Abstract
C4 photosynthesis has evolved more than 60 times as a carbon-concentrating mechanism to augment the ancestral C3 photosynthetic pathway. The rate and the efficiency of photosynthesis are greater in the C4 than C3 type under atmospheric CO2 depletion, high light and temperature, suggesting these factors as important selective agents. This hypothesis is consistent with comparative analyses of grasses, which indicate repeated evolutionary transitions from shaded forest to open habitats. However, such environmental transitions also impact strongly on plant–water relations. We hypothesize that excessive demand for water transport associated with low CO2, high light and temperature would have selected for C4 photosynthesis not only to increase the efficiency and rate of photosynthesis, but also as a water-conserving mechanism. Our proposal is supported by evidence from the literature and physiological models. The C4 pathway allows high rates of photosynthesis at low stomatal conductance, even given low atmospheric CO2. The resultant decrease in transpiration protects the hydraulic system, allowing stomata to remain open and photosynthesis to be sustained for longer under drying atmospheric and soil conditions. The evolution of C4 photosynthesis therefore simultaneously improved plant carbon and water relations, conferring strong benefits as atmospheric CO2 declined and ecological demand for water rose.
Keywords: C4 photosynthesis, C3 photosynthesis, atmospheric CO2, plant evolution, drought, hydraulics
1. Photosynthetic conservatism and diversity
Photosynthesis has evolved only once, and every photoautotrophic organism on Earth uses the same ‘C3 pathway’ [1]. This biochemical cycle employs the enzyme Rubisco to fix CO2 into a five-carbon acceptor molecule, producing three-carbon organic acids, upon which ATP and NADPH produced from the light reactions are deployed to generate sugars and to regenerate the acceptor molecule. The pigments and proteins involved in C3 photosynthesis are highly conserved across photosynthetic organisms, and this pathway operates unmodified in the majority of species, termed ‘C3 plants’. However, the basic C3 pathway has also been augmented by carbon-concentrating mechanisms (CCMs) in multiple lineages, many of which evolved during the Early Neogene following a massive depletion of atmospheric CO2 [2–5].
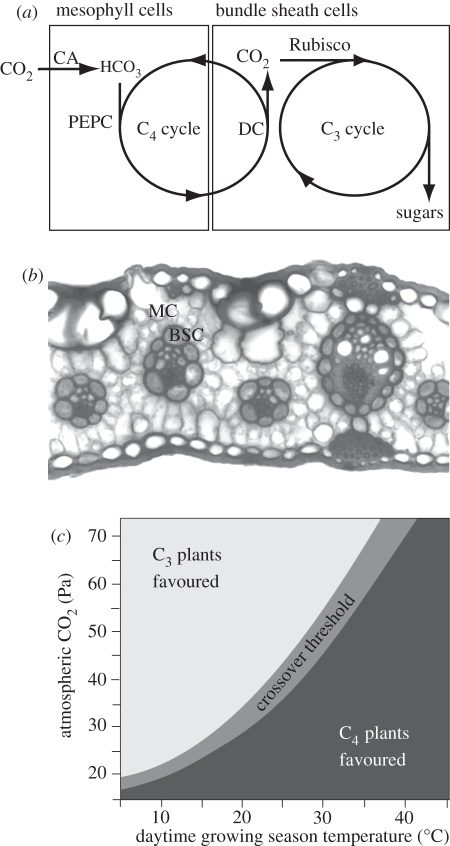
‘C4 photosynthesis’ is a collective term for CCMs which initially fix carbon into four-carbon organic acids via the enzyme phosphoenolpyruvate carboxylase (PEPC) [6], and then liberate CO2 from these C4 acids to feed the C3 pathway within a compartment of the cell or leaf [7,8] (figure 1a). The compartment is isolated from the atmosphere and resists CO2-leakage, so that CO2 is enriched at the active site of Rubisco [12] (figure 1a). Coordination of C4 and C3 biochemical pathways requires complex changes to metabolism, and the compartmentation of these pathways usually relies on a specialized leaf anatomy (figure 1b). C4 photosynthesis is therefore a complex trait based on the transcriptional regulation of hundreds of genes [13], coupled with post-transcriptional regulation [14] and the adaptation of protein-coding sequences [15].
