubisco [13]. The energized conversion of CO2 to 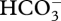 in the cyanobacterial cytosol could increase the chance of any CO2 that leaks out of the carboxysomes being trapped as
in the cyanobacterial cytosol could increase the chance of any CO2 that leaks out of the carboxysomes being trapped as 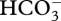 in the cytosol. In all other cases, a CCM is most energetically efficient with minimal CO2 flux from the compartment in which it is accumulated back to the medium, i.e. with very low membrane permeabilities to CO2.
in the cytosol. In all other cases, a CCM is most energetically efficient with minimal CO2 flux from the compartment in which it is accumulated back to the medium, i.e. with very low membrane permeabilities to CO2.
The energetic savings that could result from a low CO2 permeability of the plasmalemma, and of the inner plastid envelope (if that is the membrane involved in active transport of inorganic carbon), in eukaryotes with CCMs based on active transport of an inorganic carbon species would, if verified, suggest a phylogenetic, and in many cases, an acclimatory (changes from growth in high to low CO2) decrease in CO2 permeability. This question has been addressed by a number of workers [103,108], who found relatively high CO2 permeability in the eukaryotic algal membranes examined, regardless of whether the algae had been cultured in high (CCM repressed) or low (CCM expressed) inorganic carbon concentrations. The permeability values for CO2 of the plasmalemma of high-CO2 and low-CO2-grown cells of Chlamydomonas reinhardtii range from 0.76 to 1.8.10−3 cm s−1 [108], while for four species of diatom the range is 15–56.10−3 cm s−1 [103], with the range of values probably related to methodological as well as phylogenetic differences. While models for CCMs in diatoms consistent with the available data show relatively modest energy losses during CCM operation, they do involve constraints such as the membrane(s) at which active inorganic carbon transport occurs, and the chemical species involved in this active transport [103]. The same argument applies to the protein shell of the carboxysome, for which a restriction on CO2 diffusion, but not on the diffusion of anionic substrates ( and ribulose-1,5-bisphosphate) and product (3-phosphoglycerate) has been demonstrated [109].
and ribulose-1,5-bisphosphate) and product (3-phosphoglycerate) has been demonstrated [109].
Most CCMs also involve one or more carbonic anhydrase enzymes: an exception is a C4 mechanism which involves, as indicated above, 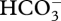 entry to the cytosol, (C3 + C1) carboxylation using
entry to the cytosol, (C3 + C1) carboxylation using  , and (C4 – C1) decarboxylation in the plastid stroma with CO2 as the inorganic carbon product [13,92,95,104,110]. All other well-investigated CCMs seem to involve ‘normal’ carbonic anhydrases, i.e. those catalysing the equilibration of CO2 and
, and (C4 – C1) decarboxylation in the plastid stroma with CO2 as the inorganic carbon product [13,92,95,104,110]. All other well-investigated CCMs seem to involve ‘normal’ carbonic anhydrases, i.e. those catalysing the equilibration of CO2 and 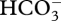 [11,67,111]: this is the case for ‘active CO2 influx’ in cyanobacteria, which involves a carbonic anhydrase in the carboxysome as well as the energized conversion of CO2 to
[11,67,111]: this is the case for ‘active CO2 influx’ in cyanobacteria, which involves a carbonic anhydrase in the carboxysome as well as the energized conversion of CO2 to 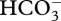 at the thylakoid membrane, which is effectively a unidirectional carbonic anhydrase [56].
at the thylakoid membrane, which is effectively a unidirectional carbonic anhydrase [56].
The functioning of CCMs is influenced by a number of factors other than the availability of inorganic carbon (and, in some cases, O2), e.g. photosynthetically active radiation, UV-B radiation, the form and the concentration of combined nitrogen, the phosphate concentration and the iron concentration [66,67,74,105]. The influence of these factors on the expression and functioning of CCMs is presented in table 3 [66,67,74]. There are also predicted effects of expression of CCMs rather than reliance on diffusive entry of CO2 on the resource costs of synthesis of the photosynthetic apparatus, and of its operation; these are discussed below. We next discuss the possible influence of these interactions on the evolution of CCMs, their retention through any high-CO2 episodes between their origin in a low-CO2 habitat and today, and their fate in a future higher CO2 and warmer world.
Table 3.
Influence of environmental conditions on the expression of CCMs and the resource cost of CCM operation versus diffusive entry of CO2. Effects on CCMs of environmental factors, and the direction of change of these environmental factors in algal and aquatic plant habitats with global environmental change between icehouse episodes. Modified from [10]. Further details and references are given in the text and in [10–12,101,105,106,110,112–120]. Predicted resource costs of synthesizing and operating a photosynthetic apparatus using a CCM relative to one relying on entry of CO2 by diffusion [66,68–71,73,91,105,121]. DOC, dissolved organic carbon; n.a., not applicable.
| factor | change to algal environment caused by variation in the factor | effects on expression of CCMs and on their affinity for CO2 | predicted effect of CCM expression on resource costs of synthesis and (for PAR) operation of the photosynthetic apparatus |
|---|---|---|---|
| CO2 | increase in CO2 in essentially all environments, although less predictable effect in freshwaters which can be out of equilibrium with the atmosphere | decreased inorganic carbon affinity with growth at high CO2; can be a switch to diffusive CO2 entry in some eukaryotes | no general effect on carbon cost of synthesis of the photosynthetic apparatus |
| temperature | increase in temperature in all environments | prediction of increased CCM expression, or increased fraction of organisms with CCMs, at higher temperatures as a result of lower CO2 solubility, is not uniformly supported by the available data. | inapplicable: temperature (Boltzmann energy) is not resource consumed in the synthesis of the photosynthetic apparatus, in its operation or in its maintenance |
| PAR | increase in PAR in pelagic planktonic environments | decreased inorganic carbon affinity with growth at low PAR | possible decreased energy needed in synthesis of photosynthetic apparatus which uses a CCM. Energy saving in operation of a CCM if there is low CO2 leakage and a low CO2 affinity and low CO2:O2 selectivity |
| nitrogen | decrease in combined nitrogen in upper mixed layer of lotic environments | generally increased inorganic carbon affinity with growth at low 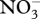 . One example each of decreased carbon affinity with growth at lowest . One example each of decreased carbon affinity with growth at lowest  concentration tested, and with growth over entire concentration tested, and with growth over entire  range tested. range tested. |
decreased nitrogen cost of synthesis of the photosynthetic apparatus incorporating a CCM if the savings in the synthesis of smaller amounts per cell of Rubisco and of the PCOC enzymes are not offset by the nitrogen cost of the synthesis of CCM components. No consumption of nitrogen in the operation of the CCM |
| phosphorus | decrease in phosphate in upper mixed layer of lotic environments | two examples of increased inorganic carbon affinity, two examples of decreased inorganic carbon affinity, with growth at low phosphate | where there is a decreased protein content in the photosynthetic apparatus there could be a corresponding decrease in the need for phosphorus needed to synthesize the photosynthetic apparatus as a result of decreased requirement for RNA |
| iron | probable decrease in iron in upper mixed layer of lotic environments | one example of increased inorganic carbon affinity with growth at low iron, one example of no effect | decreased Fe content of the photosynthetic apparatus if the decreased requirement for NADPH in the near-absence of Rubisco oxygenase activity, and the PCOC, with correspondingly lower content of non-cyclic electron transport components, is not offset by the additional thylakoid components needed for the additional ATP requirement for CCM operation, especially if this additional ATP is made using cyclic photophosphorylation using only phosystem I with its high Fe content |
| zinc | as for iron | no direct measurements | variable predictions of relative zinc requirements depending on CCM mechanism |
| UV-A | increase in UV-A in lotic planktonic environments, but decrease with higher concentration of DOC | no data | n.a. |
| UV-B | increase in UV-B in lotic planktonic environments, but decrease with higher concentration of DOC | variable responses of CCMs with increased UV-B flux for growth | n.a. |
7. The origins of co2-concentrating mechanisms
The ‘why’ of the origin of CCMs presumably concerns the occurrence of low CO2, both in absolute terms and in relation to O2, which was indicated above as requiring additional protein (hence RNA and phosphorus) in more Rubisco and in enzymes metabolizing 2-phosphoglycolate to sugar phosphate, as well as additional energy input per net CO2 assimilated by diffusive CO2 entry of CO2 to Rubisco and metabolism of 2-phosphoglycolate. Depending on the circumstances, e.g. the form of Rubisco present and the environmental conditions as well as the mechanism of the particular CCM [10,34,68–71,74,79,91,103,105], a CCM could require less energy, nitrogen, phosphorus, zinc and iron for its synthesis, and less energy for its operation, than diffusive CO2 entry with metabolism of 2-phosphoglycolate (table 2). Other factors that could have influenced the evolution of CCMs include the decreasing UV-B flux with the increased stratospheric O3 resulting from the build-up of O2, which is itself an influence on the evolution of CCMs [79]. UV-B radiation causes damage to Rubisco and to photosystem II, but has less effect on photosystem I. The limited data available suggest that UV-B has little effect on CCM activity in the green alga Dunaliella tertiolecta [122] but elevated CO2 can increase the sensitivity of microalgae to UV-B [123,124]. There is no information about the possibility of a differential impact of UV-B on the various forms of Rubisco, though it would be interesting to know if changes in UV-B in the past relate to the evolution of different Rubiscos.
