Abstract
Variation in atmospheric [CO2] is a prominent feature of the environmental history over which vascular plants have evolved. Periods of falling and low [CO2] in the palaeo-record appear to have created selective pressure for important adaptations in modern plants. Today, rising [CO2] is a key component of anthropogenic global environmental change that will impact plants and the ecosystem goods and services they deliver. Currently, there is limited evidence that natural plant populations have evolved in response to contemporary increases in [CO2] in ways that increase plant productivity or fitness, and no evidence for incidental breeding of crop varieties to achieve greater yield enhancement from rising [CO2]. Evolutionary responses to elevated [CO2] have been studied by applying selection in controlled environments, quantitative genetics and trait-based approaches. Findings to date suggest that adaptive changes in plant traits in response to future [CO2] will not be consistently observed across species or environments and will not be large in magnitude compared with physiological and ecological responses to future [CO2]. This lack of evidence for strong evolutionary effects of elevated [CO2] is surprising, given the large effects of elevated [CO2] on plant phenotypes. New studies under more stressful, complex environmental conditions associated with climate change may revise this view. Efforts are underway to engineer plants to: (i) overcome the limitations to photosynthesis from today's [CO2] and (ii) benefit maximally from future, greater [CO2]. Targets range in scale from manipulating the function of a single enzyme (e.g. Rubisco) to adding metabolic pathways from bacteria as well as engineering the structural and functional components necessary for C4 photosynthesis into C3 leaves. Successfully improving plant performance will depend on combining the knowledge of the evolutionary context, cellular basis and physiological integration of plant responses to varying [CO2].
Keywords: adaptation, climate change, evolution, yield, fitness
1. Introduction: why take an evolutionary perspective?
Assimilation of CO2 from the atmosphere into biomass by higher plants is fundamental to: (i) providing food, fuel and fibre for human consumption; (ii) supplying energy to terrestrial ecosystems; and (iii) regulating the concentration of atmospheric CO2 ([CO2]) and climate. In almost all higher plants, photosynthetic CO2 fixation (A) and stomatal conductance (gs) are instantaneously sensitive to variation in [CO2] over the range of past to present and/or predicted future [CO2]. The rise in [CO2] starting during the Industrial Revolution and continuing today is notable for how quickly it is altering plant function. Changes in A and gs caused by increasing [CO2] initiate a set of cellular and physiological responses, which typically increase growth and can increase reproductive output. Genotypic variation in almost all elements of these responses creates the potential for ecological and evolutionary consequences over a wide range of timescales.
Plant and ecosystem responses to varying [CO2] are currently best understood at the physiological and ecological levels and on timescales of one generation or less. Investigating plant responses to varying atmospheric [CO2] in an evolutionary context is important because variations in [CO2] over geological timescales are believed to have played important roles in the evolution of ecologically and economically important traits in extant species. In addition, if plants evolve in response to twenty-first century [CO2], changes in future ecosystem structure, function and services will extend beyond what can be predicted from knowledge of physiological and ecological responses to elevated [CO2]. From a practical perspective, there is the possibility that despite major breeding successes, present elite crop varieties may not be adapted for optimal performance under present and future [CO2]. Accordingly, improving plant productivity in high [CO2] environments may be an open opportunity for biotechnology or breeding to improve crop performance now and in the future. This paper builds on previous reviews [1–4] to synthesize the present knowledge on each of these topics. It discusses how an evolutionary perspective could advance efforts to understand and manage plant and ecosystem responses to rising [CO2] by addressing the following five questions:
(1) Have plants evolved in response to varying [CO2] on a geological timescale?
(2) Have plants evolved by natural or artificial selection in response to contemporary increases in [CO2]?
(3) Will rising [CO2] drive natural selection in the future, and if so how?
(4) What traits are favoured under high [CO2]?
(5) How does evolutionary history impact and inform efforts to engineer crops for improved performance in present and future [CO2]?
Detailed evaluation of the approaches available for integrating evolutionary biology with physiology and ecology are reviewed authoritatively elsewhere [5–7].
2. Question 1: have plants evolved in response to varying [co2] on a geological timescale?
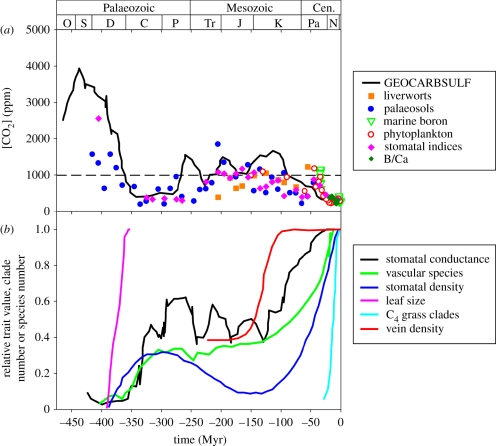
Variation in [CO2] is proposed to have played a key role in driving the evolution of plants since they colonized the land 400 Ma [8–12]. Estimates of the palaeo-[CO2] record have been generated by modelling the weathering and burial of Ca–Mg silicates and organic carbon [13], as well as a range of proxies including stomatal characteristics of fossil leaves [14] and the stable isotope composition of pedogenic carbonates, marine organic matter and fossil bryophytes [15–18]. There is significant uncertainty associated with each individual methodology and variation across methods [13,16,19,20]. Nevertheless, the following general trend emerges. The earliest land plants became established at high [CO2] (1500–3000 ppm) before a period of low [CO2] (less than or equal to 1000 ppm), which started ∼350 Ma and lasted ∼50–100 Myr (figure 1a). Between ∼250–100 Ma [CO2] appears to have been maintained at approximately 1000 ppm. With the exception of a period in the Eocene ∼40–50 Ma, all proxies and models indicate [CO2] of less than 1000 ppm for the last 100 Myr (figure 1a).
Figure 1.
Comparative time courses over most of the Phanerozoic of: (a) estimated atmospheric [CO2] predicted from a geochemical model of the carbon cycle (GEOCARBSULF, adapted from Berner [13]) and multiple proxies of [CO2] (stomatal indices and isotope analysis of liverworts, palaeosols, marine boron, phytoplankton and B/Ca, updated from the compilation of Royer [21]), with a dashed line at 1000 ppm indicating the atmospheric [CO2] above which photosynthesis is saturated in most modern plants [22]; (b) estimated maximum stomatal conductance (adapted from Franks & Beerling [23]), estimated vascular species richness (adapted from Knoll & Niklas [24]), stomatal density (redrawn from Royer et al. [20]), Devonian and Carboniferous leaf size (adapted from Osborne et al. [25]), C4 grass clade richness (adapted from Edwards et al. [9]) and angiosperm vein density (adapted from Brodribb & Feild [26]), all of which are expressed relative to the maximum value in the individual records of each parameter from the cited studies.
Periods of falling or low [CO2] have been linked to the evolution of a number of important plant traits as well as diversification of the vascular flora (figure 1b) [8,27]. The development of megaphyll leaves in multiple independent lineages was coincident with the transition from high to low [CO2] during the Devonian and Carboniferous 400–350 Ma [25,28]. As [CO2] dropped, rates of A would have become increasingly limited by the resistance to diffusion of CO2 from the atmosphere to the site of fixation by Rubisco. Changes in stomatal density and stomatal size (a combination of pore depth and pore cross-sectional area determined from measurements of the entire guard cell complex, which better predicts gs than pore cross-sectional area alone) observed in fossil leaves have been used to drive models predicting increases in maximum gs at this time [14,23,29], which would have relieved resistance to CO2 diffusion through the epidermis and maintained rates of A [30]. The rise in gs is proposed to have increased the capacity for evaporative cooling of leaves, allowing greater leaf areas to develop for intercepting radiation without causing overheating [25,28]. These changes in the structure and function of leaves coincided with the first major increase in the number of vascular plant species [24]. Although most data indicate that periods of low [CO2] are associated with novel adaptations and diversification, Willis & McElwain [12] found that over geological timescales, periods of high [CO2] corresponded with greater origination rates of fossil species. These two observations appear contradictory on first examination. However, origination rates are defined as the rate at which new species appear in the fossil record, and this could differ from the rate of change of overall species richness. If this is the case, a combination of decreasing rates of species gain in low [CO2] with even greater decreases in the rate of species loss could be the basis of the observed patterns.
The second major period of falling and low [CO2] (from 100 Ma until the present day) also overlapped with increases in stomatal density and decreases in individual stomatal size that suggest plants were developing greater maximum gs to counteract the CO2-limitation of photosynthesis (figure 1b) [14,23,29]. This was preceded by a rapid and significant increase of vein density in angiosperm leaves that started ∼150 Ma [26]. Greater leaf hydraulic conductance resulting from greater vein density would have allowed plants to potentially achieve greater A by delivering more water to the leaf in order to sustain greater stomatal conductance. Therefore, increasing vein density has been proposed to have been a major fitness advantage for angiosperms and contributed to their subsequent radiation (figure 1a,b) [26,31–33]. The magnitude of the benefit to A from greater hydraulic conductance supporting greater gs is negatively correlated with [CO2]. Modelling these relationships suggests that increases in hydraulic conductance on the scale observed would support several fold greater A at [CO2] of 280 ppm, but be of more modest benefit (+13%) at [CO2] of 1000 ppm [26]. The interdependence of the reported variations in [CO2], vein density and stomatal characteristics is hard to determine. The change in vein density appears to have significantly predated the decrease in [CO2] during the Cretaceous, as well as changes in stomatal characteristics (figure 1a,b) [34]. Uncertainties in the palaeo-[CO2] record and dating of samples in fossil studies could contribute to this disparity. This leaves open the possibility that additional data will demonstrate that the three events were coordinated, as understanding of physiology in extant species would lead us to expect. On the other hand, the majority of papers in this field openly acknowledge that other environmental factors also varied during these key periods of plant evolution and could have been the selective agent for adaptive traits. A further possibility related to this scenario is that greater hydraulic capacity was an exaptation [35] that developed under some other selective force but then resulted in further fitness gains when [CO2] subsequently decreased.
An important constraint on the role of varying [CO2] in driving plant evolution that has been rarely discussed is the nonlinearity of [CO2] effects on plant performance and the concentration at which [CO2] is saturating. A survey of a wide variety of modern plant species, including angiosperms and gymnosperms, woody and herbaceous species, indicates that A is almost universally saturated at intercellular [CO2] of less than 700 ppm, which corresponds to atmospheric [CO2] of 1000 ppm [22]. Changes in leaf water use and energy balance associated with altered gs are also minimal above 1000 ppm [36]. If this saturating [CO2] was maintained across the course of plant evolutionary history, it would set a threshold above which variations in [CO2] would have no consequence for plant physiology or fitness (figure 1). For example, in this scenario, the initial decline in [CO2] during the Devonian from peak values of 1500–3000 ppm would have no direct effect on plants until dropping below 1000 ppm ∼350 Ma (figure 1a). However, the saturating [CO2] for early plants may have been much higher than for modern species. This is partly because the resistance for CO2 to pass through the epidermis was much greater, owing to their large stomatal sizes and lower stomatal densities [23,29]. In addition, it is possible that cell wall and chloroplast envelope structures of ancient species reduced mesophyll conductance relative to modern species [37]. Both of these factors would increase the saturating atmospheric [CO2] for photosynthesis [38,39] and, therefore, the threshold for atmospheric [CO2] effects on plant fitness. This alternative scenario is consistent with the fact that stomatal conductance and leaf size both started to increase ∼390 Ma, when [CO2] estimates from a variety of proxies ranged from 1500 to 3000 ppm. Establishing that the saturating [CO2] for early plants is that high will require further experimental and modelling analysis. Further investigation of changes in mesophyll conductance and saturating [CO2] in plants from the late Mesozoic and Cenozoic might also clarify the role that changes in [CO2] played in triggering the evolution of high water use and photosynthetic capacity in angiosperms.