Figure 1.
Mechanism of C4 photosynthesis. (a) Simplified schematic of the C4 syndrome, showing how the C3 pathway is isolated from the atmosphere in most C4 species within a specialized tissue composed of bundle sheath cells (BSC). The C4 pathway captures CO2 within mesophyll cells (MC) using the enzymes carbonic anyhdrase (CA) and phosphoenolpyruvate carboxylase (PEPC). It then transports fixed carbon to the BSC where decarboxylase enzymes (DC) liberate CO2, which accumulates to high concentrations around Rubisco. (b) Transverse section of a C4 leaf, showing the arrangement of MC and BSC. In this picture, the BSC are thicker walled, are stained darker and form rings surrounding the veins (adapted from Watson & Dallwitz [9]). Enlarged BSC relative to MC and close vein spacing are typical of C4 leaves, and this pattern is referred to as ‘Kranz anatomy’. (c) Explanation of how variation in CO2 and temperature favours C3 or C4 species [10,11]. The schematic shows the range of CO2 partial pressures and temperatures predicted to favour the growth of either C3 or C4 species, based on the maximum quantum yield of photosynthesis. Quantum yield is a measure of photosynthetic efficiency, which declines in C3 plants as photorespiration increases at low CO2 and high temperature, (reproduced with permission from Edwards et al. [3], which was adapted from Ehleringer et al. [11]).
Despite the complexity of C4 photosynthesis, it has been recorded in more than 60 plant lineages [16]. This striking evolutionary convergence probably arises because the pathway is constructed from numerous pre-existing gene networks [17], and altered levels and patterns of expression of enzymes that are already present in C3 leaves [18–20]. Many of the known C4 lineages occur in clusters, suggesting that they share early steps on the evolutionary trajectory towards C4 photosynthesis, taking an independent path only during later stages of the process [21].
In recent years, evidence from genomics, molecular genetics, physiology, ecology, biogeography, evolutionary biology and geosciences has enriched our understanding of when, where and how C4 photosynthesis evolved. Here, we extend previous work that focused on why the pathway evolved, based on the environmental conditions that drove natural selection. We begin by reviewing the well-established current hypothesis that atmospheric CO2 depletion, high temperatures and open environments selected for the C4 pathway as a means of directly improving photosynthetic rate and efficiency. Our main objective is to introduce a new dimension to these ideas, by proposing that these same environmental conditions—CO2 depletion, high temperatures and open environments—as well as seasonal drought, would also select for C4 photosynthesis for an additional reason; i.e. to compensate for the strain on the plant hydraulic system resulting from excessive demand for water transport. Our hypothesis is consistent with recent comparative analyses of ecological niche evolution and plant physiology, which suggest important effects of the C4 pathway on plant–water relations. We support our proposal with evidence from the literature, and mechanistic models that integrate the latest understanding of how hydraulics, stomata and photosynthesis are coordinated within leaves. Our central focus is on grasses, but we complement our analysis with additional evidence from eudicots.
2. Environmental selection on photosynthetic efficiency
Atmospheric CO2 depletion has long been advocated as the primary selection pressure for C4 CCMs, through its differential effects on the efficiency of C3 and C4 photosynthesis [22]. This difference arises because the active site of Rubisco is unable to discriminate completely between CO2 and O2, and catalyses the fixation of both molecules [23,24]. The oxygenation reaction generates toxic intermediates that must be metabolized via photorespiration to render them harmless and to recover carbon. Oxygenation renders photosynthesis less efficient because it competes directly with carboxylation (CO2-fixation), and because photorespiration consumes the products of photochemistry and liberates CO2. In a C3 plant, the ratio of carboxylation to oxygenation (and therefore photosynthetic efficiency) decreases rapidly with declining atmospheric CO2, especially at high temperatures [25]. In contrast, by fixing the bicarbonate ion rather than CO2, the C4 pathway does not confuse CO2 with O2 (figure 1a). Furthermore, by isolating Rubisco from the atmosphere and concentrating CO2 at its active site [26], it also minimizes oxygenation in the C3 pathway. However, energy required to run the C4 CCM imposes a cost on photosynthetic efficiency under all conditions [27,28]. This means that the efficiency of C4 photosynthesis is only greater than the C3 type under conditions that promote high rates of photorespiration [19]. Low atmospheric CO2 and high temperatures are considered especially important in favouring C4 photosynthesis, with a ‘crossover threshold’ for CO2 of 35–55 Pa and temperatures of 25–30°C (figure 1c; [10,11]).