The ‘how’ of the origin of CCMs concerns the ancestry of the various components of the pathway. For the active transport components, H+ pumps are ubiquitous and anion transporters/pumps (hence 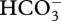 transporters/pumps) are also widespread, as could be the ancestors of CO2 pumps [10,13,34,51–53,56,79,102,104]. Facilitators of downhill transmembrane CO2 transport, yielding permeabilities in excess of those owing to the lipid phase alone, are required for cyanobacterial active transporters and for the mechanism involving an acidified compartment generating CO2 from
transporters/pumps) are also widespread, as could be the ancestors of CO2 pumps [10,13,34,51–53,56,79,102,104]. Facilitators of downhill transmembrane CO2 transport, yielding permeabilities in excess of those owing to the lipid phase alone, are required for cyanobacterial active transporters and for the mechanism involving an acidified compartment generating CO2 from  with subsequent transmembrane CO2 diffusion to Rubisco. Such facilitators would presumably have originally been components of the diffusive pathway for CO2 from the medium to Rubisco. Carbonic anhydrases could have had a number of roles prior to their co-option into CCMs, including that of facilitating diffusion of CO2 (as
with subsequent transmembrane CO2 diffusion to Rubisco. Such facilitators would presumably have originally been components of the diffusive pathway for CO2 from the medium to Rubisco. Carbonic anhydrases could have had a number of roles prior to their co-option into CCMs, including that of facilitating diffusion of CO2 (as 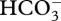 ) in the diffusive entry of CO2 from the medium to Rubisco. Cyanobacterial carboxysomes are part of a larger family of bacterial micro-compartments [125]. This brief view may be over-optimistic as to the ease of co-opting existing mechanisms into CCMs [34], and does not address the origin of the eukaryotic pyrenoid [14]; however, it does indicate some possibilities.
) in the diffusive entry of CO2 from the medium to Rubisco. Cyanobacterial carboxysomes are part of a larger family of bacterial micro-compartments [125]. This brief view may be over-optimistic as to the ease of co-opting existing mechanisms into CCMs [34], and does not address the origin of the eukaryotic pyrenoid [14]; however, it does indicate some possibilities.
There are a number of options as to the ‘when’ of the evolution of CCMs. We initially consider times of relatively low CO2, based on low palaeotemperatures (with the requirement that greenhouse gases corrected for the faint young Sun) or on biogeochemical or biological proxies. Glacial/low CO2 episodes occurred 2.4–2.1 Ga and at 750, 650 and 320–270 Ma, as well as the Pleistocene (last 2.4 Myr) [10]. All but the earliest of these times would have been relevant to at least some of the eukaryotic as well as cyanobacterial oxygenic photosynthetic organisms (table 1).There is no direct fossil evidence as to the origin of CCMs, and little help from molecular clocks [10,67,79], although recent work by Young et al. [107] shows episodes of positive selection of form ID Rubisco in diatoms and haptophytes which correspond to low-CO2 episodes and hence possibly relate to the origin of CCMs. Assuming, as seems very likely, that cyanobacterial and at least some algal CCMs evolved before the Pleistocene, they would have had to have survived intervening period(s) of higher CO2 and higher temperatures. The mechanisms of retention of CCMs in these apparently unfavourable environments are now considered in the context of the response of present day CCMs to such environments.
8. Retention of co2-concentrating mechanisms in high-co2 episodes
It is possible to argue for long-lasting low-CO2 micro-habitats, e.g. in benthic microbial mats, including stromatolites, where inorganic carbon diffusion from the bulk medium into the cells is restricted by thick diffusion boundary layers [79]. Biogeochemically more important are planktonic habitats where such low CO2 refuges are less plausible. CCM retention is considered in the context of present work on the response of phytoplankton to current and expected environmental change, and especially increasing CO2 and the associated warming.
More widespread (planktonic) retention of CCMs in relatively high CO2 concentrations has been argued by Raven et al. [10] in the context of what might happen with increasing CO2 and temperature over the next several decades. The argument here is that the increased buoyancy of warmer surface waters will lead to a shoaling of the thermocline, which will decrease fluxes of combined nitrogen and of phosphorus from the deeper ocean where mineralization of sinking particulate organic matter occurs, which have been modelled as decreasing global marine primary productivity [126–128]. This decreased nutrient supply, with the increased mean flux of photosynthetically active radiation (and UV-B) incident on the cells in the shallower upper mixed layer will, on the basis of observations on extant phytoplankton (table 3), favour retention of CCMs despite higher CO2 concentrations [10].
A further relevant consideration which was not mentioned by Raven et al. [10] is that a warmer upper mixed layer has decreased oxygen solubility. For the same rate of net oxygen production at two temperatures, there will be greater degree of oxygen superaturation and hence a greater loss of oxygen to the atmosphere at the higher temperature. Together with the decreased solute transfer between the upper mixed layer and deeper ocean, there is less transfer of oxygen below the thermocline [129–131]. The widespread, but not universal, decrease in calcification by calcified plankton [132–134], and the much smaller effect on silicification by diatoms [135] in a higher CO2, warmer ocean means less ballasting of sinking particulate organic matter, hence slower sinking and more microbial mineralization just below the thermocline ([136], cf. [137]). While this higher nutrient concentration just below the thermocline might be expected to partly offset the lower rate constant for nutrient transfer to the upper mixed layer, another factor must be considered. The combination of more microbial respiration and increased oxygen supply can lead to hypoxia and even anoxia in certain sub-surface waters, with implications for loss of the nitrate and nitrite forms of combined nitrogen produced from organic matter by mineralization and nitrification in less deoxygenated zones followed by denitrification or the anammox reaction in more deoxygenated places [131], with a decrease in the nitrogen : phosphorus ratio in the nutrients reaching the upper mixed layer. Although, in the long term (thousands of years and more), a warmer world would heat the ocean interior as well as the upper mixed layer and potentially decrease the extent to which the thermocline shoals, there is a well-established correlation of widespread deep-ocean anoxia (and even euxinia) with warmer, high-CO2 episodes in Earth history [138], so at least a decreased upward flux of combined nitrogen across the thermocline would continue, favouring retention of CCMs.