Plants with C4 photosynthesis appear to have emerged during the most recent period of low [CO2] (less than 1000 ppm), before becoming ecologically important in many ecosystems 3–8 Ma [9]. Molecular clock data calibrated using fossils suggest that the origins of all the known C4 grass clades occurred between ∼3 and 32 Ma, and it has been proposed that this was driven by a mid-Oligocene drop in [CO2] that favoured C4 species over C3 species because of their greater photosynthetic efficiency at low [CO2], particularly when combined with high temperatures and drought stress (figure 1a,b) [40–42]. Carbon isotope analysis of pollen grains suggests that a significant fraction (more than 25%) of grass species were C4 ∼15–33 Ma [43]. Isotopic evidence from organic matter in abyssal sediments of the Atlantic Ocean were initially proposed to suggest that C4 species might have originated earlier, as much as 90 Ma, but could alternatively be explained by expansion of marine archaea at that time [44,45]. Crassulacean acid metabolism (CAM) photosynthesis is another adaptation that involves a CO2 concentrating mechanism to maximize water-use efficiency, and it is estimated to have also evolved in the orchids during the period of relatively low [CO2] (∼65 Ma) [46], while the cacti diverged from their closest relatives ∼35 Ma [47].
In summary, there are several lines of evidence that falling and low [CO2] creates selective pressure for two major classes of adaptation. First, adaptations to acquire and use water in exchange for [CO2] (smaller stomata, greater stomatal density, megaphyll leaves and greater vein density), which were presumably restricted to plants existing in mesic environments. Second, adaptations for CO2 concentrating mechanisms that increase photosynthetic efficiency and maximize water-use efficiency (C4 and CAM photosynthesis), which were presumably favoured in hot and dry environments. The potential strength of low [CO2] as an agent of selection on plant traits has been highlighted by work on plants growing 8–55 Ka, which included a glacial period with very low [CO2] (∼180–220 ppm). Isotopic evidence indicates that the ratio of intercellular [CO2] to atmospheric [CO2] was similar to that observed in modern plants, and therefore glacial trees were operating close to the photosynthetic CO2 compensation point where carbon starvation is experienced [48]. In contrast, periods of rising or high [CO2] have not widely been proposed to drive major events in plant evolution. The relative rarity of major evolutionary events during periods of high [CO2] is unlikely to result from the absence of genetic variation in plant sensitivity to high [CO2], or heritability of key traits controlling plant response to [CO2] (see Question 3) [49]. Alternatively, it might reflect that fitness and selection were more strongly driven by genetic variation in plant responses to other environmental influences when [CO2] was high and imposing little or no limitation on photosynthesis and productivity.
3. Question 2: have plants evolved by natural or artificial selection in response to contemporary increases in [co2]?
Anthropogenically driven global environmental change since the mid-twentieth century has been detectable against the background variability in climate and atmospheric composition [50]. In addition, biological responses to global environmental change are detectable in both natural and agricultural ecosystems [51–54]. Included in these biological responses are evolutionary changes across a range of taxa in response to air pollutants, drought and temperature [55–58]. However, there is still no unequivocal evidence that plants have evolved in response to contemporary increases in [CO2]. Stomatal density and stomatal size of nine diverse Floridian plant species have changed over the last 150 years, causing a decrease in maximal gs as [CO2] has risen from 290 to 390 ppm [51]. However, these changes were interpreted as being driven by an acclimation response, not genetic changes. This is consistent with numerous other studies on diverse taxa, finding that physiological adjustments play more significant roles than evolutionary responses to recent environmental change [59].
It is possible that crop breeding programmes will have incidentally selected for genotypes with improved responsiveness to elevated [CO2]. This possibility has not been intensively studied, but the available evidence suggests that it is not the case. In fact, the opposite scenario where [CO2] responsiveness has been selected against may have occurred. Two experimental comparisons of wheat genotypes released at different dates over the nineteenth and twentieth centuries both showed that stimulation of yield by elevated [CO2] predicted for mid- to late twenty-first century compared with ambient or pre-industrial [CO2] was greater in genotypes with earlier release dates (figure 2) [60,61]). There is also no evidence for a greater CO2-fertilization effect on yield of more recently released soybean genotypes in the US Department of Agriculture germplasm collection (R. Nelson & E. A. Ainsworth 2011, unpublished data). These findings have suggested that optimizing the performance of crops under [CO2] today and in the decades to come will not happen incidentally. Accordingly, high yield under the elevated [CO2] predicted in the future needs to be included as a target in crop breeding and biotechnology programmes [1,62].
Figure 2.
Seed yield as a function of growth at [CO2] ranging from subambient (293 ppm) to ambient (385 ppm) and elevated [CO2] (715 ppm) for four different Spring wheat lines released in (a) 1903 (Marquis), (b) 1921 (Thatcher), (c) 1965 (Chris) and (d) 1996 (Oxen). Treatment means are adapted from Ziska et al. [60].
4. Question 3: will rising [co2] drive natural selection in the future, and if so how?
While palaeoeological studies have implied evolutionary responses to past changes in atmospheric [CO2], recent quantitative genetic and selection experiments have tested whether predicted future elevated [CO2] concentrations will cause further evolutionary change [63–71]. The evolutionary effects of rising atmospheric [CO2] are likely to be fundamentally different from evolutionary effects of other types of anthropogenic environmental change because the rise in [CO2] occurs almost uniformly across the globe. Whereas evolution in response to other types of global change, such as global warming, may be facilitated by spatial variation (e.g. populations from warmer regions may possess genes that facilitate adaptation to warming climates), [CO2] does not vary substantially across species ranges, and, therefore, little genetic differentiation in [CO2] responsiveness among populations across a species range is expected. Still, because the traits that mediate [CO2] responsiveness are influenced by a wide variety of abiotic environmental conditions that vary both spatially and temporally (e.g. drought and temperature), genetic variation for the physiological traits underlying [CO2] response may exist in both natural [72] and crop species [73,74]. In this section, we review quantitative genetic studies that test how elevated [CO2] will affect predicted future evolutionary trajectories, what traits are likely to change in response to further increases in [CO2], and constraints on adaptation to future, elevated [CO2]. In addition, we highlight some of the challenges to predicting evolutionary responses to future increases in [CO2].
Evolutionary responses depend on: (i) selection, or whether elevated [CO2] alters the relationship between plant traits and plant fitness; and (ii) heritability and genetic covariance, or whether trait and fitness responses to [CO2] are passed to subsequent generations. Two approaches have been employed to understand how varying [CO2] will influence plant evolution. The first approach uses ‘selection in controlled environments’ experiments (sensu [75] e.g. [68,71]). Replicated plant populations are grown for multiple generations under ambient [CO2] or elevated [CO2] predicted for mid- to late twenty-first century. Offspring from populations that had evolved under ambient [CO2] conditions versus elevated [CO2] conditions are then compared, ideally in both ambient [CO2] and elevated [CO2] environments. Any divergence between populations can be attributed to genetic changes in plant traits in response to the [CO2] environment, provided that maternal environmental effects are controlled for. Increased fitness of populations that had evolved under elevated [CO2] conditions compared with populations evolved under ambient [CO2] conditions when grown in elevated [CO2] environments is evidence for adaptation to elevated [CO2]. The second approach employs quantitative genetics to compare predicted evolution in ambient [CO2] versus elevated [CO2] environments [63–65,69]. This approach involves estimating components of the evolutionary process (selection, heritability and/or genetic covariances) on plant populations grown in ambient [CO2] or elevated [CO2]. The advantage of the selection in a controlled environment approach is that it specifically tests for whether an evolutionary response occurs; however, the mechanisms underlying the response cannot be identified. The advantage of the quantitative genetic approach is that it identifies how the mechanisms of evolutionary change (altered patterns of natural selection, heritabilities or genetic covariances between traits) are affected by [CO2] and also can identify specific traits underlying adaptation to elevated [CO2] (see Question 4). Predicting the effects of [CO2] on long-term evolutionary change with this method, however, is complicated by assumptions that heritabilities and covariances remain constant over time [76].
Most studies that employ the selection in controlled environments approach find limited evidence that plants adapt to elevated [CO2], even though genetic changes in plant traits are often observed. Potvin & Tousignant [68] simultaneously manipulated [CO2] concentration and temperature to simulate future environmental conditions by increasing [CO2] concentrations from 370 to 650 ppm and temperature from 20°C to 23.6°C over seven generations. They detected little evidence that populations of Brassica juncea adapted to simulated future environments. Although half of the 14 traits measured had diverged between populations that had evolved under present versus future environmental conditions, only one measured trait showed an adaptive response, and no fitness measures showed a pattern of local adaptation. Similarly, Ward et al. [71] isolated the effects of [CO2] by artificially selecting on fecundity in replicate Arabidopsis thaliana populations grown under subambient [CO2] (200 ppm) and elevated [CO2] (700 ppm) for five generations. They found that subambient populations had adapted to low [CO2] and produced more seed than lines selected under elevated [CO2] when grown at 200 ppm [CO2]; however, elevated [CO2] populations had not adapted to elevated [CO2]—populations that had evolved under 200 ppm and 700 ppm did not differ significantly in seed production in elevated [CO2] environments. Moreover, elevated [CO2] selection lines actually increased biomass less in response to elevated [CO2] compared with control lines that were not artificially selected, suggesting that the biomass increases commonly observed in response to elevated [CO2] in single generation studies will not be increased by evolutionary change. Finally, Collins & Bell [77] used the model organism Chlamydomonas to investigate evolutionary responses over 1000 generations in either constant [CO2] environments (430 ppm) or steadily increasing [CO2] (430–1050 ppm). As with the earlier mentioned studies, no Chlamydomonas populations showed evidence for adaptation to elevated [CO2] even though changes in photosynthesis and respiration rates occurred. These changes reduced the fitness of populations evolved under elevated [CO2] when they were grown in lower [CO2] environments but did not affect fitness when they were grown in elevated [CO2]. Similar results were observed in natural Chlamydomonas populations found in CO2 springs [78]. Collins & Bell attribute their findings to the fixation of conditionally neutral mutations in the carbon concentration mechanism—these changes had no effect when [CO2] was saturating, but reduced growth and fitness when [CO2] was limiting. Together, these studies suggest that genetic changes may occur in response to elevated [CO2], but that these changes do not necessarily result in increased fitness or productivity in elevated [CO2] environments.
Similar to the results observed from selection in controlled environment studies, quantitative genetic experiments also find little evidence that elevated [CO2] has large effects on predicted evolutionary trajectories. For example, Lau et al. [64] failed to detect evidence that elevated [CO2] alters patterns of natural selection on plant traits, heritabilities or genetic covariances, despite employing a statistically powerful, well-replicated experiment on a highly variable population. Both Steinger et al. [69] and Bazzaz et al. [63] found evidence that elevated [CO2] alters patterns of natural selection and/or heritabilities; however, effects of [CO2] on evolutionary processes typically were small in magnitude. All of these studies focus primarily on down-stream traits, such as phenology and growth. Selection acting on physiological traits is rarely measured, in part because of the difficulty of measuring physiological traits on the hundreds or thousands of individuals necessary for rigorous quantitative genetics analyses. This is unfortunate given that: (i) physiological traits might be expected to respond most strongly to elevated [CO2]; (ii) several studies have shown that [CO2] alters phenotypic integration and the trade-offs between plant traits [70,79]; and (iii) results from selection in controlled environment studies suggest genetic changes in physiological traits in response to variation in [CO2] [77,80,81].