A further physiological difference between C3 and C4 species arises because the carboxylation reaction of Rubisco is strongly limited by its substrate when CO2 falls below approximately 70 Pa at the active site [29]. Resistances to CO2 diffusion from the atmosphere to the chloroplast mean that this limitation arises when atmospheric CO2 levels fall below approximately 100 Pa. In contrast, significant CO2-limitation only occurs in C4 plants at atmospheric CO2 levels of less than 20 Pa [30]. The CO2-saturation of Rubisco in C4 photosynthesis means that in high light environments the enzyme reaches its saturated catalytic rate, maximizing the photosynthetic difference between C3 and C4 species. As a consequence, in open habitats, the ‘crossover threshold’ is raised to higher CO2 concentrations and lower temperatures [31]. Conversely, the cool shade of forest understorey environments offers little benefit for the C4 pathway over the C3 type [32], although once C4 evolves in a lineage, descendent species may invade shaded habitats by modifying their tissue costs, allowing them to maintain an advantage in photosynthetic rate over co-occurring C3 species [33].
Water relations have previously been proposed to influence the evolution of C4 photosynthesis in an indirect way. For terrestrial vascular plants, the loss of water is an inevitable cost of photosynthesis, regulated by turgor-mediated decreases in the aperture of stomatal pores. The partial closure of stomata to conserve water in arid and saline soils or dry atmospheric conditions (characterized by high vapour pressure deficit (VPD)) has been hypothesized to select for the C4 pathway via indirect effects on photosynthetic efficiency [34]. Thus, reduced stomatal aperture (i) restricts the CO2 supply to photosynthesis and (ii) decreases transpiration, thereby reducing latent heat loss and raising leaf temperature. Both effects increase photorespiration, depressing the efficiency of C3 photosynthesis, and favouring the C4 type.
Therefore, the general expectation based on physiological evidence is that declining atmospheric CO2 should select for C4 photosynthesis in hot, open and dry or saline environments, where photorespiration is especially high in C3 species [19].
3. Ecological drivers of c4 pathway evolution
Recent data on the timing of C4 origins and geological history have supported these ideas for the importance of low CO2 and high temperature and irradiance, but also highlight the importance of aridity. First, the modelling of evolutionary transitions from C3 to C4 photosynthesis using time-calibrated molecular phylogenies indicated that C4 origins have all occurred over the past 30 Myr, with no difference in timing between monocot and eudicot lineages (figure 2) [35–37]. Secondly, multiple geological proxies suggested that atmospheric CO2 has remained below 60 Pa for most of this 30 Myr interval (figure 2), after a dramatic decline during the Oligocene (23–34 Ma) that corresponds to the onset of Antarctic glaciation [4]. Therefore, C4 photosynthesis does seem to have evolved in a CO2-depleted atmosphere, within the ranges of uncertainty that are inherent to phylogenetic and geological evidence [4,37].
Figure 2.