These arguments are based on the response of extant algae to the changes occurring, and predicted, in their environment as a result of increased CO2 and temperature, with downstream effects on the marine and inland water inorganic system, the mixed layer depth and water body oxygenation. This can be used to inform us of how CCMs were retained in past episodes of high CO2. Dealing first with the organisms studied, almost all of the work has been carried out with organisms which have only been exposed to the increased CO2 and associated changes in temperature and the availability of other resources (table 3) for time periods of days to weeks. This time period allows 1–100 generation, meaning that only regulatory (altering the existing proteome by post-translational modification, and changes in the metabolome) and acclimatory (altering the expressed proteome based on the existing genome) [139] responses of extant algae can be expressed. In very few cases has relevant evolutionary evidence been sought in laboratory experiments for increased CO2 [139–146] and, using different methods, higher temperatures [147]. An example of where evolution has been unable to cope with natural CO2 enrichment present for several decades concerns calcified red algae growing on seagrass leaves near an underwater vent in the Mediterranean [148]. Even for studies of regulation and acclimation there can be problems with the length of time that an alga has been in culture and frequently exposed to CO2, nitrogen, phosphorus, PAR, UV and temperature which has little relevance to their natural environment [149]. Furthermore, there is a relative lack of studies in which changes in CO2 have been combined with other relevant environmental changes: table 3 and Raven et al. [10] analyse and give references to the important work in which such interactions have been studied. Finally, there are some relatively poorly constrained factors of ocean chemistry in the past [80,150–154], and until recently little consensus as to the appropriate methodology [134,155–158] for laboratory and mesocosm experimentation on increased CO2.
Despite these reservations, which also apply to other models of past and future atmosphere–ocean–organism interactions, the suggestions of Raven et al. [27] provide a lead into further studies in how CCMs could be retained in lengthy episodes between shorter low-CO2 episodes. Any retention of CCMs in high-CO2 episodes would be a further complication in the use of stable carbon isotope ratios of phytoplankton-derived organic carbon from marine sediments as a palaeobarometer for CO2, since most marine phytoplankton today have CCMs [67,98,159,160]. Organisms lacking CCMs, e.g. terrestrial liverworts and mosses, do not suffer from this problem when used in palaeobarometry of CO2 [161].
9. Conclusions
The changing CO2 and O2 concentration over the last 2.4 Gyr have had significant effects on the physiology and ecology of cyanobacteria and algae. From the presumed ancestral diffusive CO2 entry to Rubisco, all extant cyanobacteria have CCMs, Rubiscos with high CO2-saturated catalytic activity and low CO2 affinity and CO2/O2 selectivity, and an essential role for the capacity to convert the 2-phosphoglycolate formed as a very small fraction of the total carbon flux into triose phosphates. Most eukaryotic algae have CCMs: a greater fraction has CCMs in the sea than in freshwaters, and there is no strong relationship to water temperatures. The evolution of CCMs can apparently be related to decreased CO2 availability and to the presence of oxygen, modulated by the kinetics of the form of Rubisco in the organisms, with some components of the CCMs adapted in evolution from the roles in other pathways. The retention of CCMs during the high-CO2 episodes predominant through Earth history could have been related in part to the interaction of CCM expression with other environmental factors which change in high-CO2 water bodies.
Acknowledgements
The University of Dundee is a registered Scottish Charity, No SC10596. J.A.R.'s research on inorganic carbon acquisition was funded by BBSRC UK and NERC UK. J.A.R. thanks David Beerling for the invitation to lecture at the meeting at the Kavli Royal Society International Centre from which this paper, and the Theme Issue, arose, and also Anthony Cohen for the invitation to the Ocean Deoxygenation meeting at the Kavli Royal Society International Centre which expanded his reading on ocean deoxygenation. J.B. is grateful to the Australian Research Council for their support of his work on inorganic carbon acquisition in relation to climate change. M.G.'s research on CCMs was partially funded by the Fondazione Cariverona, Italy, the Southeast Wisconsin Energy Technology Research Center, USA and the Ministry for Agriculture and Forestry (MIPAF), Italy (project Bioforme).
We thank Ian McCulloch for his help in converting the references from Harvard to Vancouver.
References
- 1.Rasmussen B., Fletcher I. R., Brocks J. J., Kilburn M. R. 2008. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 10.1038/nature07381 (doi:10.1038/nature07381) [DOI] [PubMed] [Google Scholar]
- 2.Butterfield N. J. 2000. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26, 386–404 (doi:10.1666/0094-8373(2000)026<0386:bpngns<2.0.co;2) [DOI] [Google Scholar]
- 3.Butterfield N. J. 2004. A vaucheriacean alga from the middle Neoproterozoic of Spitsbergen: implications for the evolution of Proterozoic eukaryotes and the Cambrian explosion. Paleobiology 30, 231–252 (doi:10.1666/0094-8373(2004)030<0231:avaftm<2.0.co;2) [DOI] [Google Scholar]
- 4.Butterfield N. J., Knoll A. H., Swett K. 1988. Exceptional preservation of fossils in an upper Proterozoic shale. Nature 334, 424–427 10.1038/334424a0 (doi:10.1038/334424a0) [DOI] [PubMed] [Google Scholar]
- 5.Strother P. K., Battison L., Brasier M. D., Wellman C. H. 2011. Earth's earliest non-marine eukaryotes. Nature 473, 505–509 10.1038/nature09943 (doi:10.1038/nature09943) [DOI] [PubMed] [Google Scholar]
- 6.Taylor T. N., Taylor E. L., Krings M. 2009. Paleobotany: the biology and evolution of fossil plants, 2nd edn. London, UK: Academic [Google Scholar]
- 7.Graham L. E., Wilcox L. W. 2000. Algae. Upper Saddle River, NJ: Prentice Hall [Google Scholar]
- 8.Van den Hoek C., Mann D. G., Jahns H. M. 1995. Algae: an introduction to phycology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Maberly S. C., Ball L. A., Raven J. A., Sueltemeyer D. 2009. Inorganic carbon acquisition by chrysophytes. J. Phycol. 45, 1052–1061 10.1111/j.1529-8817.2009.00734.x (doi:10.1111/j.1529-8817.2009.00734.x) [DOI] [PubMed] [Google Scholar]
- 10.Raven J. A., Giordano M., Beardall J., Maberly S. C. 2011. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 109, 1–16 10.1007/s11120-011-9632-6 (doi:10.1007/s11120-011-9632-6) [DOI] [PubMed] [Google Scholar]
- 11.Raven J. A., et al. 2002. Seaweeds in cold seas: evolution and carbon acquisition. Ann. Bot. 90, 525–536 10.1093/aob/mcf171 (doi:10.1093/aob/mcf171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raven J. A., et al. 2002. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Funct. Plant Biol. 29, 355–378 10.1071/pp01201 (doi:10.1071/pp01201) [DOI] [PubMed] [Google Scholar]