Together, the selection in controlled environment studies and quantitative genetic studies conducted to date indicate that adaptive evolutionary responses to elevated [CO2] will be weak relative to ecological and physiological responses. The lack of evidence for strong evolutionary responses is surprising, given the large effects of elevated [CO2] on plant phenotypes. However, it is consistent with mixed results from studies of populations growing along gradients of [CO2] at natural CO2 springs. Many studies fail to find evidence for adaptation to elevated [CO2] or evidence for genetic changes towards increased productivity, even though reduced allocation to photosynthetic apparatus is sometimes observed in populations with an evolutionary history of high [CO2] [78,82,83] (but see [84]). One recent example in which adaptation to elevated [CO2] was observed documented genetic divergence between Plantago asiatica populations growing near or far from natural CO2 springs [83]. When reared in common environments, genotypes collected from locations near the springs (greater [CO2]) had lower photosynthetic capacity and gs compared with genotypes far away from the springs (lesser [CO2]), but also had greater shoot-to-root ratios and achieved greater productivity. Interestingly, the study populations included in these experiments had experienced [CO2] concentrations ranging from 380 to 5338 µmol mol−1. Genetic differences in phenotypic traits were observed between populations that experienced [CO2] between 380 and 1044 ppm. In contrast, there was no additional differentiation between the populations experiencing [CO2] of 1044 and 5338 ppm. This is consistent with the idea presented in Question 1 that [CO2] will cease to be an agent of selection above the [CO2] (approx. 1000 ppm) at which physiological responses are saturated. Moreover, the physiological and growth stimulation effects of elevated [CO2] begin to attenuate at [CO2] even lower than 1000 ppm, potentially explaining the minimal evolutionary effects of elevated [CO2] but larger evolutionary responses to subambient [CO2] [3,83].
Although most quantitative genetic studies have been conducted in relatively simplistic growth chamber and greenhouse environments where both abiotic and biotic stressors are absent, some studies suggest that the evolutionary effects of [CO2] may be heightened in the presence of competitors or herbivores [63,65]. The effects of [CO2] in more complex communities may result through two processes. First, if elevated [CO2] alters the intensity or likelihood of biotic interactions and biotic interactions are strong agents of natural selection, then elevated [CO2] may alter evolution when those interactors are present, even if elevated [CO2] has minimal direct effects on evolutionary processes. For example, if a plant is grown in the presence of competitors and elevated [CO2] alters the outcome of competition because species vary in the magnitude of their growth response to [CO2], then the strength of competition as a selective agent may be altered. Lau et al. [65] provide empirical evidence in support of this mechanism; elevated [CO2] reduces the fitness effects of competition on A. thaliana. Because competition is a strong agent of selection on A. thaliana size traits, elevated [CO2] minimizes the selective effects of competition, and differences in patterns of natural selection are observed between populations grown in ambient [CO2] versus elevated [CO2] environments when competitors are present, even though [CO2] has no direct effects on predicted evolutionary trajectories in the absence of competitors. Second, elevated [CO2] may alter evolutionary process if elevated [CO2] affects the expression of traits that mediate interactions with other species. For example, Vannette & Hunter [85] find that five genotypes of Asclepias syrica respond differently to elevated [CO2] in terms of the expression of defensive chemicals but not growth or reproductive traits. If herbivores negatively impact plant fitness, then the effects of elevated [CO2] on defence trait expression could translate into differential effects on plant fitness when herbivores are abundant, even though elevated [CO2] is unlikely to affect evolutionary processes when herbivores are absent.
In sum, the available evidence to date suggests that evolutionary responses to elevated [CO2] will not be consistently observed or large in magnitude relative to ecological and physiological responses. This is despite substantial evidence indicating that subambient [CO2] concentrations are an important selective agent [11,71], potentially responsible for large evolutionary changes in a wide variety of plant traits and even the diversification of vascular plants (see Question 1). Most studies to date, however, have been conducted in relatively simplistic environmental conditions, where biotic and abiotic stress is minimal. Given that some evolutionary effects have been observed or are predicted when plants experience competition [63,65,69] or herbivory [85,86], evolutionary effects may be more likely in more stressful biotic environments. Similarly, evolutionary effects of elevated [CO2] also may be more likely when plants experience abiotic stress, such as drought or nutrient limitation. Under stressful environments, it is possible that the genetic changes in physiological traits observed in numerous studies may change from being conditionally neutral to beneficial, thereby resulting in differential effects on growth and fitness. Few studies, however, have investigated evolutionary consequences of rising atmospheric [CO2] in suboptimal environmental conditions. Given that temperature and potentially drought stress will increase simultaneously with [CO2], such studies are needed to identify evolutionary effects and traits under selection in future environments.
5. Question 4: what traits are favoured under high [co2]?
Physiological, palaeoecological and quantitative genetics experiments suggest that leaf and photosynthetic traits are responsive to [CO2] and, therefore, may play a key role in mediating adaptive evolutionary responses to elevated [CO2]. Moreover, both inter- and intraspecific comparisons reveal variations in [CO2] response (reviewed by Poorter & Navas [87], see table 1 in Lau et al. [64]). Still, we have a rather limited understanding of which plant traits are most likely to produce higher yields or increased fitness in the elevated [CO2] environments predicted for the future, as well as which specific combinations of traits are necessary for strong growth enhancement responses to elevated [CO2] [88]. The recent advent of trait-based approaches and associated multi-species trait datasets, combined with intraspecific comparisons and genetic or phenotypic manipulations of traits, may provide improved methods to more thoroughly understand the traits underlying [CO2] responsiveness and to predict which genotypes and species will be favoured in an elevated [CO2] world.
To date, most studies attempting to identify traits underlying variation in [CO2] response have used interspecific comparisons and have focused on differences among broad functional groups (e.g. C3 forbs, C3 grasses, C4 grasses and legumes). Such studies typically find that C3 species have greater responses to elevated [CO2] than C4 species and that legumes have relatively higher CO2 responses than non-legumes, provided that other nutrients are not limiting [89,90]. Fewer studies have focused on more specific growth, phenological or physiological traits. Traits underlying adaptation to elevated [CO2] may be identified by growing species under ambient or elevated [CO2] conditions and correlating phenotypic traits with the growth stimulation effects of elevated [CO2] or fitness/yield in elevated [CO2] environments. In one such study, Atkin et al. [91] compared the growth stimulation effects of elevated [CO2] on ten Acacia species that varied in relative growth rate (RGR), and found that fast-growing species (greater RGR) responded more to elevated [CO2] than slow-growing species. However, later studies on the same set of Acacia species found no relationship between RGR or leaf traits (specific foliage area) and CO2 response [92]. Furthermore, studies on other systems have found the opposite pattern [93]. Such empirical studies have the advantage of manipulating [CO2] on multiple species grown in common environmental conditions, but are limited by the small number of taxa that can be considered in any one experiment. In addition, manipulating [CO2] in a single environment may be problematic given that environmental conditions affect [CO2] responses and these environmental effects may vary across genotypes. In the study by Atkin et al., for example, the [CO2] manipulation took place under optimal nutrient and water conditions, an environment that favours the fast-growing species. Slow-growing species typically inhabit more stressful environments, and a very different finding may have resulted if the experimental environment more closely matched environments to which slow-growing species were adapted.
More recently, meta-analyses have been conducted on data from hundreds of existing empirical studies to look for broad patterns in [CO2] response across taxonomic scales. Poorter & Navas [87] conducted a meta-analysis on 350 different experiments that examined the growth stimulation effects of elevated [CO2] on 350 different plant species. Surprisingly, only 18 per cent of the variation in growth response was explained by species, possibly because CO2 stimulation effects also depend on ontogeny, environment and intraspecific variation. Still, the meta-analysis confirmed expectations: C3 plants exhibited the strongest growth response to elevated [CO2]; C4 plants showed the smallest growth response; and CAM plants demonstrated intermediate responses. These functional group classifications based on photosynthetic mechanisms explained only 10 per cent of the variation among species in CO2 response. This may reflect the importance of ontogeny, environment and genotypes as mentioned earlier, but could also indicate that other traits play strong roles in mediating productivity responses to [CO2]. For example, among C3 forbs, fast growers responded more strongly to elevated [CO2] than slow growers and differences were also observed between dicots and monocots as well as legumes versus non-legumes [87]. Other meta-analyses and reviews have focused on other traits. Kerstiens [94] found that biomass responses to elevated [CO2] were greater for shade-tolerant species than shade-intolerant species. Similarly, Niinements [39] observed that plants with more robust leaves (i.e. evergreen schlerophylls) responded more positively to elevated [CO2] than species with less robust leaves, emphasizing the importance of mesophyll conductance and its role in influencing [CO2] supply to chloroplasts.
Similar to the interspecific comparisons described earlier, intraspecific variation in plant traits also can be correlated with growth responses to elevated [CO2] or high fitness/yield in elevated [CO2] environments. A study measuring RGR on 29 Picea glauca genotypes found no association between RGR and stimulation of productivity at elevated [CO2] [95]. Moreover, P. glauca growth at elevated [CO2] was tightly correlated with growth at ambient [CO2]. In other words, the most productive genotypes in ambient [CO2] were also the most productive genotypes/highest yielders in elevated [CO2]. This result was used to argue that the association between RGR and stimulation of productivity by elevated [CO2] observed in prior interspecific comparisons was due to traits correlated with both RGR and enhancement of productivity by elevated [CO2], rather than a direct relationship between RGR and enhancement of productivity by elevated [CO2]. Similarly, Liu et al. [96] observed that four provenances of Populus tremuloides exhibited different responses to elevated [CO2] in terms of gs and transpiration rate, but these differences did not translate into differences among the provenances in biomass response to elevated [CO2].
Conclusions reached from both intra- and interspecific approaches illustrate an important challenge to identifying traits associated with adaptation to elevated [CO2] environments. To pinpoint exactly what traits are responsible for high growth responses to elevated [CO2] or high fitness/yield in elevated [CO2] environments, all relevant traits must be measured. If all relevant traits are included in the regression model, then the particular traits responsible for adaptation to elevated [CO2] can be identified. In an intraspecific context, this is essentially what is done in the phenotypic selection analysis approach developed by Lande & Arnold [97]. This approach uses multiple regression to account for correlations among traits. As a result, it can differentiate between traits that are directly associated with fitness versus traits that are indirectly associated with fitness due to correlations with other phenotypic traits. It should be noted, however, that there are several statistical and biological challenges to be dealt with when applying this approach (summarized by Mitchell-Olds & Shaw [98]), including issues with identifying traits directly under selection due to correlations between measured traits and unmeasured traits that are under selection. In the selection analyses in ambient [CO2] versus elevated [CO2] environments conducted to date, however, there is little evidence suggesting that different traits are associated with high fitness in ambient [CO2] versus elevated [CO2] [64]. It is important to note, however, that most such studies focus on growth or phenological traits rather than on physiological traits. Similarly, comparisons among recombinant inbred lines (as suggested by Zhang et al. [95]), mutant-wild-type comparisons [99] and experimental manipulations of phenotypic traits [100] also may effectively identify traits involved in CO2 response because focal traits segregate independently of genetic background. Alternatively, modelling approaches [101] can be used to predict which traits are important to fitness/yields in future environments, identifying focal traits for further investigation in empirical studies.
Similar approaches can be applied to interspecific comparisons [102]. While previous interspecific comparisons were largely limited to coarse-scale comparisons among functional groups or to studies on few species in a single clade, the advent of new trait databases, which include physiological, as well as morphological and phenological traits, such as TRY [103], may allow for robust multi-trait analyses on the hundreds of species for which [CO2] responses have been measured. Although such trait-based approaches have not yet been used to investigate traits underlying [CO2] response, they have been employed to predict changes in community composition in response to other anthropogenic environmental changes (e.g. habitat fragmentation [104]).
A challenge to all studies focused on finding traits or genes underlying adaptation to elevated [CO2] is that multiple traits are probably involved. For example, interspecific variation in photosynthetic efficiency is not due to one trait, but is instead a result of several physiological traits each with relatively small effects [105]. Moreover, these traits are often correlated and may have synergistic effects [72]. For example, hypothetically, having low Amass and high Nmass is expected to yield very unfit genotypes because the high cost of maintaining Rubisco would exceed carbon gains [72]. Many such correlations are probably maintained by selection, suggesting that the correlations may change in response to novel environmental conditions. Although it would require large sample sizes and many trait measurements, in the quantitative genetics framework, correlational selection studies could be used to identify how the adaptive value of one trait depends on other trait values. Similar approaches could be applied to interspecific trait datasets. Alternatively, cluster analyses of morpho-physiological diversity may be used to identify suites of correlated traits underlying adaptation [102]. Cluster analyses have been used to identify traits and potential diversity for improved agronomic breeding to novel stressors, such as drought tolerance [106].