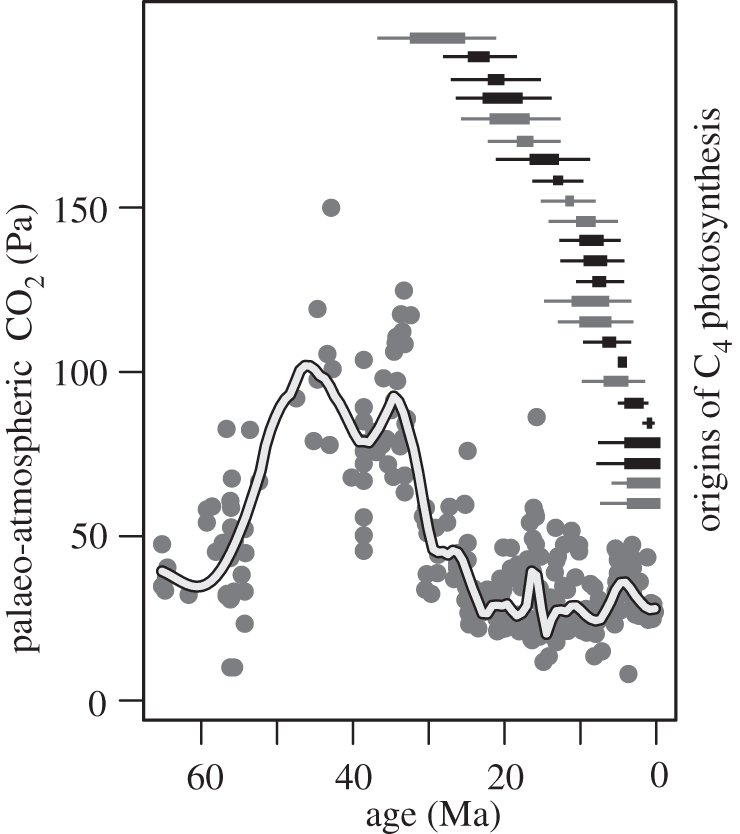
Geological history of atmospheric CO2 and the estimated ages of C4 evolutionary origins. The palaeo-atmospheric CO2 history of the Cenozoic is reconstructed from multiple independent proxies (pale grey circles), with a smoothed line of best fit encompassing all of the evidence (reproduced with permission from Beerling & Royer [4]). The estimated ages of C4 evolutionary origins in grasses (dark grey horizontal bars) and eudicots (black horizontal bars) were obtained using phylogenetic inference and calibration to fossils [35]. Thick bars represent uncertainty in the position of each C4 evolutionary origin on the phylogeny, while thin bars indicate uncertainty in dating of the phylogeny (reproduced with permission from Christin et al. [35]).
The hypothesis that high temperatures select for C4 photosynthesis has been supported for 30 years by invoking the observation that species richness of C4 grasses increases along latitudinal and altitudinal temperature gradients [32,38,39]. However, phylogenetic comparative analyses have shown that C4 species do not live in warmer environments than their closest C3 relatives. First, Edwards & Still [40] showed that the absence of C4 species from high altitudes in the predominantly exotic grass flora of Hawaii [39] is better explained by phylogenetic history than photosynthetic pathway. The exclusively C3 lineage Pooideae is most speciose at cool, high elevations. However, species of the PACMAD lineage are most numerous in the warm lowlands, irrespective of whether they use C3 or C4 photosynthesis. Edwards & Smith [41] further investigated this pattern by reconstructing the evolution of temperature niche in the world's grasses. Their analysis indicated that grasses originated in the tropics. The C3 and C4 species of the PACMAD clade have remained predominantly in these warm environments, irrespective of photosynthetic pathway, while Pooideae have adapted to and radiated in low-temperature environments. Thus, C4 photosynthesis did evolve at high temperatures, but the major innovation that caused ecological sorting of grasses along temperature gradients was the adaptation of certain C3 lineages to cold conditions [41].
Comparative analyses also support the hypothesis that the C4 pathway in grasses evolved in open environments. First, by modelling the rate of evolutionary transitions between C3 and C4 photosynthesis, and shaded and open habitats, Osborne & Freckleton [42] showed that C4 origins were significantly more likely to have occurred in open than shaded environments. Secondly, Edwards & Smith [41] quantified the shift in environmental niche that is correlated with evolutionary transitions from C3 to C4 photosynthesis. The origins of C4 photosynthesis were generally associated with migration from an aseasonal tropical niche into a seasonal, sub-tropical one, an ecological shift consistent with a transition from moist tropical forest to drier, more open woodland or savannah habitats [41]. These complementary analyses suggest that C4 photosynthesis evolved in C3 species that had migrated out of their ancestral niche in tropical forests, and invaded open sub-tropical woodland or savannah habitats. The C4 pathway, therefore, seems to be a key adaptation to one of the most important ecological transitions in the evolutionary history of grasses [43], which ultimately enabled the assembly of the tropical grassland biome.