- 13.Raven J. A. 2010. Inorganic carbon acquisition by eukaryotic algae: four current questions. Photosynth. Res. 106, 123–134 10.1007/s11120-010-9563-7 (doi:10.1007/s11120-010-9563-7) [DOI] [PubMed] [Google Scholar]
- 14.Badger M. R., Andrews T. J., Whitney S. M., Ludwig M., Yellowlees D. C., Leggat W., Price G. D. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. Rev. Can. Bot. 76, 1052–1071 10.1139/cjb-76-6-1052 (doi:10.1139/cjb-76-6-1052) [DOI] [Google Scholar]
- 15.Sanchez-Baracaldo P., Hayes P. K., Blank C. E. 2005. Morphological and habitat evolution in the Cyanobacteria using a compartmentalization approach. Geobiology 3, 145–165 10.1111/j.1472-4669.2005.00050.x (doi:10.1111/j.1472-4669.2005.00050.x) [DOI] [Google Scholar]
- 16.Blank C. E., Sanchez-Baracaldo P. 2010. Timing of morphological and ecological innovations in the cyanobacteria—a key to understanding the rise in atmospheric oxygen. Geobiology 8, 1–23 10.1111/j.1472-4669.2009.00220.x (doi:10.1111/j.1472-4669.2009.00220.x) [DOI] [PubMed] [Google Scholar]
- 17.Schirrmeister B. E., Antonelli A., Bagheri H. C. 2011. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 11, 45.(doi:10.1186/1471-2148-11-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teyssedre B. 2006. Are the green algae (phylum Viridiplantae) two billion years old? Carnets Geol. 3, 1–15 [Google Scholar]
- 19.Becker B., Marin B. 2009. Streptophyte algae and the origin of embryophytes. Ann. Bot. 103, 999–1004 10.1093/aob/mcp044 (doi:10.1093/aob/mcp044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis L. A., McCourt R. M. 2004. Green algae and the origin of land plants. Am. J. Bot. 91, 1535–1556 10.3732/ajb.91.10.1535 (doi:10.3732/ajb.91.10.1535) [DOI] [PubMed] [Google Scholar]
- 21.Derenne S., Metzger P., Largeau C., Vanbergen P. F., Gatellier J. P., Damste J. S. S., Deleeuw J. W., Berkaloff C. 1992. Similar morphological and chemical variations of Gloeocapsomorpha prisca in Ordovician sediments and cultured Botryococcus braunii as a response to changes in salinity. Org. Geochem. 19, 299–313 10.1016/0146-6380(92)90001-e (doi:10.1016/0146-6380(92)90001-e) [DOI] [Google Scholar]
- 22.Senousy H. H., Beakes G. W., Hack E. 2004. Phylogenetic placement of Botryococcus braunii (Trebouxiophyceae) and Botryococcus sudeticus isolate UTEX 2629 (Chlorophyceae). J. Phycol. 40, 412–423 10.1046/j.1529-8817.2004.03173.x (doi:10.1046/j.1529-8817.2004.03173.x) [DOI] [Google Scholar]
- 23.Zhang Z., Metzger P., Sachs J. P. 2007. Biomarker evidence for the co-occurrence of three races (A, B and L) of Botryococcus braunii in El Junco Lake, Galápagos. Org. Geochem. 38, 1459–1478 10.1016/j.orggeochem.2007.05.015 (doi:10.1016/j.orggeochem.2007.05.015) [DOI] [Google Scholar]
- 24.Rubinstein C. V., Gerrienne P., de la Puente G. S., Astini R. A., Steemans P. 2010. Early Middle Ordovician evidence for land plants in Argentina (Eastern Gondwana). New Phytol. 188, 365–369 10.1111/j.1469-8137.2010.03433.x (doi:10.1111/j.1469-8137.2010.03433.x) [DOI] [PubMed] [Google Scholar]
- 25.Wellman C. H. 2010. The invasion of the land by plants: when and where? New Phytol. 188, 306–309 10.1111/j.1469-8137.2010.03471.x (doi:10.1111/j.1469-8137.2010.03471.x) [DOI] [PubMed] [Google Scholar]
- 26.Xiao S. H., Knoll A. H., Yuan X. L., Pueschel C. M. 2004. Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae. Am. J. Bot. 91, 214–227 10.3732/ajb.91.2.214 (doi:10.3732/ajb.91.2.214) [DOI] [PubMed] [Google Scholar]
- 27.Yuan X., Chen Z., Xiao S., Zhou C., Hua H. 2011. An early Ediacaran assemblage of macroscopic and morphologically differentiated eukaryotes. Nature 470, 389–392 10.1038/nature09810 (doi:10.1038/nature09810) [DOI] [PubMed] [Google Scholar]
- 28.Raven J. A., Waite A. M. 2004. The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytol. 162, 45–61 10.1111/j.1469-8137.2004.01022.x (doi:10.1111/j.1469-8137.2004.01022.x) [DOI] [Google Scholar]
- 29.Sims P. A., Mann D. G., Medlin L. K. 2006. Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45, 361–402 10.2216/05-22.1 (doi:10.2216/05-22.1) [DOI] [Google Scholar]
- 30.Xu Z. L. 2001. New discoveries of phaeophycean fossils in the Early Cambrian, Haikou, Kunming, Yunnan, southwest China. Acta Bot. Sin. 43, 1072–1076 [Google Scholar]
- 31.Javaux E. J., Knoll A. H., Walter M. 2003. Recognizing and interpreting the fossils of early eukaryotes. Orig. Life Evol. Biosphere 33, 75–94 10.1023/a:1023992712071 (doi:10.1023/a:1023992712071) [DOI] [PubMed] [Google Scholar]
- 32.Shields-Zhou G., Och L. 2011. The case for a Neoproterozoic oxygenation event: geochemical evidence and biological consequences. GSA Today 21, 4–11 10.1130/GSATG102A.1 (doi:10.1130/GSATG102A.1) [DOI] [Google Scholar]
- 33.Berner R. A. 2006. GEOCARBSULF: a combined model for Phanerozoic atmsopheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664 10.1016/j.gca.2005.11.032 (doi:10.1016/j.gca.2005.11.032) [DOI] [Google Scholar]
- 34.Raven J. A., Giordano M., Beardall J. 2008. Insights into the evolution of CCMs from comparisons with other resource acquisition and assimilation processes. Physiol. Plant 133, 4–14 10.1111/j.1399-3054.2007.01024.x (doi:10.1111/j.1399-3054.2007.01024.x) [DOI] [PubMed] [Google Scholar]
- 35.Sheldon N. D. 2006. Precambrian paleosols and atmospheric CO2 levels. Precambrian Res. 147, 148–155 10.1016/j.precamres.2006.02.004 (doi:10.1016/j.precamres.2006.02.004) [DOI] [Google Scholar]
- 36.Riding R. 2006. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic–Cambrian changes in atmospheric composition. Geobiology 4, 299–316 10.1111/j.1472-4669.2006.00087.x (doi:10.1111/j.1472-4669.2006.00087.x) [DOI] [Google Scholar]
- 37.Bao H., Lyons J. R., Zhou C. 2008. Triple oxygen isotope evidence for elevated CO2 levels after a Neoproterozoic glaciation. Nature 453, 504–506 10.1038/nature06959 (doi:10.1038/nature06959) [DOI] [PubMed] [Google Scholar]
- 38.Bjerrum C. J., Canfield D. E. 2011. Towards a quantitative understanding of the late Neoproterozoic carbon cycle. Proc. Natl Acad. Sci. USA 108, 5542–5547 10.1073/pnas.1101755108 (doi:10.1073/pnas.1101755108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riding R. 2009. An atmospheric stimulus for cyanobacterial-bioinduced calcification ca. 350 million years ago? Palaios 24, 685–696 10.2110/palo.2009.p09-033r (doi:10.2110/palo.2009.p09-033r) [DOI] [Google Scholar]
- 40.Raven J. A. 2009. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 56, 177–192 10.3354/ame01315 (doi:10.3354/ame01315) [DOI] [Google Scholar]
- 41.Bar-Even A., Noor E., Lewis N. E., Milo R. 2010. Design and analysis of synthetic carbon fixation pathways. Proc. Natl Acad. Sci. USA 107, 8889–8894 10.1073/pnas.0907176107 (doi:10.1073/pnas.0907176107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg I. A., Kockelkorn D., Ramos-Vera W. H., Say R. F., Zarzycki J., Huegler M., Alber B. E., Fuchs G. 2010. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 8, 447–460 10.1038/nrmicro2365 (doi:10.1038/nrmicro2365) [DOI] [PubMed] [Google Scholar]