The trait-based approaches described earlier may be powerful tools for identifying individual traits and suites of correlated traits underlying high fitness in future [CO2]. Ultimately, suites of traits with synergistic interactions are likely to contribute to high fitness in novel environments. As a result, large datasets consisting of many traits for each of many genotypes or species are necessary. Prior palaeoecological studies on evolutionary responses to variation in [CO2] concentration have identified several potential traits that may be involved in [CO2] responses, and modelling approaches may identify many more. Improvements in both the number of traits and number of taxa included in existing trait datasets, as well as identification of likely adaptive traits through modelling or knowledge of physiological responses, may allow for rapid advances in identifying the physiological, phenological and morphological traits that may lead to increased growth and fitness in future, elevated [CO2] environments. Moreover, crosses between distinct genotypes that differ in entire suites of traits may yield novel genotypes and trait combinations that could contribute to fitness and yield improvement in ambient [CO2] versus elevated [CO2].
6. Question 5: how does evolutionary history impact and inform efforts to engineer crops for improved performance in present and future [co2]?
Circumventing the limitations of current low [CO2] to photosynthesis in many of our main crop plants is a key target for biotechnological efforts to improve crop productivity [107–110]. In addition, there is emerging recognition that intervention to adapt crops by breeding or biotechnology for optimal performance in elevated [CO2] is needed [111]. In response, targets for selection are beginning to be identified [1], and approaches that account for the challenges of genetic and trait-based approaches described earlier (Questions 3 and 4) have been proposed [62]. Here, we review present targets for crop improvement related to present and future [CO2].
The enzyme responsible for photosynthetic fixation of CO2 in all plants, Rubisco (RibUlose-1,5-BISphosphate Carboxylase Oxygenase), is fundamentally inefficient. This stems from a relatively low CO2 affinity and low carboxylation reaction catalytic rate, which plants compensate for by synthesizing very large quantities of the enzyme, at the expense of a very large fraction of their leaf nitrogen. In addition, a significant fraction of reactions catalysed by Rubisco result in oxygenation rather than carboxylation of RuBP (RibUlose-1,5-BisPhosphate). Recycling of the toxic 2PG (2-PhosphoGlycolate) that is one of the products of the oxygenation reaction is achieved by the photorespiratory pathway, but at the expense of energy, carbon and nitrogen [109]. Efforts to circumvent these inefficiencies fall into three categories. First, engineering of the Rubisco to improve its enzymatic performance. Second, engineering of CO2 concentrating mechanisms to saturate the carboxylation reaction and suppress the oxygenation reaction. Third, modifications to the photorespiratory pathway that reduce losses of carbon, nitrogen and energy.
There has been considerable selective pressure for the evolution of more efficient Rubisco throughout the history of land plants. During the periods of sub-saturating [CO2], which—at a minimum—include 50 Myr in the Carboniferous/Permian and the last 30 Myr (figure 1a), modifications leading to greater [CO2] specificity, greater catalytic rate or impaired oxygenation without deleterious side-effects could have increased photosynthetic efficiency [110]. During periods of saturating [CO2], modifications leading to greater catalytic rate without deleterious side-effects could have increased photosynthetic efficiency. In each case, greater photosynthetic efficiency would result in greater carbon gain for the same investment in resources, or equivalent carbon gain but with greater water-use efficiency and nitrogen-use efficiency. Nevertheless, it appears that evolution of Rubisco has been constrained in a fundamental manner as it remains the limiting step in metabolism for most modern C3 plants in many growing conditions.
The factors that have constrained the evolution and engineering of ‘improved’ Rubisco up until now are becoming better understood [110]. There are three different clades of Rubisco [112]. Clade 1 includes all vascular plants along with cyanobacteria and some algae and proteobacteria. Clade 2 is found in chemoautotrophs, dinoflagellate algae and some proteobacteria. Clade 3 is exclusive to the archaea. They all probably share a common ancestor, which was a methanogenic archaea [112]. Even with considerable variation in amino acid sequence and biological function, key active site residues are conserved across the three clades [113]. This has resulted in a shared activation process and catalytic chemistry that suggests that there are considerable constraints upon modification of enzyme function [114]. One fundamental issue may be that CO2 directly binds to the RuBP enediol, rather than forming a Michaelis complex with Rubisco, which reduces the capacity for discrimination against O2 binding [115]. An important trade-off exists in which Rubiscos with greater specificity for CO2 relative to O2 have lower catalytic rates (figure 3) [116,117]. Modelling the effects of variation in Rubisco specificity on canopy photosynthesis while accounting for the constraints of this relationship indicated that the Rubisco specificity and catalytic rate of modern C3 plants is optimal for [CO2] of approximately 200 ppm [116]. This may reflect adaptation of Rubisco for optimal carbon gain at the average [CO2] of the last 400 000 years. If so, Rubisco evolution has not kept pace with anthropogenic [CO2] rise, and will become increasingly maladapted over the course of this century. In addition, the evolutionary changes that have occurred could be considered fine-scale tuning of Rubisco without solving its more fundamental inefficiencies. Transgenic Rubiscos have been produced that break the trade-off between specificity and catalytic rate, but typically the results have been reduced rather than improved enzyme performance [116]. However, the modelling analysis does emphasize that among the natural diversity of Rubisco, there are enzymes with more favourable characteristics than currently found in C3 plants. Improved understanding of the expression and assembly of Rubisco is necessary to allow the expression of non-native Rubisco in higher plants. The process is complicated because Rubisco in higher plants is a complex of four dimers of a large subunit encoded in the plastid genome plus eight small subunits encoded in the nuclear genome [110]. Progress has been made in using plastome transformation to replace tobacco Rubisco with bacterial and archaeal Rubiscos [118,119]. However, attempts to express more efficient red algal and sunflower Rubiscos in tobacco have failed owing to differences in their requirements for protein folding and assembly [110]. An additional molecular constraint to the modification of Rubisco is the need for sugar-phosphate inhibitors bound to its active site to be removed during an interaction with Rubisco activase. The identity of residues required for successful interaction with Rubisco activase has been proposed [120], but awaits experimental confirmation [110]. The absence of regulation by Rubisco activase and colocalization of genes for both Rubisco subunits and chaperone proteins on the plastome may explain why evolution of Rubisco appears to have progressed further in red algae than in higher plants [121].
Figure 3.
Assuming a fixed number of Rubisco active sites per unit leaf area and the dependence of catalytic rate per active site 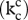 on specificity described for different photosynthetic organisms by Zhu et al. [116], the line shows, for any given atmospheric [CO2], the specificity (τ) that will give the highest light-saturated rate of leaf photosynthetic CO2 uptake (Asat). The average τ for terrestrial C3 crop plants (92.5) is indicated (τ1) together with the interpolated atmospheric [CO2] at which it would yield the maximum Asat (C1). Point τ2 is the specificity that would yield the highest Asat at the current [CO2] of the atmosphere (C2). At C2, decrease in τ from present average (τ1) to the optimum for current [CO2] (τ2) can increase light-saturated leaf photosynthetic carbon uptake by 12%. Reproduced with permission from Zhu et al. [116].
on specificity described for different photosynthetic organisms by Zhu et al. [116], the line shows, for any given atmospheric [CO2], the specificity (τ) that will give the highest light-saturated rate of leaf photosynthetic CO2 uptake (Asat). The average τ for terrestrial C3 crop plants (92.5) is indicated (τ1) together with the interpolated atmospheric [CO2] at which it would yield the maximum Asat (C1). Point τ2 is the specificity that would yield the highest Asat at the current [CO2] of the atmosphere (C2). At C2, decrease in τ from present average (τ1) to the optimum for current [CO2] (τ2) can increase light-saturated leaf photosynthetic carbon uptake by 12%. Reproduced with permission from Zhu et al. [116].
While Rubisco engineering attempts to overcome the limitations of natural evolution, an alternative strategy is derived from knowledge of successful natural evolutionary responses in which a CO2 concentrating mechanism overcomes the CO2-limitation of photosynthesis. Higher plants with C4 and CAM photosynthesis, as well as cyanobacteria with carboxysomes and algae with pyrenoids, all achieve efficient photosynthesis by concentrating CO2 around Rubisco in order to stimulate carboxylation and inhibit oxygenation. Efforts have begun to engineer these traits in C3 plants as a means to increase productivity and yield.
C4 photosynthesis is thought to have evolved as an adaptation to limit photorespiration at times of sub-saturating [CO2] [42]. Successful conversion of rice from a C3 plant to a C4 plant would likely increase A, water-use efficiency and nitrogen-use efficiency, especially in hotter and drier environments [107]. Achieving this goal will be challenging because C4 photosynthesis is a highly polygenic trait. Accordingly, it will require not just the expression of genes encoding the enzymes of the CO2 concentrating mechanism, but also engineering Kranz anatomy and transporters supporting flux between mesophyll and bundle sheath compartments [122]. This is reflected in a proposed evolutionary scheme involving a series of steps in the evolution from C3 to C4 photosynthesis: (i) general preconditioning, i.e. gene duplication; (ii) anatomical preconditioning, i.e. close veins; (iii) enhancement of bundle sheath organelles; (iv) addition of photorespiratory pump, including localization of glycine decarboxylase to the bundle sheath; (v) enhancement of Phosphoenol pyruvate carboxylase activity; (vi) integration of components; and (vii) optimization of components [42]. Linked to this knowledge, significant effort has recently focused on determining the transcriptional control of C4 leaf structural and metabolic development [123]. The fact that C4 photosynthesis has independently evolved in many genetic backgrounds on different occasions increases the likelihood that successful engineering can be achieved [107]. Specific evidence for this assertion includes that: (i) independent lineages of C4 species share common mechanisms controlling the localization of key enzymes for C4 photosynthesis in bundle sheath cells; and (ii) specific localization of enzymes in C4 leaves to bundle sheath versus mesophyll cells can be achieved by modification of trans-factors without a change in existing cis-regulation of C3 species [124].
An effort is also beginning to engineer tobacco plants with carboxysomes from cyanobacteria (http://www.nsf.gov/news/news_summ.jsp?cntn_id=119017). This could also enhance carbon gain, water-use efficiency and nitrogen-use efficiency. Carboxysomes are structures where photosynthetic enzymes are localized, creating high [CO2]. Engineering carboxysomes into chloroplasts of crop plants would effectively create an evolutionary flashback to when cyanobacteria were symbiotically recruited into host cells as the precursors of chloroplasts. While the strategy requires conglomeration of traits from now distantly related species, it benefits from being limited to manipulation of metabolism within individual cells.
An alternative in preventing photorespiratory losses by the development of CO2 concentrating mechanisms is direct manipulation of the photorespiratory pathway in order to prevent or reduce the typical losses of carbon, nitrogen and energy once the oxygention reaction has produced 2PG [109]. Two independent approaches in achieving this goal have inserted multi-enzyme pathways into plant chlorolasts. The first fully oxidizes glycolate in the chloroplast into CO2 [125]. The second inserts a bacterial pathway into the chloroplast that converts glycolate into glycerate (producing some CO2 as a by-product), which can then be phosphorylated and re-incorporated into the Calvin cycle [126]. Both modifications are beneficial because they: (i) release CO2 in the chloroplast that can be refixed by Rubisco, potentially at higher concentrations; (ii) avoid release of ammonia and the energetic cost usually associated with its reassimilation; and (iii) produce additional reducing equivalents in the chloroplast [109]. Expression of the bacterial pathway in Arabidopsis led to a 30 per cent stimulation of biomass production [127]. One potential problem that remains to be tested is whether excess reducing equivalents will be produced under high light conditions, as photorespiration can play an important role as an alternative electron sink during periods of stress that impair photosynthetic quenching [109,128].