The proportion of C4 species in eudicot floras increases along gradients of rising aridity [11]. Recent comparative analyses at the regional and global scales show that, in addition to preferring open habitats, C4 grasses have also sorted into drier environmental niches than their C3 relatives [40–42]. However, for grasses, there is no evidence that C4 photosynthesis is more likely to evolve in xeric than mesic habitats [42]. Rather, the distribution of C4 species in dry areas can be explained by two inferences from comparative analyses: (i) that C4 origins were accompanied by shifts to a drier ecological niche within the humid sub-tropics [41] and (ii) a greater likelihood that C4 than C3 lineages will invade very dry (xeric) environments [42]. In some systems, C4 grasses tolerate greater aridity than C3 species of adjacent areas; for example, it has been argued that certain C4 grasses of the Kalahari occur in areas too dry for the C3 type to persist [44]. Water relations clearly play an important role in the ecology and biogeography of both C4 monocots and C4 eudicots.
A corpus of physiological work and comparative analyses therefore supports the theory of how low atmospheric CO2 drove selection for improved photosynthetic rate and efficiency in hot and open environments over the last 30 Myr. What has been missing is an understanding of the importance of plant–water relations in differentiating photosynthetic types in such environments.
4. Strain on plant–water relations in a co2-depleted atmosphere
Multiple strands of geological, ecological and physiological evidence indicate that a restriction in water supply and an increase in evaporative demand were important ecological factors during the evolution of C4 species. First, permanent ice sheets of low CO2 ‘icehouse’ climate intervals are known to reduce atmospheric moisture levels, with cool temperatures reducing the intensity of the hydrological cycle and increasing climatic seasonality [45]. As a consequence, the CO2-depleted ‘icehouse’ climate of the last 30 Myr has caused the ecological availability of water to decline across large parts of the Earth's surface.
Paleontological evidence reveals that open woodland, savannah and grassland vegetation had begun to extend over large areas of the tropics and subtropics by the Miocene (24–6 Ma) [3,46]. By analogy with processes in the modern world, these open tropical and sub-tropical ecosystems are likely to have been generated by seasonal aridity, edaphic conditions that exclude trees (including high salinity), and disturbance by fire and large mammalian herbivores [3,47–49]. Low atmospheric CO2 may interact with each of these processes by limiting tree growth [50–52]. In fire-prone environments, this limitation means that trees are less likely to become large enough to survive surface fires [51,53]. Indeed, in contemporary mesic savannahs, woody plant cover is apparently increasing in response to rising CO2 [53]. C4 plants are generally short-statured herbs, and only trees in exceptionally rare cases (several species of Hawaiian C4 Euphorbia are fully trees, including rainforest as well as dry forest species; and Haloxylon trees of West Asia have C4 photosynthetic stems). The central role of seasonal aridity in reducing forest cover therefore means that the early C4 plants living in open tropical and sub-tropical environments may well have been subjected to fire events and/or episodes of soil drying.
The demand for water imposed by potential evapotranspiration (PET) is also significantly greater for plants in open habitats than in shaded understorey environments. This environmental contrast is caused by a suite of interrelated microclimatic effects associated with tree cover, illustrated in figure 3 using field observations and a model of leaf energy balance and evaporation (appendix A). Micrometeorological data are shown in figure 3a,b for transects crossing a rainforest edge into adjacent pasture in tropical (Mexico) and temperate (New Zealand) localities [54,55]. Closed forest canopies typically intercept greater than 95 per cent of incident shortwave radiation (figure 3a,b). A model of leaf energy balance shows that this markedly reduces the net radiation and therefore the energy available to drive evaporation (latent heat flux) at ground level (figure 3c,d). Shading of the land surface under a forest canopy also causes a decrease in the air temperature at ground level (from 28 to 25°C in the tropical example, and from 10 to 5°C in the temperate example; figure 3). Therefore, the vapour pressure gradient driving transpiration, as indicated by the VPD, also declines (figure 3a,b). Finally, windspeed is lower in forested compared with open environments (figure 3a,b). This reduces the leaf boundary layer conductance to water vapour (figure 3e,f) and further slows the modelled rate of evaporation. The overall effect of this micrometeorological gradient is a massive difference in the PET modelled for leaves in forested compared with open environments (figure 3e,f; appendix A). In the examples illustrated in figure 3, modelled PET is close to zero in the humid forest understorey, but exceeds 10 mmol H2O m−2 s−1 in the adjacent open pasture for both tropical and temperate climates (figure 3e,f).