- 43.Berg I. A. 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 77, 1925–1936 10.1128/aem.02473-10 (doi:10.1128/aem.02473-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle N. R., Morgan J. A. 2011. Computation of metabolic fluxes and efficiencies for biological carbon dioxide fixation. Metabol. Eng. 13, 150–158 10.1016/j.ymben.2011.01.005 (doi:10.1016/j.ymben.2011.01.005) [DOI] [PubMed] [Google Scholar]
- 45.Hüegler M., Sievert S. M. 2011. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annu. Rev. Mar. Sci. 3, 261–289 10.1146/annurev-marine-120709-142712 (doi:10.1146/annurev-marine-120709-142712) [DOI] [PubMed] [Google Scholar]
- 46.Ljungdhal L. G. 1986. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 40, 415–450 10.1146/annurev.mi.40.100186.002215 (doi:10.1146/annurev.mi.40.100186.002215) [DOI] [PubMed] [Google Scholar]
- 47.Tabita F. R., Hanson T. E., Li H., Satagopan S., Singh J., Chan S. 2007. Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs. Microbiol. Mol. Biol. Rev. 71, 576. 10.1128/mmbr.00015-07 (doi:10.1128/mmbr.00015-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabita F. R., Hanson T. E., Satagopan S., Witte B. H., Kreel N. E. 2008. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Phil. Trans. R. Soc. B 363, 2629–2640 10.1098/rstb.2008.0023 (doi:10.1098/rstb.2008.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabita F. R., Satagopan S., Hanson T. E., Kreel N. E., Scott S. S. 2008. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 59, 1515–1524 10.1093/jxb/erm361 (doi:10.1093/jxb/erm361) [DOI] [PubMed] [Google Scholar]
- 50.Marin B., Nowack E., Glockner G., Melkonian M. 2007. The ancestor of the Paulinella chromatophore obtained a carboxysomal operon by horizontal gene transfer from a Nitrococcus-like γ-proteobacterium. BMC Evol. Biol. 7, 85. 10.1186/1471-2148-7-85 (doi:10.1186/1471-2148-7-85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Badger M. R., Hanson D., Price G. D. 2002. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct. Plant Biol. 29, 161–173 10.1071/pp01213 (doi:10.1071/pp01213) [DOI] [PubMed] [Google Scholar]
- 52.Badger M. R., Price G. D. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 54, 609–622 10.1093/jxb/erg076 (doi:10.1093/jxb/erg076) [DOI] [PubMed] [Google Scholar]
- 53.Badger M. R., Price G. D., Long B. M., Woodger F. J. 2006. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 57, 249–265 10.1093/jxb/eri286 (doi:10.1093/jxb/eri286) [DOI] [PubMed] [Google Scholar]
- 54.Marin B., Nowack E. C. M., Melkonian M. 2005. A plastid in the making: evidence for a second primary endosymbiosis. Protist 156, 425–432 10.1016/j.protis.2005.09.001 (doi:10.1016/j.protis.2005.09.001) [DOI] [PubMed] [Google Scholar]
- 55.Nowack E. C. M., Melkonian M., Gloeckner G. 2008. Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes. Curr. Biol. 18, 410–418 10.1016/j.cub.2008.02.051 (doi:10.1016/j.cub.2008.02.051) [DOI] [PubMed] [Google Scholar]
- 56.Price G. D., Badger M. R., Woodger F. J., Long B. M. 2008. Advances in understanding the cyanobacterial CO2-concentrating mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59, 1441–1461 10.1093/jxb/erm112 (doi:10.1093/jxb/erm112) [DOI] [PubMed] [Google Scholar]
- 57.Deusch O., Landan G., Roettger M., Gruenheit N., Kowallik K. V., Allen J. F., Martin W., Dagan T. 2008. Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor. Mol. Biol. Evol. 25, 748–761 10.1093/molbev/msn022 (doi:10.1093/molbev/msn022) [DOI] [PubMed] [Google Scholar]
- 58.Janouskovec J., Horak A., Obornik M., Lukes J., Keeling P. J. 2010. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc. Natl Acad. Sci. USA 107, 10 949–10 954 10.1073/pnas.1003335107 (doi:10.1073/pnas.1003335107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin W., Schnarrenberger C. 1997. The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr. Genet. 32, 1–18 10.1007/s002940050241 (doi:10.1007/s002940050241) [DOI] [PubMed] [Google Scholar]
- 60.Reyes-Prieto A., Bhattacharya D. 2007. Phylogeny of Calvin cycle enzymes supports Plantae monophyly. Mol. Phylogenet. Evol. 45, 384–391 10.1016/j.ympev.2007.02.026 (doi:10.1016/j.ympev.2007.02.026) [DOI] [PubMed] [Google Scholar]
- 61.Kroth P. G., et al. 2008. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 3, e1426.(doi:10.1371/journal.pone.0001426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moustafa A., Beszteri B., Maier U. G., Bowler C., Valentin K., Bhattacharya D. 2009. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324, 1724–1726 10.1126/science.1172983 (doi:10.1126/science.1172983) [DOI] [PubMed] [Google Scholar]
- 63.Maberly S. C., Courcelle C., Groben R., Gontero B. 2010. Phylogenetically-based variation in the regulation of the Calvin cycle enzymes, phosphoribulokinase and glyceraldehyde-3-phosphate dehydrogenase, in algae. J. Exp. Bot. 61, 735–745 10.1093/jxb/erp337 (doi:10.1093/jxb/erp337) [DOI] [PubMed] [Google Scholar]
- 64.Groben R., Kaloudas D., Raines C. A., Offmann B., Maberly S. C., Gontero B. 2010. Comparative sequence analysis of CP12, a small protein involved in the formation of a Calvin cycle complex in photosynthetic organisms. Photosynth. Res. 103, 183–194 10.1007/s11120-010-9542-z (doi:10.1007/s11120-010-9542-z) [DOI] [PubMed] [Google Scholar]
- 65.Tcherkez G. G. B., Farquhar G. D., Andrews T. J. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl Acad. Sci. USA 103, 7246–7251 10.1073/pnas.0600605103 (doi:10.1073/pnas.0600605103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raven J. A. 2011. The cost of photoinhibition. Physiol. Plant 142, 87–104 10.1111/j.1399-3054.2011.01465.x (doi:10.1111/j.1399-3054.2011.01465.x) [DOI] [PubMed] [Google Scholar]
- 67.Giordano M., Beardall J., Raven J. A. 2005. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 56, 99–131 10.1146/annurev.arplant.56.032604.144052 (doi:10.1146/annurev.arplant.56.032604.144052) [DOI] [PubMed] [Google Scholar]
- 68.Raven J. A. 1990. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol. 116, 1–18 10.1111/j.1469-8137.1990.tb00505.x (doi:10.1111/j.1469-8137.1990.tb00505.x) [DOI] [Google Scholar]
- 69.Raven J. A. 1991. Physiology of inorganic C acquisition and implications for resource use efficiency by marine-phytoplankton—relation to increased CO2 and temperature. Plant Cell Environ. 14, 779–794 10.1111/j.1365-3040.1991.tb01442.x (doi:10.1111/j.1365-3040.1991.tb01442.x) [DOI] [Google Scholar]
- 70.Raven J. A. 1991. Implications of inorganic carbon utilization—ecology, evolution, and geochemistry. Can. J. Bot. Rev. Can. Bot. 69, 908–924 10.1139/b91-118 (doi:10.1139/b91-118) [DOI] [Google Scholar]
- 71.Raven J. A., Johnston A. M. 1991. Mechanisms of inorganic carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnol. Oceanogr. 36, 1701–1714 10.4319/lo.1991.36.8.1701 (doi:10.4319/lo.1991.36.8.1701) [DOI] [Google Scholar]
- 72.Falkowski P. G., Godfrey L. V. 2008. Electrons, life and the evolution of Earth's oxygen cycle. Phil. Trans. R. Soc. B 363, 2705–2716 10.1098/rstb.2008.0054 (doi:10.1098/rstb.2008.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raven J. A., Andrews M., Quigg A. 2005. The evolution of oligotrophy: implications for the breeding of crop plants for low input agricultural systems. Ann. Appl. Biol. 146, 261–280 10.1111/j.1744-7348.2005.040138.x (doi:10.1111/j.1744-7348.2005.040138.x) [DOI] [Google Scholar]