All of the approaches to enhancing photosynthesis described above tackle the limitation to photosynthesis by current [CO2]. They address the urgent need to boost crop production in the face of growing food insecurity. However, looking further into the future, the continuing rise of [CO2] will gradually diminish this limitation to photosynthesis and optimization of crop productivity will present a modified set of challenges. The speed of anthropogenically driven [CO2] rise means forward thinking is particularly necessary to optimize crops to their growth [CO2]. Based on the evidence reviewed earlier, natural selection for improved performance in elevated [CO2] is weak and there is unlikely to have been incidental breeding for improved performance at elevated [CO2] to date (Questions 2 and 3). However, understanding of plant cellular, physiological and agronomic responses to elevated [CO2] has allowed preliminary identification of targets for biotechnological improvement [1].
Future, elevated [CO2] will favour replacement of Rubisco in C3 crops with Rubisco that has lower specificity and greater catalytic rate (derived from C4 species or algae) even more so than under present conditions [116]. Some organisms are capable of expressing different Rubiscos whose characteristics are tailored to variation in growth conditions [127]. Engineering such a regulatory system into crops could provide additional benefits, such as expressing different Rubiscos in sun and shade leaves [116].
At elevated [CO2], A becomes limited by the capacity for regeneration of RuBp [129]. Modelling suggests that allocation of greater nitrogen resources to enzymes involved in RuBp regeneration in the Calvin cycle will stimulate photosynthesis at elevated [CO2] [130]. Transgenic tobacco overexpressing one of these enzymes, sedoheptulose-1,7-bisphosphatase, achieves enhanced photosynthesis and productivity [131].
C3 plants grown at elevated [CO2] consistently accumulate substantially larger pools of carbohydrates in leaves and other tissues, even when grown with unlimited rooting volume in the field [132]. The primary molecular response of soybean to growth at elevated [CO2] is transcriptional reprogramming of the respiratory pathway, allowing greater use of the additional available assimilate [133]. Even though soybean undergoes this metabolic rewiring and is able to match greater C fixation with enhanced N assimilation [134], there is still significant accumulation of leaf starch for much of the growing season [133,135]. This implies that greater enhancement of productivity at elevated [CO2] might be achieved by increasing utilization of photoassimilate. Despite improved understanding of how carbohydrate status drives productivity [136,137], further work is needed to determine how C utilization is controlled by interactions among sink metabolism, photoassimilate transport capacity and energy demand for photoassimilate export from source leaves [133,138–141]. Given the projected increases in temperature, drought and disease stress that crops will experience due to global environmental change, greater allocation of carbon resources to metabolites associated with stress tolerance could have multiple advantages [1]. For example, greater production of osmolytes, such as pinotol, mannitol and raffinose, can provide protection from dehydration under high temperature and drought stress, while antioxidant metabolites, such as ascorbate, reduce oxidative damage from elevated ozone and drought stress [142–144]. Greater carbon resources are typically assumed to allow plants to invest great resources in defence [145]. However, the changes in hormone signalling and secondary metabolism of soybean grown under elevated [CO2] provide an interesting exception to this rule. When grown at elevated [CO2] in the field, the inducible defence response of soybean to damage by Japanese beetle, induction of a protease inhibitor that hinders the beetles' digestive process, is impaired [146]. Changes in sugar–hormone interactions are thought to underpin the response and may provide another target for enhancing crop production in elevated [CO2].
Growth at elevated [CO2] increases nitrogen-use efficiency by stimulating A per unit leaf N and by allowing photosynthetic acclimation in which less N is allocated to Rubisco, leaving greater N resources available for other processes including growth [129,147]. Despite these physiological changes, N availability strongly limits the response of productivity to elevated [CO2] [132]. Legumes are able to achieve large increases in yield and maintain tissue C : N ratios under elevated [CO2] because they can allocate additional carbon to N-fixing nodules, provided other nutrients are not limiting [148–150]. Engineering other C3 crops with the capacity to fix N through symbiotic relationships with nodule-forming or endophytic microbes would allow them to benefit more from rising [CO2] and would be favoured by conditions of greater C availability. The biofuel crop, Miscanthus giganteus, shows the potential of such an approach, as it was recently shown to achieve its extremely high productivity by combining C4 photosynthesis with N fixation by endophytic microbes [151]. A potentially deleterious coupling between inhibition of photorespiration at elevated [CO2] and impairment of leaf N assimilation in Arabidopsis has also recently been proposed [152]. If this is the case in crop species under field conditions, and the response is not counteracted by greater root N assimilation, elucidation of the mechanism of response could yield a further target for biotechnological improvement to optimize the coupling of carbon and nitrogen metabolism and maximize productivity.
7. Conclusion
There are several lines of evidence that periods of falling and low [CO2] in the palaeo-record created selective pressure for two major classes of adaptation: (i) adaptations to acquire and use water in exchange for [CO2], which were presumably restricted to plants existing in mesic environments and (ii) adaptations for CO2 concentrating mechanisms that increase photosynthetic efficiency and maximize water-use efficiency, which were presumably favoured in hot and dry environments. Nevertheless, while contemporary global environmental change is impacting many elements of plant biology, there is still no unequivocal evidence for plant adaptation to contemporary increases in [CO2]. This includes no evidence for incidental breeding of crop varieties to achieve greater yield enhancement from future [CO2]. The studies of evolution in response to elevated [CO2] conducted to date applying selection in controlled environments, quantitative genetics and trait-based approaches suggest that the evolutionary responses of natural plant populations to future [CO2] will not be consistent or strong relative to ecological and physiological responses. This lack of evidence for strong evolutionary effects is surprising given the large effects of elevated [CO2] on plant phenotypes. Most selection and quantitative genetics studies to date, however, have been conducted in relatively simplistic environmental conditions, where biotic and abiotic stresses were avoided. Under more stressful and complex field environments, it is possible that the genetic changes in physiological traits observed in numerous studies may change from conditionally neutral to beneficial, thereby resulting in differential effects on growth and fitness. Given that temperature and potentially drought stress will increase simultaneously with [CO2], such studies are needed to identify evolutionary effects and traits under selection in future environments. Improvements in both the number of traits and number of taxa included in existing trait datasets, as well as identification of likely adaptive traits through modelling or knowledge of physiological responses, may allow for rapid advances in identifying the physiological, phenological and morphological traits that may lead to increased growth and fitness in future, elevated [CO2] environments. Already, efforts are underway to engineer plants to overcome present day [CO2] limitations to photosynthesis and carbon gain. These include efforts to tackle those inefficiencies of Rubisco that natural selection has failed to overcome, as well as attempts to mimic the evolutionary successes of CO2 concentrating mechanisms and photorespiratory shunts that allow enhanced carbon gain and greater resource-use efficiency in some higher plants, algae and bacteria. Looking further into the future, the continuing rise of [CO2] will gradually diminish this limitation to photosynthesis and optimization of crop productivity will present a modified set of challenges. Methods to tackle this challenge are available and fundamental understanding of plant cellular and physiological responses is improving such that targets for biotechnological optimization of crop performance under future [CO2] are being proposed and should be tested.
Acknowledgements
We thank David Beerling for the invitation to participate in the workshop, ‘CO2 and Plant Evolution’, which was funded and hosted by the Royal Society at the Kavli Centre, and led to the development of this manuscript. We thank Dana Royer for providing a compilation of Phanerozoic [CO2] estimates from proxy analysis. We also thank Dana Royer, Colin Osborne and an anonymous reviewer for helpful comments and suggestions that greatly improved this manuscript. This is KBS publication no. 1594.
References
- 1.Ainsworth E. A., Rogers A., Leakey A. D. B. 2008. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 147, 13–19 10.1104/pp.108.117101 (doi:10.1104/pp.108.117101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beerling D. J. 2009. Coevolution of photosynthetic organisms and the environment. Geobiology 7, 97–99 10.1111/j.1472-4669.2009.00196.x (doi:10.1111/j.1472-4669.2009.00196.x) [DOI] [PubMed] [Google Scholar]
- 3.Ward J. K., Kelly J. K. 2004. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol. Lett. 7, 427–440 10.1111/j.1461-0248.2004.00589.x (doi:10.1111/j.1461-0248.2004.00589.x) [DOI] [Google Scholar]
- 4.Ward J. K., Strain B. R. 1999. Elevated CO2 studies: past, present and future. Tree Physiol. 19, 211–220 10.1093/treephys/19.4-5.211 (doi:10.1093/treephys/19.4-5.211) [DOI] [PubMed] [Google Scholar]
- 5.Ackerly D. D., et al. 2000. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50, 979–995 10.1641/0006-3568(2000)050[0979:TEOPET]2.0.CO;2 (doi:10.1641/0006-3568(2000)050[0979:TEOPET]2.0.CO;2) [DOI] [Google Scholar]
- 6.Ackerly D. D., Monson R. K. 2003. Waking the sleeping giant: the evolutionary foundations of plant function. Int. J. Plant Sci. 164, S1–S6 10.1086/374729 (doi:10.1086/374729) [DOI] [Google Scholar]
- 7.Reusch T. B. H., Wood T. E. 2007. Molecular ecology of global change. Mol. Ecol. 16, 3973–3992 10.1111/j.1365-294X.2007.03454.x (doi:10.1111/j.1365-294X.2007.03454.x) [DOI] [PubMed] [Google Scholar]
- 8.Beerling D. J. 2005. Leaf evolution: gases, genes and geochemistry. Ann. Bot. 96, 345–352 10.1093/aob/mci186 (doi:10.1093/aob/mci186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards E. J., Osborne C. P., Strömberg C. A. E., Smith S. A. and C4 Grasses Consortium. 2010 The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591 10.1126/science.1177216 (doi:10.1126/science.1177216) [DOI] [PubMed] [Google Scholar]