Figure 3.
Micrometeorological gradient spanning the transition from forested to open habitats. Data are shown for (a,c,e) pasture at the edge of tropical rainforest and (b,d,f) temperate rainforest, plotted against distance into the forest (negative values for distance = pasture; positive values = forest). Micrometeorological observations of photosynthetically active radiation (PAR), vapour pressure deficit (VPD) and surface windspeed are shown for (a) Mexico [54] and (b) New Zealand [55]. From these data, net radiation, latent and sensible heat fluxes are calculated using the model outlined in appendix A for the (c) tropical and (d) temperate forests. Calculated values of potential evapotranspiration (PET) and boundary layer conductance (ga) are also shown for the same (e) tropical and (f) temperate localities.
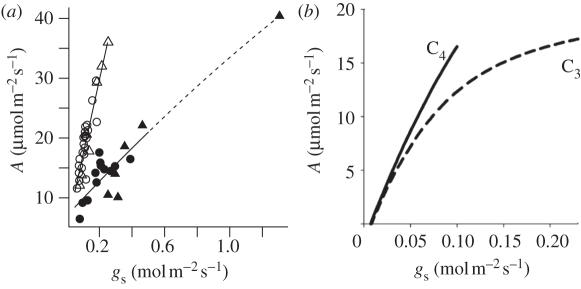
In fact, the risk of colonizing exposed habits depends on the actual rate of leaf transpiration (E), which is lower than PET, being determined by the coupling of micrometeorological effects with the leaf stomatal conductance to water vapour (gs). The gs is controlled by changes in stomatal aperture, which are regulated by leaf water status, photosynthetic rate and chemical signals reflecting the soil water status transmitted from roots to leaves [56]. The gs plays a pivotal role in leaf gas exchange, limiting both the efflux of water and influx of CO2, and the C4 CCM causes a large shift in this trade-off between carbon gain and water loss. For any given value of gs, the net rate of leaf photosynthetic CO2 uptake (A) is greater for C4 than C3 species. This contrast is illustrated in figure 4a for interspecific comparisons of closely related C3 and C4 PACMAD grass species. Measurements were made under modern atmospheric CO2 levels, and in high light and temperature conditions representative of a warm, open environment. The contrast is also modelled in figure 4b using models of leaf gas exchange for idealized C3 [59] and C4 [29] species (see appendix B). In the interspecific comparison, photosynthesis at 30°C can span the same range in C3 and C4 species, with one exceptionally high value of A in the C3 species exceeding the highest values observed in the C4 species (figure 4a; [57]). However, high A for C3 species comes at enormous cost in terms of gs (figure 4a,b), and elevated rates of CO2-fixation are therefore achieved with far greater water economy in C4 than C3 leaves (i.e. with greater water-use efficiency (WUE), defined as A relative to E). The ecological significance of this difference is underscored by the fact that the exceptionally high C3 value of A in figure 4a was measured in the wetland species Phragmites australis, whereas the highest C4 value was in the savannah species Eriachne aristidea [57].
Figure 4.