- 74.Raven J. A., Brown K., Mackay M., Beardall J., Giordano M., Granum E., Leegood R. C., Kilminster K., Walker D. I. 2005. Iron, nitrogen, phosphorus and zinc cycling and consequences for primary productivity in the oceans. In Micro-organisms and earth systems—advances in geomicrobiology (eds Gadd G. M., Semple K. T., Lappin-Scott H. M.), pp. 247–272 Cambridge, UK: Cambridge University Press [Google Scholar]
- 75.Grime J. P. 2001. Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Chichester, UK: John Wiley [Google Scholar]
- 76.Reynolds C. S. 2006. Ecology of phytoplankton. Cambridge, UK: Cambridge University Press [Google Scholar]
- 77.Flynn K. J. 2009. Going for the slow burn: why should possession of a low maximum growth rate be advantageous for microalgae? Plant Ecol. Diversity 2, 179–189 10.1080/17550870903207268 (doi:10.1080/17550870903207268) [DOI] [Google Scholar]
- 78.Lambers H., Raven J. A., Shaver G. R., Smith S. E. 2008. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 23, 95–103 10.1016/j.tree.2007.10.008 (doi:10.1016/j.tree.2007.10.008) [DOI] [PubMed] [Google Scholar]
- 79.Raven J. A., Cockell C. S., De La Rocha C. L. 2008. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Phil. Trans. R. Soc. B 363, 2641–2650 10.1098/rstb.2008.0020 (doi:10.1098/rstb.2008.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falkowski P. G., Raven J. A. 2007. Aquatic photosynthesis, 2nd edn. Princeton, NJ: Princeton University Press [Google Scholar]
- 81.Raven J. A., Larkum A. W. D. 2007. Are there ecological implications for the proposed energetic restrictions on photosynthetic oxygen evolution at high oxygen concentrations? Photosynth. Res. 94, 31–42 10.1007/s11120-007-9211-z (doi:10.1007/s11120-007-9211-z) [DOI] [PubMed] [Google Scholar]
- 82.Shevela D., Beckmann K., Clausen J., Junge W., Messinger J. 2011. Membrane-inlet mass spectrometry reveals a high driving force for oxygen production by photosystem II. Proc. Natl Acad. Sci. USA 108, 3602–3607 10.1073/pnas.1014249108 (doi:10.1073/pnas.1014249108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans D. A., Beukes N. J., Kirschvink J. L. 1997. Low-latitude glaciation in the Palaeoproterozoic era. Nature 386, 262–266 10.1038/386262a0 (doi:10.1038/386262a0) [DOI] [Google Scholar]
- 84.Schwarte S., Bauwe H. 2007. Identification of the photorespiratory 2-phosphoglycolate phosphatase, PGLP1, in Arabidopsis. Plant Physiol. 144, 1580–1586 10.1104/pp.107.099192 (doi:10.1104/pp.107.099192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bauwe H., Hagemann M., Fernie A. R. 2010. Photorespiration: players, partners and origin. Trends Plant Sci. 15, 330–336 10.1016/j.tplants.2010.03.006 (doi:10.1016/j.tplants.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 86.Kim Y. C., et al. 2004. Structure- and function-based characterization of a new phosphoglycolate phosphatase from Thermoplasma acidophilum. J. Biol. Chem. 279, 517–526 10.1074/jbc.M306054200 (doi:10.1074/jbc.M306054200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellicer M. T., Nunez M. F., Aguilar J., Badia J., Baldoma L. 2003. Role of 2-phosphoglycolate phosphatase of Escherichia coli in metabolism of the 2-phosphoglycolate formed in DNA repair. J. Bacteriol. 185, 5815–5821 10.1128/jb.185.19.5815-5821.2003 (doi:10.1128/jb.185.19.5815-5821.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kern R., Bauwe H., Hagemann M. 2011. Evolution of enzymes involved in the photorespiratory 2-phosphoglycolate cycle from cyanobacteria via algae toward plants. Photosynth. Res. 109, 1–12 10.1007/s11120-010-9615-z (doi:10.1007/s11120-010-9615-z) [DOI] [PubMed] [Google Scholar]
- 89.Eisenhut M., et al. 2006. The plant-like C2 glycolate cycle and the bacterial-like glycerate pathway cooperate in phosphoglycolate metabolism in cyanobacteria. Plant Physiol. 142, 333–342 10.1104/pp.106.082982 (doi:10.1104/pp.106.082982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisenhut M., Ruth W., Haimovich M., Bauwe H., Kaplan A., Hagemann M. 2008. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 105, 17 199–17 204 10.1073/pnas.0807043105 (doi:10.1073/pnas.0807043105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raven J. A., Kubler J. E., Beardall J. 2000. Put out the light, and then put out the light. J. Mar. Biol. Assoc. UK 80, 1–25 10.1017/s0025315499001526 (doi:10.1017/s0025315499001526) [DOI] [Google Scholar]
- 92.Roberts K., Granum E., Leegood R., Raven J. 2007. Carbon acquisition by diatoms. Photosynth. Res. 93, 79–88 10.1007/s11120-007-9172-2 (doi:10.1007/s11120-007-9172-2) [DOI] [PubMed] [Google Scholar]
- 93.Bartsch O., Hagemann M., Bauwe H. 2008. Only plant-type (GLYK) glycerate kinases produce d-glycerate-3-phosphate. FEBS Lett. 582, 3025–3028 10.1016/j.febslet.2008.07.038 (doi:10.1016/j.febslet.2008.07.038) [DOI] [PubMed] [Google Scholar]
- 94.Boldt R., Edner C., Kolukisaoglu U., Hagemann M., Weckwerth W., Wienkoop S., Morgenthal K., Bauwe H. 2005. d-Glycerate-3-kinase, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell 17, 2413–2420 10.1105/tpc.105.033993 (doi:10.1105/tpc.105.033993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts K., Granum E., Leegood R. C., Raven J. A. 2007. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol. 145, 230–235 10.1104/pp.107.102616 (doi:10.1104/pp.107.102616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holland H. D. 1978. The chemistry of the atmosphere and oceans. New York, NY: Wiley [Google Scholar]
- 97.Lenton T. M., Watson A. J. 2000. Redfield revisited: 2 What regulates the oxygen content of the atmosphere? Global Biogeochem. Cycles 14, 249–268 10.1029/1999gb900076 (doi:10.1029/1999gb900076) [DOI] [Google Scholar]
- 98.Johnston A. M., Kennedy H. 1998. Carbon isotope fractionation in marine systems: open ocean studies and laboratory studies. In Stable isotopes: integration of biological, ecological and geochemical processes (ed. Griffiths H.), pp. 239–256 Oxford, UK: BIOS Scientific [Google Scholar]
- 99.Maberly S. C. 1990. Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 26, 439–449 10.1111/j.0022-3646.1990.00439.x (doi:10.1111/j.0022-3646.1990.00439.x) [DOI] [Google Scholar]
- 100.Hepburn C. D., Pritchard D. W., Cornwall C. E., McLeod R. J., Beardall J., Raven J. A., Hurd C. L. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biol. 17, 2488–2497 10.1111/j.1365-2486.2011.02411.x (doi:10.1111/j.1365-2486.2011.02411.x) [DOI] [Google Scholar]
- 101.Marconi M., Giordano M., Raven J. A. 2011. Impact of taxonomy, geography and depth of on δ13C and δ15N variation in a large collection of macroalgae. J. Phycol. 47, 1023–1035. (doi:10.1111/j.1529-88172011.01045.x) [DOI] [PubMed] [Google Scholar]
- 102.Raven J. A. 2011. Carbon. In Ecology of cyanobacteria (ed. Whitton B. A.), 2nd edn Berlin, Germany: Springer [Google Scholar]