- 10.Feild T. S., Arens N. C. 2005. Form, function and environments of the early angiosperms: merging extant phylogeny and ecophysiology with fossils. New Phytol. 166, 383–408 10.1111/j.1469-8137.2005.01333.x (doi:10.1111/j.1469-8137.2005.01333.x) [DOI] [PubMed] [Google Scholar]
- 11.Gerhart L. M., Ward J. K. 2010. Plant responses to low [CO2] of the past. New Phytol. 188, 674–695 10.1111/j.1469-8137.2010.03441.x (doi:10.1111/j.1469-8137.2010.03441.x) [DOI] [PubMed] [Google Scholar]
- 12.Willis K. J., McElwain J. C. 2002. The evolution of plants. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Berner R. A. 2006. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664 10.1016/j.gca.2005.11.032 (doi:10.1016/j.gca.2005.11.032) [DOI] [Google Scholar]
- 14.Royer D. L. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 114, 1–28 10.1016/S0034-6667(00)00074-9 (doi:10.1016/S0034-6667(00)00074-9) [DOI] [PubMed] [Google Scholar]
- 15.Cerling T. E. 1991. Carbon dioxide in the atmosphere-evidence from Cenozoic and Mesozoic paleosols. Am. J. Sci. 291, 377–400 10.2475/ajs.291.4.377 (doi:10.2475/ajs.291.4.377) [DOI] [Google Scholar]
- 16.Fletcher B. J., Brentnall S. J., Anderson C. W., Berner R. A., Beerling D. J. 2008. Atmospheric carbon dioxide linked with Mesozoic and early Cenozoic climate change. Nat. Geosci. 1, 43–48 10.1038/ngeo.2007.29 (doi:10.1038/ngeo.2007.29) [DOI] [Google Scholar]
- 17.Freeman K. H., Hayes J. M. 1992. Fractionation of carbon isolopes by phytoplankton and estimates of ancient carbon dioxide levels. Glob. Biogeochem. Cycles 6, 185–198 10.1029/92GB00190 (doi:10.1029/92GB00190) [DOI] [PubMed] [Google Scholar]
- 18.Pagani M., Freeman K. H., Arthur M. A. 1999. Late Miocene atmospheric CO2 concentrations and the expansion of C4 grasses. Science 285, 876–879 10.1126/science.285.5429.876 (doi:10.1126/science.285.5429.876) [DOI] [PubMed] [Google Scholar]
- 19.Pagani M., Zachos J. C., Freeman K. H., Tipple B., Bohaty S. 2005. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science 309, 600–603 10.1126/science.1110063 (doi:10.1126/science.1110063) [DOI] [PubMed] [Google Scholar]
- 20.Royer D. L., Berner R. A., Beerling D. J. 2001. Phanerozoic atmospheric CO2 change: evaluating geochemical and paleobiological approaches. Earth Sci. Rev. 54, 349–392 10.1016/S0012-8252(00)00042-8 (doi:10.1016/S0012-8252(00)00042-8) [DOI] [Google Scholar]
- 21.Royer D. 2006. CO2-forced climate thresholds during the Phanerozoic. Geochim. Cosmochim. Acta 70, 5665–5675 10.1016/j.gca.2005.11.031 (doi:10.1016/j.gca.2005.11.031) [DOI] [Google Scholar]
- 22.Wullschleger S. D. 1993. Biochemical limitations to carbon assimilation in C3 plants: a retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 44, 907–920 10.1093/jxb/44.5.907 (doi:10.1093/jxb/44.5.907) [DOI] [Google Scholar]
- 23.Franks P. J., Beerling D. J. 2009. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7, 227–236 10.1111/j.1472-4669.2009.00193.x (doi:10.1111/j.1472-4669.2009.00193.x) [DOI] [PubMed] [Google Scholar]
- 24.Knoll A. H., Niklas K. J. 1987. Adaptation, plant evolution, and the fossil record. Rev. Palaeobot. Palynol. 50, 127–149 10.1016/0034-6667(87)90043-1 (doi:10.1016/0034-6667(87)90043-1) [DOI] [PubMed] [Google Scholar]
- 25.Osborne C. P., Beerling D. J., Lomax B. H., Chaloner W. G. 2004. Biophysical constraints on the origin of leaves inferred from the fossil record. Proc. Natl Acad. Sci. USA 101, 10 360–10 362 10.1073/pnas.0402787101 (doi:10.1073/pnas.0402787101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodribb T. J., Feild T. S. 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175–183 10.1111/j.1461-0248.2009.01410.x (doi:10.1111/j.1461-0248.2009.01410.x) [DOI] [PubMed] [Google Scholar]
- 27.Haworth M., Elliott-Kingston C., McElwain J. C. 2011. Stomatal control as a driver of plant evolution. J. Exp. Bot. 62, 2419–2423 10.1093/jxb/err086 (doi:10.1093/jxb/err086) [DOI] [PubMed] [Google Scholar]
- 28.Beerling D. J., Osborne C. P., Chaloner W. G. 2001. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410, 352–354 10.1038/35066546 (doi:10.1038/35066546) [DOI] [PubMed] [Google Scholar]
- 29.Franks P. J., Beerling D. J. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl Acad. Sci. USA 106, 10 343–10 347 10.1073/pnas.0904209106 (doi:10.1073/pnas.0904209106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodward W. I. 1998. Do plants really need stomata? J. Exp. Bot. 49, 471–480 10.1093/jexbot/49.suppl_1.471 (doi:10.1093/jexbot/49.suppl_1.471) [DOI] [Google Scholar]
- 31.Boyce C. K., Brodribb T. J., Field T. S., Zwienieki M. A. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B 276, 1771–1776 10.1098/rspb.2008.1919 (doi:10.1098/rspb.2008.1919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beerling D. J., Franks P. J. 2010. The hidden cost of transpiration. Nature 464, 495–496 10.1038/464495a (doi:10.1038/464495a) [DOI] [PubMed] [Google Scholar]
- 33.McKown A. D., Cochard H., Sack L. 2010. Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am. Nat. 175, 447–460 10.1086/650721 (doi:10.1086/650721) [DOI] [PubMed] [Google Scholar]
- 34.Sack L., Holbrook N. M. 2006. Leaf hydraulics. Annu. Rev. Plant Biol. 57, 361–381 10.1146/annurev.arplant.56.032604.144141 (doi:10.1146/annurev.arplant.56.032604.144141) [DOI] [PubMed] [Google Scholar]
- 35.Gould S. J., Vrba E. S. 1982. Exaptation: a missing term in the science of form. Paleobiology 8, 4–15 [Google Scholar]
- 36.Anderson L. J., Maherali H., Johnson H. B., Polley H. W., Jackson R. B. 2001. Gas exchange and photosynthetic acclimation over subambient to elevated CO2 in a C3–C4 grassland. Global Change Biol. 7, 693–707 10.1046/j.1354-1013.2001.00438.x (doi:10.1046/j.1354-1013.2001.00438.x) [DOI] [Google Scholar]
- 37.Tholen D., Zhu X. G. 2011. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol. 156, 90–105 10.1104/pp.111.172346 (doi:10.1104/pp.111.172346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farquhar G. D., Sharkey T. D. 1982. Stomatal conductance and photosynthesis. Annu. Rev. Plant Phys. Plant Mol. Biol. 33, 317–345 10.1146/annurev.pp.33.060182.001533 (doi:10.1146/annurev.pp.33.060182.001533) [DOI] [Google Scholar]
- 39.Niinemets U., Flexas J., Penuelas J. 2011. Evergreens favored by higher responsiveness to increased CO2. Trends Ecol. Evol. 26, 136–142 10.1016/j.tree.2010.12.012 (doi:10.1016/j.tree.2010.12.012) [DOI] [PubMed] [Google Scholar]
- 40.Christin P. A., Besnard G., Samaritani E., Duvall M. R., Hodkinson T. R., Savolainen V., Salamin N. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr. Biol. 18, 37–43 10.1016/j.cub.2007.11.058 (doi:10.1016/j.cub.2007.11.058) [DOI] [PubMed] [Google Scholar]
- 41.Ehleringer J. R., Cerling T. E., Helliker B. R. 1997. C4 photosynthesis, atmospheric CO2 and climate. Oecologia 112, 285–299 10.1007/s004420050311 (doi:10.1007/s004420050311) [DOI] [PubMed] [Google Scholar]
- 42.Sage R. F. 2004. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 10.1111/j.1469-8137.2004.00974.x (doi:10.1111/j.1469-8137.2004.00974.x) [DOI] [PubMed] [Google Scholar]
- 43.Urban M. A., Nelson D. M., Jimenez-Moreno G., Chateauneuf J. J., Pearson A., Hu F. S. 2010. Isotopic evidence of C4 grasses in southwestern Europe during the Early Oligocene–Middle Miocene. Geology 38, 1091–1094 10.1130/G31117.1 (doi:10.1130/G31117.1) [DOI] [Google Scholar]
- 44.Kuypers M. M. M., Pancost R. D., Damste J. S. S. 1999. A large and abrupt fall in atmospheric CO2 concentration during Cretaceous times. Nature 399, 342–345 10.1038/20659 (doi:10.1038/20659) [DOI] [Google Scholar]
- 45.Kuypers M. M. M., Blokker P., Erbacher J., Kinkel H., Pancost R. D., Schouten S., Damste J. S. S. 2001. Massive expanasion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science 239, 92–94 10.1126/science.1058424 (doi:10.1126/science.1058424) [DOI] [PubMed] [Google Scholar]
- 46.Silvera K., Santiago L. S., Cushman J. C., Winter K. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol. 149, 1838–1847 10.1104/pp.108.132555 (doi:10.1104/pp.108.132555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arakaki M., Christin P.-A., Nyffeler R., Lendel A., Eggli U., Ogburn R. M., Spriggs E., Moore M. J., Edwards E. J. 2011. Contemporaneous and recent radiations of the world's major succulent plant lineages. Proc. Natl Acad. Sci. USA 108, 8379–8384 10.1073/pnas.1100628108 (doi:10.1073/pnas.1100628108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward J. K., Harris J. M., Cerling T. E., Wiedenhoeft A., Lott M. J., Dearing M. D., Coltrain J. B., Ehleringer J. R. 2005. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc. Natl Acad. Sci. USA 102, 690–694 10.1073/pnas.0408315102 (doi:10.1073/pnas.0408315102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geber M. A., Dawson T. E. 1997. Genetic variation in stomatal and biochemical limitations to photosynthesis in the annual plant, Polygonum arenastrum. Oecologia 109, 535–546 10.1007/s004420050114 (doi:10.1007/s004420050114) [DOI] [PubMed] [Google Scholar]
- 50.Intergovernmental Panel on Climate Change. 2007. Climate Change 2007: The Physical Science Basis—Summary for Policymakers, 18 pp. Cambridge, UK: Cambridge University Press [Google Scholar]
- 51.Lammertsma E. I., de Boer H. J., Dekker S. C., Dilcher D. L., Lotter A. F., Wagner-Cremer F. 2011. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl Acad. Sci. USA 108, 4035–4040 10.1073/pnas.1100371108 (doi:10.1073/pnas.1100371108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lobell D. B., Schlenker W., Costa-Roberts J. 2011. Climate trends and global crop production since 1980. Science 333, 616–620 10.1126/science.1204531 (doi:10.1126/science.1204531) [DOI] [PubMed] [Google Scholar]
- 53.van Mantgem P. J., et al. 2009. Widespread increase of tree mortality rates in the Western United States. Science 323, 521–524 10.1126/science.1165000 (doi:10.1126/science.1165000) [DOI] [PubMed] [Google Scholar]
- 54.Welch J. R., Vincent J. R., Auffhammer M., Moya P. F., Dobermann A., Dawe D. 2010. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl Acad. Sci. USA 107, 14 562–14 567 10.1073/pnas.1001222107 (doi:10.1073/pnas.1001222107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barnes J., Bender J., Lyons T., Borland A. 1999. Natural and man-made selection for air pollution resistance. J. Exp. Bot. 50, 1423–1435 10.1093/jexbot/50.338.1423 (doi:10.1093/jexbot/50.338.1423) [DOI] [Google Scholar]
- 56.Davison A. W., Reiling K. 1995. A rapid change in ozone resistance of Plantago major after summers with high ozone concentrations. New Phytol. 131, 337–344 10.1111/j.1469-8137.1995.tb03069.x (doi:10.1111/j.1469-8137.1995.tb03069.x) [DOI] [Google Scholar]
- 57.Franks S. J., Sim S., Weis A. E. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282 10.1073/pnas.0608379104 (doi:10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulido F., Berthold P. 2010. Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proc. Natl Acad. Sci. USA 107, 7341–7346 10.1073/pnas.0910361107 (doi:10.1073/pnas.0910361107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann A. A., Sgro C. M. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485 10.1038/nature09670 (doi:10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 60.Ziska L. H., Morris C. F., Goins E. W. 2004. Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance? Global Change Biol. 10, 1810–1819 10.1111/j.1365-2486.2004.00840.x (doi:10.1111/j.1365-2486.2004.00840.x) [DOI] [Google Scholar]