Illustration of the relationships of photosynthesis (A) to stomatal conductance (gs) in C3 and C4 species [57,58]. (a) Interspecific comparison of PACMAD grass species under high light and the current ambient CO2 level (filled symbols, C3; open symbols, C4; circles from Taylor et al. [58], photosynthetic photon flux density (PPFD) = 1300 µmol m−2 s−1, temperature = approx. 25°C, VPD = 1 kPa; triangles from Taylor et al. [57], PPFD = 2500 µmol m−2 s−1, temperature = 30°C, VPD = 0.8–1.5 kPa). Fitting a general linear model to these data showed that the slope of A on gs was significantly steeper among C4 than C3 species (125 versus 29 µmol CO2 mol−1 H2O, respectively; F1,42 = 55.8, p < 0.0001), with a significant quadratic term that shows no interaction with photosynthetic pathway. (b) Intraspecific response simulated for a C3 and a C4 species by prescribing variation in gs, and modelling A using the approach described in appendix B. Note the saturation response in C3 and linear response in C4, indicating the greater sensitivity of A to stomatal closure in C4, despite achieving higher maximum A at its maximum gs.
The greater WUE of C4 compared with C3 photosynthesis arises from both differences in stomatal aperture and the kinetic properties of the carboxylase enzymes employed by each pathway (figure 1a). PEPC in C4 plants is able to fix carbon at a much higher rate than Rubisco in C3 plants at in vivo substrate concentrations [29]. This allows the C4 pathway to generate a much steeper air–leaf CO2 gradient and higher rates of photosynthesis for a given value of gs. The lower E and greater WUE of C4 than C3 plants was first recognized more than 40 years ago [60], although it is subject to ecological adaptation that can lead to significant interspecific variation and thus overlaps in the ranges of photosynthesis and transpiration for the two photosynthetic types [61]. However, recent work sampling multiple independent lineages of C4 grasses from a range of different habitats showed that gs is significantly lower in each lineage of C4 species than in closely related lineages of C3 grasses [57,58].
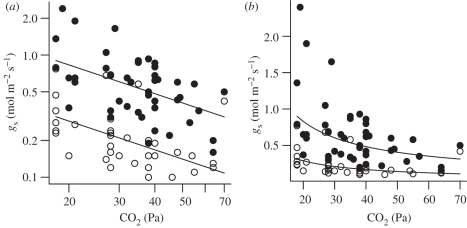
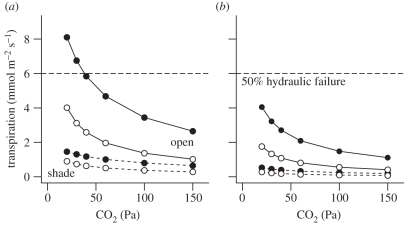
The need for a lower gs to conserve water is especially strong when CO2 levels fall and leaves tend to open stomata to maintain photosynthesis. Data compiled from five independent experiments demonstrate that gs in both C3 and C4 plants increases dramatically in response to the depletion of atmospheric CO2 to sub-ambient levels (figure 5). This negative relationship of gs to CO2 is apparently independent of the responsiveness of gs to VPD [68], and partially or fully offsets the CO2-limitation of photosynthesis at the cost of greater water use. A general linear model fitted to ln-transformed data shows a significant effect of photosynthetic pathway on the intercept of this relationship of gs to CO2 depletion, supporting the generality of higher gs in C3 than C4 species (figure 5a). However, there is no difference in the slope of this relationship between C3 and C4 species (figure 5), indicating a similar relative increase in gs with declining atmospheric CO2 for C3 and C4 species. This finding is consistent with previous meta-analyses showing similar relative declines in gs with CO2 enrichment in C3 and C4 grasses [66,67]. Notably, because gs is typically higher in C3 than C4 species, a similar relative response to CO2 translates into a larger absolute effect (figure 5b). For example, for the model fitted in figure 5b, a depletion of atmospheric CO2 from 100 to 30 Pa equivalent to the Oligocene CO2 drop (figure 2) results in a rise in gs from 0.23 to 0.61 mol H2O m−2 s−1 in C3, but only from 0.08 to 0.21 mol H2O m−2 s−1 in C4 species.
Figure 5.