- 103.Hopkinson B. M., Dupont C. L., Allen A. E., Morel F. M. M. 2011. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Natl Acad. Sci. USA 108, 3830–3837 10.1073/pnas.1018062108 (doi:10.1073/pnas.1018062108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reinfelder J. R. 2011. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 3, 291–315 10.1146/annurev-marine-120709-142720 (doi:10.1146/annurev-marine-120709-142720) [DOI] [PubMed] [Google Scholar]
- 105.Raven J. A., Ball L. A., Beardall J., Giordano M., Maberly S. C. 2005. Algae lacking carbon-concentrating mechanisms. Can. J. Bot.-Rev. Can. Bot. 83, 879–890 10.1139/b05-074 (doi:10.1139/b05-074) [DOI] [Google Scholar]
- 106.Spijkerman E. 2011. The expression of a carbon concentrating mechanism in Chlamydomonas acidophila under variable phosphorus, iron, and CO2 concentrations. Photosynth. Res. 109, 1–11 10.1007/s11120-010-9607-z (doi:10.1007/s11120-010-9607-z) [DOI] [PubMed] [Google Scholar]
- 107.Young J. N., Rickaby R. E. M., Kapralov M. V., Filatov D. A. 2012. Adaptive signals in algal Rubisco reveal a history of ancient atmospheric carbon dioxide. Phil. Trans. R. Soc. B 367, 483–492 10.1098/rstb.2011.0145 (doi:10.1098/rstb.2011.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sultemeyer D., Rinast K. A. 1996. The CO2 permeability of the plasma membrane of Chlamydomonas reinhardtii: mass-spectrometric 18O exchange measurements from CO2 13C-18O in suspensions of carbonic anhydrase-loaded plasma-membrane vesicles. Planta 200, 358–368 10.1007/BF00200304 (doi:10.1007/BF00200304) [DOI] [Google Scholar]
- 109.Dou Z., Heinhorst S., Williams E. B., Murin C. D., Shively J. M., Cannon G. C. 2008. CO2 fixation kinetics of Halothiobacillus neapolitanus mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2. J. Biol. Chem. 283, 10 377–10 384 10.1074/jbc.M709285200 (doi:10.1074/jbc.M709285200) [DOI] [PubMed] [Google Scholar]
- 110.Reinfelder J. R., Kraepiel A. M. L., Morel F. M. M. 2000. Unicellular C4 photosynthesis in a marine diatom. Nature 407, 996–999 10.1038/35039612 (doi:10.1038/35039612) [DOI] [PubMed] [Google Scholar]
- 111.Giordano M., Norici A., Forssen M., Eriksson M., Raven J. A. 2003. An anaplerotic role for mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol. 132, 2126–2134 10.1104/pp.103.023424 (doi:10.1104/pp.103.023424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beardall J., Giordano M. 2002. Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Funct. Plant Biol. 29, 335–347 10.1071/pp01195 (doi:10.1071/pp01195) [DOI] [PubMed] [Google Scholar]
- 113.Beardall J., Stojkovic S., Larsen S. 2009. Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecol. Diversity 2, 191–205 10.1080/17550870903271363 (doi:10.1080/17550870903271363) [DOI] [Google Scholar]
- 114.Finkel Z. V., Beardall J., Flynn K. J., Quigg A., Rees T. A. V., Raven J. A. 2010. Phytoplankton in a changing world: cell size and elemental stoichiometry. J. Plankton Res. 32, 119–137 10.1093/plankt/fbp098 (doi:10.1093/plankt/fbp098) [DOI] [Google Scholar]
- 115.Gao G., Gao K., Giordano M. 2009. Responses to solar UV radiation of the diatom Skeletonema costatum (Bacillariophyceae) grown at different Zn2+ concentrations. J. Phycol. 45, 119–129 10.1111/j.1529-8817.2008.00616.x (doi:10.1111/j.1529-8817.2008.00616.x) [DOI] [PubMed] [Google Scholar]
- 116.Gao K., Zheng Y. 2010. Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta). Global Change Biol. 16, 2388–2398 10.1111/j.1365-2486.2009.02113.x (doi:10.1111/j.1365-2486.2009.02113.x) [DOI] [Google Scholar]
- 117.Ihnken S., Roberts S., Beardall J. 2011. Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability. Phycologia 50, 182–193 10.2216/10-11.1 (doi:10.2216/10-11.1) [DOI] [Google Scholar]
- 118.Kohinata T., Nishino H., Fukuzawa H. 2008. Significance of zinc in a regulatory protein, CCM1, which regulates the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell. Physiol. 49, 273–283 10.1093/pcp/pcn003 (doi:10.1093/pcp/pcn003) [DOI] [PubMed] [Google Scholar]
- 119.Morel F. M. M., Cox E. H., Kraepiel A. M. L., Lane T. W., Milligan A. J., Schaperdoth I., Reinfelder J. R., Tortell P. D. 2002. Acquisition of inorganic carbon by the marine diatom Thalassiosira weissflogii. Funct. Plant Biol. 29, 301–308 10.1071/pp01199 (doi:10.1071/pp01199) [DOI] [PubMed] [Google Scholar]
- 120.Raven J. A., Geider R. J. 1988. Temperature and algal growth. New Phytol. 110, 441–461 10.1111/j.1469-8137.1988.tb00282.x (doi:10.1111/j.1469-8137.1988.tb00282.x) [DOI] [Google Scholar]
- 121.Raven J. A., Evans M. C. W., Korb R. E. 1999. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 60, 111–149 10.1023/a:1006282714942 (doi:10.1023/a:1006282714942) [DOI] [Google Scholar]
- 122.Shelly K., Heraud P., Beardall J. 2002. Nitrogen limitation in Dunaliella tertiolecta (Chlorophyceae) leads to increased susceptibility to damage by ultraviolet-B radiation but also increased repair capacity. J. Phycol. 38, 713–720 10.1046/j.1529-8817.2002.01147.x (doi:10.1046/j.1529-8817.2002.01147.x) [DOI] [Google Scholar]
- 123.Sobrino C., Neale P. J., Phillips-Kress J. D., Moeller R. E., Porter J. A. 2009. Elevated CO2 increases sensitivity to ultraviolet radiation in lacustrine phytoplankton assemblages. Limnol. Oceanogr. 54, 2448–2459 10.4319/lo.2009.54.6_part_2.2448 (doi:10.4319/lo.2009.54.6_part_2.2448) [DOI] [Google Scholar]
- 124.Sobrino C., Ward M. L., Neale P. J. 2008. Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: effects on growth, photosynthesis, and spectral sensitivity of photoinhibition. Limnol. Oceanogr. 53, 494–505 10.4319/lo.2008.53.2.0494 (doi:10.4319/lo.2008.53.2.0494) [DOI] [Google Scholar]
- 125.Bobik T. A. 2006. Polyhedral organelles compartmenting bacterial metabolic processes. Appl. Microbiol. Biotechnol. 70, 517–525 10.1007/s00253-005-0295-0 (doi:10.1007/s00253-005-0295-0) [DOI] [PubMed] [Google Scholar]
- 126.Boyce D. G., Lewis M. R., Worm B. 2010. Global phytoplankton decline over the past century. Nature 466, 591–596 10.1038/nature09268 (doi:10.1038/nature09268) [DOI] [PubMed] [Google Scholar]
- 127.Richardson A. J. 2008. In hot water: zooplankton and climate change. ICES J. Mar. Sci. 65, 279–295 10.1093/icesjms/fsn028 (doi:10.1093/icesjms/fsn028) [DOI] [Google Scholar]
- 128.Steinacher M., et al. 2010. Projected 21st century decrease in marine productivity: a multi-model analysis. Biogeosciences 7, 979–1005 10.5194/bg-7-979-2010 (doi:10.5194/bg-7-979-2010) [DOI] [Google Scholar]
- 129.Froelicher T. L., Joos F., Plattner G. K., Steinacher M., Doney S. C. 2009. Natural variability and anthropogenic trends in oceanic oxygen in a coupled carbon cycle-climate model ensemble. Global Biogeochem. Cycles 23, GB1003.(doi:10.1029/2008gb003316) [Google Scholar]
- 130.Keeling R. F., Körtzinger A., Gruber N. 2010. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2, 199–229 10.1146/annurev.marine.010908.163855 (doi:10.1146/annurev.marine.010908.163855) [DOI] [PubMed] [Google Scholar]
- 131.Tyrrell T. 2011. Anthropogenic modification of the oceans. Phil. Trans. R. Soc. A 369, 887–908 10.1098/rsta.2010.0334 (doi:10.1098/rsta.2010.0334) [DOI] [PubMed] [Google Scholar]