- 61.Manderscheid R., Weigel H. J. 1997. Photosynthetic and growth responses of old and modern spring wheat cultivars to atmospheric CO2 enrichment. Agric. Ecosyst. Environ. 64, 65–73 10.1016/S0167-8809(97)00020-0 (doi:10.1016/S0167-8809(97)00020-0) [DOI] [Google Scholar]
- 62.Ainsworth E. A., et al. 2008. Next generation of elevated [CO2] experiments with crops: a critical investment for feeding the future world. Plant Cell Environ. 31, 1317–1324 10.1111/j.1365-3040.2008.01841.x (doi:10.1111/j.1365-3040.2008.01841.x) [DOI] [PubMed] [Google Scholar]
- 63.Bazzaz F. A., Jasienski M., Thomas S. C., Wayne P. 1995. Microevolutionary responses in experimental populations of plants to CO2-enriched environments: parallel results from two model systems. Proc. Natl Acad. Sci. USA 92, 8161–8165 10.1073/pnas.92.18.8161 (doi:10.1073/pnas.92.18.8161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau J. A., Shaw R. G., Reich P. B., Shaw F. H., Tiffin P. 2007. Strong ecological but weak evolutionary effects of elevated CO2 on a recombinant inbred population of Arabidopsis thaliana. New Phytol. 175, 351–362 10.1111/j.1469-8137.2007.02108.x (doi:10.1111/j.1469-8137.2007.02108.x) [DOI] [PubMed] [Google Scholar]
- 65.Lau J. A., Shaw R. G., Reich P. B., Tiffin P. 2010. Species interactions in a changing environment: elevated CO2 alters the ecological and potential evolutionary consequences of competition. Evol. Ecol. Res. 12, 435–455 [Google Scholar]
- 66.Maxon-Smith J. W. 1977. Selection for response to CO2-enrichment in glasshouse lettuce. Hort. Res. 17, 15–22 [Google Scholar]
- 67.Mycroft E. E., Zhang J. Y., Adams G., Reekie E. 2009. Elevated CO2 will not select for enhanced growth in white spruce despite genotypic variation in response. Basic Appl. Ecol. 10, 349–357 10.1016/j.baae.2008.08.005 (doi:10.1016/j.baae.2008.08.005) [DOI] [Google Scholar]
- 68.Potvin C., Tousignant D. 1996. Evolutionary consequences of simulated global change: genetic adaptation or adaptive phenotypic plasticity. Oecologia 108, 683–693 10.1007/BF00329043 (doi:10.1007/BF00329043) [DOI] [PubMed] [Google Scholar]
- 69.Steinger T., Stephan A., Schmid B. 2007. Predicting adaptive evolution under elevated atmospheric CO2 in the perennial grass Bromus erectus. Global Change Biol. 13, 1028–1039 10.1111/j.1365-2486.2007.01328.x (doi:10.1111/j.1365-2486.2007.01328.x) [DOI] [Google Scholar]
- 70.Tonsor S. J., Scheiner S. M. 2007. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. Am. Nat. 169, E119–E140 10.1086/513493 (doi:10.1086/513493) [DOI] [PubMed] [Google Scholar]
- 71.Ward J. K., Antonovics J., Thomas R. B., Strain B. R. 2000. Is atmospheric CO2 a selective agent on model C3 annuals? Oecologia 123, 330–341 10.1007/s004420051019 (doi:10.1007/s004420051019) [DOI] [PubMed] [Google Scholar]
- 72.Donovan L. A., Maherali H., Caruso C. M., Huber H., de Kroon H. 2011. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 26, 88–95 10.1016/j.tree.2010.11.011 (doi:10.1016/j.tree.2010.11.011) [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez J. A., Bruno M., Valoy M., Prado F. E. 2011. Genotypic variation of gas exchange parameters and leaf stable carbon and nitrogen isotopes in ten Quinoa cultivars grown under drought. J. Agron. Crop Sci. 197, 81–93 10.1111/j.1439-037X.2010.00446.x (doi:10.1111/j.1439-037X.2010.00446.x) [DOI] [Google Scholar]
- 74.Khan A. S., Allah S. U., Sadique S. 2010. Genetic variability and correlation among seedling traits of wheat (Triticum aestivum) under water stress. Int. J. Agric. Biol. 12, 247–250 [Google Scholar]
- 75.Conner J. K. 2003. Artificial selection: a powerful tool for ecologists. Ecology 84, 1650–1660 10.1890/0012-9658(2003)084[1650:ASAPTF]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1650:ASAPTF]2.0.CO;2) [DOI] [Google Scholar]
- 76.Holt R. D. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 10.1016/0169-5347(90)90088-U (doi:10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 77.Collins S., Bell G. 2004. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431, 566–569 10.1038/nature02945 (doi:10.1038/nature02945) [DOI] [PubMed] [Google Scholar]
- 78.Collins S., Bell G. 2006. Evolution of natural algal populations at elevated CO2. Ecol. Lett. 9, 129–135 10.1111/j.1461-0248.2005.00854.x (doi:10.1111/j.1461-0248.2005.00854.x) [DOI] [PubMed] [Google Scholar]
- 79.Bidart-Bouzat M. G., Portnoy S., DeLucia E. H., Paige K. N. 2004. Elevated CO2 and herbivory influence trait integration in Arabidopsis thaliana. Ecol. Lett. 7, 837–847 10.1111/j.1461-0248.2004.00648.x (doi:10.1111/j.1461-0248.2004.00648.x) [DOI] [Google Scholar]
- 80.Collins S., Sultemeyer D., Bell G. 2006. Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2. Plant Cell Environ. 29, 1812–1819 10.1111/j.1365-3040.2006.01559.x (doi:10.1111/j.1365-3040.2006.01559.x) [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez-Meler M. A., Blanc-Betes E., Flower C. E., Ward J. K., Gomez-Casanovas N. 2009. Plastic and adaptive responses of plant respiration to changes in atmospheric CO2 concentration. Physiol. Plant. 137, 473–484 10.1111/j.1399-3054.2009.01262.x (doi:10.1111/j.1399-3054.2009.01262.x) [DOI] [PubMed] [Google Scholar]
- 82.Cook A. C., Tissue D. T., Roberts S. W., Oechel W. C. 1998. Effects of long-term elevated [CO2] from natural CO2 springs on Nardus stricta: photosynthesis, biochemistry, growth and phenology. Plant Cell Environ. 21, 417–425 10.1046/j.1365-3040.1998.00285.x (doi:10.1046/j.1365-3040.1998.00285.x) [DOI] [Google Scholar]
- 83.Nakamura I., Onoda Y., Matsushima N., Yokoyama J., Kawata M., Hikosaka K. 2011. Phenotypic and genetic differences in a perennial herb across a natural gradient of CO2 concentration. Oecologia 165, 809–818 10.1007/s00442-010-1900-1 (doi:10.1007/s00442-010-1900-1) [DOI] [PubMed] [Google Scholar]
- 84.Onoda Y., Hirose T., Hikosaka K. 2009. Does leaf photosynthesis adapt to CO2-enriched environments? An experiment on plants originating from three natural CO2 springs. New Phytol. 182, 698–709 10.1111/j.1469-8137.2009.02786.x (doi:10.1111/j.1469-8137.2009.02786.x) [DOI] [PubMed] [Google Scholar]
- 85.Vannette R. L., Hunter M. D. 2011. Genetic variation in expression of defense phenotype may mediate evolutionary adaptation of Asclepias syriaca to elevated CO2. Global Change Biol. 17, 1277–1288 10.1111/j.1365-2486.2010.02316.x (doi:10.1111/j.1365-2486.2010.02316.x) [DOI] [Google Scholar]
- 86.Lau J. A., Tiffin P. 2009. Elevated carbon dioxide concentrations indirectly affect plant fitness by altering plant tolerance to herbivory. Oecologia 161, 401–410 10.1007/s00442-009-1384-z (doi:10.1007/s00442-009-1384-z) [DOI] [PubMed] [Google Scholar]
- 87.Poorter H., Navas L. 2003. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol. 157, 175–198 10.1046/j.1469-8137.2003.00680.x (doi:10.1046/j.1469-8137.2003.00680.x) [DOI] [PubMed] [Google Scholar]
- 88.Diaz S. 1995. Elevated CO2 responsiveness, interactions at the community level and plant functional types. J. Biogeogr. 22, 289–295 10.2307/2845923 (doi:10.2307/2845923) [DOI] [Google Scholar]
- 89.Poorter H. 1993. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104, 77–97 10.1007/BF00048146 (doi:10.1007/BF00048146) [DOI] [Google Scholar]
- 90.Tang J. J., Chen J., Chen X. 2006. Response of 12 weedy species to elevated CO2 in low-phosphorus-availability soil. Ecol. Res. 21, 664–670 10.1007/s11284-006-0161-2 (doi:10.1007/s11284-006-0161-2) [DOI] [Google Scholar]
- 91.Atkin O. K., Schortemeyer M., McFarlane N., Evans J. R. 1999. The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia 120, 544–554 10.1007/s004420050889 (doi:10.1007/s004420050889) [DOI] [PubMed] [Google Scholar]
- 92.Evans J. R., Schortemeyer M., McFarlane N., Atkin O. K. 2000. Photosynthetic characteristics of 10 Acacia species grown under ambient and elevated atmospheric CO2. Aust. J. Plant Physiol. 27, 13–25 10.1071/PP99126 (doi:10.1071/PP99126) [DOI] [Google Scholar]
- 93.Ghannoum O., Phillips N. G., Conroy J. P., Smith R. A., Attard R. D., Woodfield R., Logan B. A., Lewis J. D., Tissue D. T. 2010. Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Global Change Biol. 16, 303–319 10.1111/j.1365-2486.2009.02003.x (doi:10.1111/j.1365-2486.2009.02003.x) [DOI] [Google Scholar]
- 94.Kerstiens G. 2001. Meta-analysis of the interaction between shade-tolerance, light environment and growth response of woody species to elevated CO2. Acta Oecol. Int. J. Ecol. 22, 61–69 10.1016/S1146-609X(00)01096-1 (doi:10.1016/S1146-609X(00)01096-1) [DOI] [Google Scholar]
- 95.Zhang J. Y., Mycroft E. E., Adams G., Reekie E. 2011. Why do genotypes of Picea glauca differ in their growth response to elevated CO2? Tree Physiol. 31, 16–21 10.1093/treephys/tpq097 (doi:10.1093/treephys/tpq097) [DOI] [PubMed] [Google Scholar]
- 96.Liu N., Dang Q. L., Parker W. H. 2006. Genetic variation of Populus tremuloides in ecophysiological responses to CO2 elevation. Can. J. Bot. 84, 294–302 10.1139/b05-171 (doi:10.1139/b05-171) [DOI] [Google Scholar]
- 97.Lande R., Arnold S. J. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226 10.2307/2408842 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 98.Mitchell-Olds T., Shaw R. G. 1987. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41, 1149–1161 10.2307/2409084 (doi:10.2307/2409084) [DOI] [PubMed] [Google Scholar]
- 99.Gray J. E., Holroyd G. H., van der Lee F. M., Bahrami A. R., Sijmons P. C., Woodward F. I., Schuch W., Heterington A. M. 2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408, 713–716 10.1038/35047071 (doi:10.1038/35047071) [DOI] [PubMed] [Google Scholar]
- 100.Lewis J. D., Wang X., Griffin K. L., Tissue D. T. 2003. Age at flowering differentially affects vegetative and reproductive responses of a determinate annual plant to elevated carbon dioxide. Oecologia 135, 194–201 10.1007/s00442-003-1186-7 (doi:10.1007/s00442-003-1186-7) [DOI] [PubMed] [Google Scholar]
- 101.Ludwig F., Asseng S. 2010. Potential benefits of early vigor and changes in phenology in wheat to adapt to warmer and drier climates. Agric. Syst. 103, 127–136 10.1016/j.agsy.2009.11.001 (doi:10.1016/j.agsy.2009.11.001) [DOI] [Google Scholar]
- 102.Ackerly D. 2004. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 74, 25–44 10.1890/03-4022 (doi:10.1890/03-4022) [DOI] [Google Scholar]
- 103.Kattge J., et al. 2011. TRY—a global database of plant traits. Global Change Biol. 17, 2905–2935 10.1111/j.1365-2486.2011.02451.x (doi:10.1111/j.1365-2486.2011.02451.x) [DOI] [Google Scholar]
- 104.Verheyen K., Honnay O., Motzkin G., Hermy M., Foster D. R. 2003. Response of forest plant species to land-use change: a life-history trait-based approach. J. Ecol. 91, 563–577 10.1046/j.1365-2745.2003.00789.x (doi:10.1046/j.1365-2745.2003.00789.x) [DOI] [Google Scholar]
- 105.Hikosaka K., Shigeno A. 2009. The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160, 443–451 10.1007/s00442-009-1315-z (doi:10.1007/s00442-009-1315-z) [DOI] [PubMed] [Google Scholar]