- 132.Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 10.1146/annurev.marine.010908.163834 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 133.Raven J. A. 2011. Effects on marine algae of changed seawater chemistry with increasing atmospheric CO2. Biol. Environ. Proc. R. Ir. Acad. 111B, 1–17 10.3318/bioe.2011.01 (doi:10.3318/bioe.2011.01) [DOI] [Google Scholar]
- 134.Riebesell U., Koertzinger A., Oschlies A. 2009. Sensitivities of marine carbon fluxes to ocean change. Proc. Natl Acad. Sci. USA 106, 20 602–20 609 10.1073/pnas.0813291106 (doi:10.1073/pnas.0813291106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Milligan A. J., Varela D. E., Brzezinski M. A., Morel F. M. M. 2004. Dynamics of silicon metabolism and silicon isotopic discrimination in a marine diatom as a function of pCO2. Limnol. Oceanogr. 49, 322–329 10.4319/lo.2004.49.2.0322 (doi:10.4319/lo.2004.49.2.0322) [DOI] [Google Scholar]
- 136.Hofmann M., Schellnhuber J. 2009. Oceanic acidification affects marine carbon pump and triggers extended marine oxygen holes. Proc. Natl Acad. Sci. USA 106, 3017–3022 10.1073/pnas.0813384106 (doi:10.1073/pnas.0813384106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Devol A. H., Hartnett H. E. 2001. Role of the oxygen-deficient zone in transfer of organic carbon to the deep ocean. Limnol. Oceanogr. 46, 1684–1690 10.4319/lo.2001.46.7.1684 (doi:10.4319/lo.2001.46.7.1684) [DOI] [Google Scholar]
- 138.Jenkyns H. C. 2010. Geochemistry of oceanic anoxic events. Geochem. Geophys. Geosyst. 11, 1–30 10.1029/2009gc002788 (doi:10.1029/2009gc002788) [DOI] [Google Scholar]
- 139.Raven J. A., Geider R. D. 2003. Adaptation, acclimation and regulation of photosynthesis in algae. In Photosynthesis in algae (eds Larkum A. W. D., Douglas S. E., Raven J. A.), pp. 385–412 Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- 140.Bell G., Collins S. 2008. Adaptation, extinction and global change. Evol. Appl. 1, 3–16 10.1111/j.1752-4571.2007.00011.x (doi:10.1111/j.1752-4571.2007.00011.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Collins S. 2011. Competition limits adaptation and productivity in a photosynthetic alga at elevated CO2. Proc. R. Soc. B 278, 247–255 10.1098/rspb.2010.1173 (doi:10.1098/rspb.2010.1173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Collins S., Bell G. 2004. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431, 566–569 10.1038/nature02945 (doi:10.1038/nature02945) [DOI] [PubMed] [Google Scholar]
- 143.Collins S., Bell G. 2006. Rewinding the tape: selection of algae adapted to high CO2 at current and pleistocene levels of CO2. Evolution 60, 1392–1401 10.1111/j.0014-3820.2006.tb01218.x (doi:10.1111/j.0014-3820.2006.tb01218.x) [DOI] [PubMed] [Google Scholar]
- 144.Collins S., Bell G. 2006. Evolution of natural algal populations at elevated CO2. Ecol. Lett. 9, 129–135 10.1111/j.1461-0248.2005.00854.x (doi:10.1111/j.1461-0248.2005.00854.x) [DOI] [PubMed] [Google Scholar]
- 145.Collins S., Gardner A. 2009. Integrating physiological, ecological and evolutionary change: a Price equation approach. Ecol. Lett. 12, 744–757 10.1111/j.1461-0248.2009.01340.x (doi:10.1111/j.1461-0248.2009.01340.x) [DOI] [PubMed] [Google Scholar]
- 146.Collins S., Sueltemeyer D., Bell G. 2006. Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2. Plant Cell Environ. 29, 1812–1819 10.1111/j.1365-3040.2006.01559.x (doi:10.1111/j.1365-3040.2006.01559.x) [DOI] [PubMed] [Google Scholar]
- 147.Huertas I. E., Rouco M., López-Rodas V., Costas E. 2011. Warming will affect phytoplankton differently: evidence through a mechanistic approach. Proc. R. Soc. B 278, 3534–3543(doi:10.1098/rspb.2011.0160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall-Spencer J. M., Rodolfo-Metalpa R., Martin S., Ransome E., Fine M., Turner S. M., Rowley S. J., Tedesco D., Buia C. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 10.1038/nature07051 (doi:10.1038/nature07051) [DOI] [PubMed] [Google Scholar]
- 149.Mercado J., Gordillo F. 2011. Inorganic carbon acquisition in algal communities: are the laboratory data relevant to the natural ecosystems? Photosynth. Res. 109, 1–11 10.1007/s11120-011-9646-0 (doi:10.1007/s11120-011-9646-0) [DOI] [PubMed] [Google Scholar]
- 150.Bots P., Benning L. G., Rickaby R. E. M., Shaw S. 2011. The role of SO4 in the switch from calcite to aragonite seas. Geology 39, 331–334 10.1130/g31619.1 (doi:10.1130/g31619.1) [DOI] [Google Scholar]
- 151.Lee J., Morse J. W. 2010. Influences of alkalinity and pCO2 on CaCO3 nucleation from estimated Cretaceous composition seawater representative of ‘calcite seas’. Geology 38, 115–118 10.1130/g30537.1 (doi:10.1130/g30537.1) [DOI] [Google Scholar]
- 152.Ries J. B. 2009. Effects of secular variation in seawater Mg/Ca ratio (calcite-aragonite seas) on CaCO3 sediment production by the calcareous algae Halimeda, Penicillus and Udotea—evidence from recent experiments and the geological record. Tera Nov. 21, 323–339 10.1111/j.1365-3121.2009.00899.x (doi:10.1111/j.1365-3121.2009.00899.x) [DOI] [Google Scholar]
- 153.Zachos J. C., et al. 2005. Rapid acidification of the ocean during the Paleocene–Eocene thermal maximum. Science 308, 1611–1615 10.1126/science.1109004 (doi:10.1126/science.1109004) [DOI] [PubMed] [Google Scholar]
- 154.Zeebe R. E., Wolf-Gladrow D. A. 2001. CO2 in Seawater: equilibrium, kinetics, isotopes. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 155.Hurd C. L., Hepburn C. D., Currie K. I., Raven J. A., Hunter K. A. 2009. Testing the effects of ocean acidification on algal metabolism: considerations for experimental designs. J. Phycol. 45, 1236–1251 10.1111/j.1529-8817.2009.00768.x (doi:10.1111/j.1529-8817.2009.00768.x) [DOI] [PubMed] [Google Scholar]
- 156.Iglesias-Rodriguez M. D., et al. 2008. Phytoplankton calcification in a high-CO2 world. Science 320, 336–340 10.1126/science.1154122 (doi:10.1126/science.1154122) [DOI] [PubMed] [Google Scholar]
- 157.Riebesell U., Fabry V. J., Hansson L., Gattuso J.-P. (eds) 2010. Guide to best practices for ocean acidification research and data reporting. Luxembourg: Publications Office of the European Union [Google Scholar]
- 158.Shi D., Xu Y., Morel F. M. M. 2009. Effects of the pH/pCO2 control method on medium chemistry and phytoplankton growth. Biogeosciences 6, 1199–1207 10.5194/bg-6-1199-2009 (doi:10.5194/bg-6-1199-2009) [DOI] [Google Scholar]
- 159.Laws E. A., Popp B. N., Cassar N., Tanimoto J. 2002. 13C discrimination patterns in oceanic phytoplankton: likely influence of CO2 concentrating mechanisms, and implications for palaeoreconstructions. Funct. Plant Biol. 29, 323–333 10.1071/pp01183 (doi:10.1071/pp01183) [DOI] [PubMed] [Google Scholar]
- 160.Raven J. A., Johnston A. M., Turpin D. H. 1993. Influence of changes in CO2 concentration and temperature on marine phytoplankton 13C/12C ratios—an analysis of possible mechanisms. Glob. Planet Change 8, 1–12 10.1016/0921-8181(93)90058-v (doi:10.1016/0921-8181(93)90058-v) [DOI] [Google Scholar]
- 161.Fletcher B. J., Beerling D. J., Brentnall S. J., Royer D. L. 2005. Fossil bryophytes as recorders of ancient CO2 levels: experimental evidence and a Cretaceous case study. Global Biogeochem. Cycles 19, GB3012. 10.1029/2005gb002495 (doi:10.1029/2005gb002495) [DOI] [Google Scholar]