- 106.Ali M. A., Jabran K., Awan S. I., Abbas A., Ehsanullah Zulkiffal M., Acet T., Farooq J., Rehman A. 2011. Morpho-physiological diversity and its implications for improving drought tolerance in grain sorghum at different growth stages. Aust. J. Crop Sci. 5, 308–317 [Google Scholar]
- 107.Hibberd J. M., Sheehy J. E., Langdale J. A. 2008. Using C4 photosynthesis to increase the yield of rice: rationale and feasibility. Curr. Opin. Plant Biol. 11, 228–231 10.1016/j.pbi.2007.11.002 (doi:10.1016/j.pbi.2007.11.002) [DOI] [PubMed] [Google Scholar]
- 108.Long S. P., Zhu X. G., Naidu S. L., Ort D. R. 2006. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29, 315–330 10.1111/j.1365-3040.2005.01493.x (doi:10.1111/j.1365-3040.2005.01493.x) [DOI] [PubMed] [Google Scholar]
- 109.Peterhansel C., Maurino V. G. 2011. Photorespiration redesigned. Plant Physiol. 155, 49–55 10.1104/pp.110.165019 (doi:10.1104/pp.110.165019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Whitney S. M., Houtz R. L., Alonso H. 2011. Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco. Plant Physiol. 155, 27–35 10.1104/pp.110.164814 (doi:10.1104/pp.110.164814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Long S. P., Ainsworth E. A., Leakey A. D. B., Nosberger J., Ort D. R. 2006. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921 10.1126/science.1114722 (doi:10.1126/science.1114722) [DOI] [PubMed] [Google Scholar]
- 112.Tabita F. R., Hanson T. E., Satagopan S., Witte B. H., Kreel N. E. 2008. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Phil. Trans. R. Soc. B 363, 2629–2640 10.1098/rstb.2008.0023 (doi:10.1098/rstb.2008.0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Andersson I., Backlund A. 2008. Structure and function of Rubisco. Plant Phys. Biochem. 46, 275–291 10.1016/j.plaphy.2008.01.001 (doi:10.1016/j.plaphy.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 114.Mueller-Cajar O., Whitney S. M. 2008. Directing the evolution of Rubisco and Rubisco activase: first impressions of a new tool for photosynthesis research. Photosynth. Res. 98, 667–675 10.1007/s11120-008-9324-z (doi:10.1007/s11120-008-9324-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kannappan B., Gready J. E. 2008. Redefinition of Rubisco carboxylase reaction reveals origin of water for hydration and new roles for active-site residues. J. Am. Chem. Soc. 130, 15 063–15 080 10.1021/ja803464a (doi:10.1021/ja803464a) [DOI] [PubMed] [Google Scholar]
- 116.Zhu X. G., Portis A. R., Long S. P. 2004. Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ. 27, 155–165 10.1046/j.1365-3040.2004.01142.x (doi:10.1046/j.1365-3040.2004.01142.x) [DOI] [Google Scholar]
- 117.Tcherkez G. G. B., Farquhar G. D., Andrews T. J. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl Acad. Sci. USA 103, 7246–7251 10.1073/pnas.0600605103 (doi:10.1073/pnas.0600605103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alonso H., Blayney M. J., Beck J. L., Whitney S. M. 2009. Substrate-induced assembly of Methanococcoides burtonii D-ribulose-1,5-bisphosphate carboxylase/oxygenase dimers into decamers. J. Biol. Chem. 284, 33 876–33 882 10.1074/jbc.M109.050989 (doi:10.1074/jbc.M109.050989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whitney S. M., Andrews T. J. 2001. Plastome-encoded bacterial ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) supports photosynthesis and growth in tobacco. Proc. Natl Acad. Sci. USA 98, 14 738–14 743 10.1073/pnas.261417298 (doi:10.1073/pnas.261417298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Portis A. R., Li C. S., Wang D. F., Salvucci M. E. 2008. Regulation of Rubisco activase and its interaction with Rubisco. J. Exp. Bot. 59, 1597–1604 10.1093/jxb/erm240 (doi:10.1093/jxb/erm240) [DOI] [PubMed] [Google Scholar]
- 121.Pearce F. G. 2006. Catalytic by-product formation and ligand binding by ribulose bisphosphate carboxylases from different phylogenies. Biochem. J. 399, 525–534 10.1042/BJ20060430 (doi:10.1042/BJ20060430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu X. G., Shan L. L., Wang Y., Quick W. P. 2010. C4 rice: an ideal arena for systems biology research. J. Integr. Plant Biol. 52, 762–770 10.1111/j.1744-7909.2010.00983.x (doi:10.1111/j.1744-7909.2010.00983.x) [DOI] [PubMed] [Google Scholar]
- 123.Brautigam A., et al. 2011. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol. 155, 142–156 10.1104/pp.110.159442 (doi:10.1104/pp.110.159442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown N. J., Newell C. A., Stanley S., Chen J. E., Perrin A. J., Kajala K., Hibberd J. M. 2011. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331, 1436–1439 10.1126/science.1201248 (doi:10.1126/science.1201248) [DOI] [PubMed] [Google Scholar]
- 125.Maurino V. G., Flugge U. I. 2009. Means for improving agrobiological traits in a plant by providing a plant cell comprising in its chloroplasts enzymatic activities for converting glycolate into malate. US Patent Application WO2009103782 [Google Scholar]
- 126.Kebeish R., et al. 2007. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 25, 593–599 10.1038/nbt1299 (doi:10.1038/nbt1299) [DOI] [PubMed] [Google Scholar]
- 127.Yoshizawa Y., Toyoda K., Arai H., Ishii M., Igarashi Y. 2004. CO2-responsive expression and gene organization of three ribulose-1,5-bisphosphate carboxylase/oxygenase enzymes and carboxysomes in Hydrogenovibrio marinus strain MH-110. J. Bacteriol. 186, 5685–5691 10.1128/JB.186.17.5685-5691.2004 (doi:10.1128/JB.186.17.5685-5691.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ort D. R., Baker N. R. 2002. A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 5, 193–198 10.1016/S1369-5266(02)00259-5 (doi:10.1016/S1369-5266(02)00259-5) [DOI] [PubMed] [Google Scholar]
- 129.Leakey A. D. B., Ainsworth E. A., Bernacchi C. J., Rogers A., Long S. P., Ort D. R. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876 10.1093/jxb/erp096 (doi:10.1093/jxb/erp096) [DOI] [PubMed] [Google Scholar]
- 130.Zhu X. G., de Sturler E., Long S. P. 2007. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 145, 513–526 10.1104/pp.107.103713 (doi:10.1104/pp.107.103713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lefebvre S., Lawson T., Zakhleniuk O. V., Lloyd J. C., Raines C. A. 2005. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 138, 451–460 10.1104/pp.104.055046 (doi:10.1104/pp.104.055046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ainsworth E. A., Long S. P. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–371 10.1111/j.1469-8137.2004.01224.x (doi:10.1111/j.1469-8137.2004.01224.x) [DOI] [PubMed] [Google Scholar]
- 133.Leakey A. D. B., Xu F., Gillespie K. M., McGrath J. M., Ainsworth E. A., Ort D. R. 2009. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc. Natl Acad. Sci. USA 106, 3597–3602 10.1073/pnas.0810955106 (doi:10.1073/pnas.0810955106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rogers A., Gibon Y., Stitt M., Morgan P. B., Bernacchi C. J., Ort D. R., Long S. P. 2006. Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ. 29, 1651–1658 10.1111/j.1365-3040.2006.01549.x (doi:10.1111/j.1365-3040.2006.01549.x) [DOI] [PubMed] [Google Scholar]
- 135.Rogers A., et al. 2004. Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ. 27, 449–458 10.1111/j.1365-3040.2004.01163.x (doi:10.1111/j.1365-3040.2004.01163.x) [DOI] [Google Scholar]
- 136.Sulpice R., et al. 2009. Starch as a major integrator in the regulation of plant growth. Proc. Natl Acad. Sci. USA 106, 10 348–10 353 10.1073/pnas.0903478106 (doi:10.1073/pnas.0903478106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sulpice R., et al. 2010. Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22, 2872–2893 10.1105/tpc.110.076653 (doi:10.1105/tpc.110.076653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ainsworth E. A., Bush D. R. 2011. Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 155, 64–69 10.1104/pp.110.167684 (doi:10.1104/pp.110.167684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ainsworth E. A., Rogers A., Nelson R., Long S. P. 2004. Testing the ‘source-sink’ hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. Forest Meteorol. 122, 85–94 10.1016/j.agrformet.2003.09.002 (doi:10.1016/j.agrformet.2003.09.002) [DOI] [Google Scholar]
- 140.Davidson A., Keller F., Turgeon R. 2011. Phloem loading, plant growth form, and climate. Protoplasma 248, 153–163 10.1007/s00709-010-0240-7 (doi:10.1007/s00709-010-0240-7) [DOI] [PubMed] [Google Scholar]
- 141.Graf A., Smith A. M. 2011. Starch and the clock: the dark side of plant productivity. Trends Plant Sci. 16, 169–175 10.1016/j.tplants.2010.12.003 (doi:10.1016/j.tplants.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 142.Bartels D., Sunkar R. 2005. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 24, 23–58 10.1080/07352680590910410 (doi:10.1080/07352680590910410) [DOI] [Google Scholar]
- 143.Busch W., Wunderlich M., Schoffl F. 2005. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 41, 1–14 10.1111/j.1365-313X.2004.02272.x (doi:10.1111/j.1365-313X.2004.02272.x) [DOI] [PubMed] [Google Scholar]
- 144.Streeter J. G., Lohnes D. G., Fioritto R. J. 2001. Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant Cell Environ. 24, 429–438 10.1046/j.1365-3040.2001.00690.x (doi:10.1046/j.1365-3040.2001.00690.x) [DOI] [Google Scholar]
- 145.Herms D. A., Mattson W. J. 1992. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 10.1086/417659 (doi:10.1086/417659) [DOI] [Google Scholar]
- 146.Zavala J. A., Casteel C. L., DeLucia E. H., Berenbaum M. R. 2008. Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc. Natl Acad. Sci. USA 105, 5129–5133 10.1073/pnas.0800568105 (doi:10.1073/pnas.0800568105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Long S. P., Ainsworth E. A., Rogers A., Ort D. R. 2004. Rising atmospheric carbon dioxide: plants face the future. Annu. Rev. Plant Biol. 55, 591–628 10.1146/annurev.arplant.55.031903.141610 (doi:10.1146/annurev.arplant.55.031903.141610) [DOI] [PubMed] [Google Scholar]
- 148.de Graaff M. A., van Groenigen K. J., Six J., Hungate B., van Kessel C. 2006. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biol. 12, 2077–2091 10.1111/j.1365-2486.2006.01240.x (doi:10.1111/j.1365-2486.2006.01240.x) [DOI] [Google Scholar]
- 149.Rogers A., Ainsworth E. A., Leakey A. D. B. 2009. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 151, 1009–1016 10.1104/pp.109.144113 (doi:10.1104/pp.109.144113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taub D. R., Miller B., Allen H. 2008. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol. 14, 565–575 10.1111/j.1365-2486.2007.01511.x (doi:10.1111/j.1365-2486.2007.01511.x) [DOI] [Google Scholar]
- 151.Davis S. C., Parton W. J., Dohleman F. G., Smith C. M., Del Grosso S., Kent A. D., DeLucia E. H. 2010. Comparative biogeochemical cycles of bioenergy crops reveal nitrogen-fixation and low greenhouse gas emissions in a Miscanthus x giganteus agro-ecosystem. Ecosystems 13, 144–156 10.1007/s10021-009-9306-9 (doi:10.1007/s10021-009-9306-9) [DOI] [Google Scholar]
- 152.Bloom A. J., Burger M., Rubio-Asensio J. S., Cousins A. B. 2010. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899–903 10.1126/science.1186440 (doi:10.1126/science.1186440) [DOI] [PubMed] [Google Scholar]