Stomatal conductance (gs) for the leaves of C3 and C4 plants grown and measured under a range of different CO2 partial pressures, with an emphasis on experiments investigating the effects of CO2 below the current ambient level of approximately 40 Pa (data sources: [30,62–65]; electronic supplementary material). The data compilation is based on literature searches for studies reporting the leaf gas exchange of plants under sub-ambient CO2. However, values for elevated CO2 were included when they were reported as part of the same CO2-gradient studies. The fitted curve for the C3 species is ln(gs) = 2.16 − 0.78 ln(CO2), and for the C4 is ln(gs) = 1.10 − 0.78 ln(CO2). Data and curves are shown on (a) log and (b) linear plots to illustrate relative and absolute sensitivity to CO2, respectively. The fitted curves produce effect sizes for gs at elevated CO2 in C3 and C4 grasses that fall within confidence intervals of previous meta-analyses [66,67]. Filled circles, C3; open circles, C4.
We explored the implications of these contrasting stomatal responses to CO2 in C3 and C4 leaves by modelling leaf transpiration and energy balance (appendix A). Simulations with the model made the simplifying assumption that stomata respond only directly to CO2, with no hydraulic or hormonal feedback on gs. We compared the modelled response of E to CO2 under four sets of micrometeorological conditions representing either an open pasture or a shaded rainforest understorey, in a humid tropical or temperate climate (using the data from figure 3). Our model simulations showed that falling atmospheric CO2 drove larger increases of gs and E in C3 than C4 species (figure 6a,b); this contrast was especially pronounced in open, tropical environments (figure 6a). For the simulations shown in figure 6a, we used a high relative humidity of 80 per cent (VPD = 0.6 kPa), which is typical of the moist tropics and the summer growing season in the humid subtropics (e.g. figure 3a). Under these conditions, simulated E in the C3 species exceeded 6 mmol H2O m−2 s−1 Mpa−1 at a CO2 level of less than 40 Pa (figure 6a). Assuming a leaf-specific whole- plant hydraulic conductance (Kplant) of 12 mmol H2O m−2 s−1 in grasses, and a 50 per cent decline in Kplant under a soil–leaf water potential gradient of −1 MPa [69], this transpiration rate would be sufficient to cause 50 per cent failure of the hydraulic system. The threshold was not reached in a C4 leaf under these conditions. In a drier atmosphere of 60 per cent relative humidity (VPD = 1.5 kPa), the threshold for 50 per cent hydraulic failure in the C3 leaf was reached at CO2 < 80 Pa, and in the C4 at < 20 Pa (data not shown). The contrast was yet more pronounced at a VPD of 3.0 kPa, which is typical of hot, arid climates. Here, 50 per cent hydraulic failure occurred for the C3 leaf at CO2 < 150 Pa, and for the C4 leaf at CO2 < 50 Pa (data not shown).
Figure 6.
Modelled response of transpiration to CO2 for C3 and C4 leaves in open (solid lines) and shaded rainforest understorey (dashed lines) conditions in either (a) humid tropical or (b) humid temperate climates. The model is described in appendix A. Horizontal dashed lines indicate the transpiration rate that would cause 50% hydraulic failure in the absence of feedbacks on stomatal conductance. Filled circles, C3; open circles, C4.
Thus, to avoid failure of the vascular system in open, tropical environments, hydraulic regulation of stomata, investment in hydraulic supply, or a reduction in leaf area [62], would be required at a considerably higher atmospheric CO2 partial pressure in C3 than C4 species. Coupled with the inference that open tropical habitats selected for the C4 pathway in grasses, this observation points strongly to plant–water relations as a potential driver of C4 evolution.
5. Hypothesis for a central importance of hydraulics in c4 evolution
The current consensus model for the evolution of C4 photosynthesis has been developed over 25 years [19]. The evolutionary trajectory from C3 to C4 photosynthesis, via C3–C4 intermediates, is framed in terms of distinct phases for the sake of clarity; however, in reality, these are likely to overlap, and certain developments may occur earlier or later in the sequence. A simplified model of the phases is outlined in table 1, and Sage [19] provides both a comprehensive review of the evidence underpinning this model and the detailed mechanisms proposed. As with the evolution of any complex trait, each step must provide a selective advantage over the previous phase or be selectively neutral.
Table 1.
Hypothetical model for the evolution of C4 photosynthesis, showing three phases that are each observed in extant C3–C4 intermediates. The scheme is a simplified version of that presented by Sage [19], incorporating new evidence [70].
| phase 1. Evolution of ‘proto-Kranz anatomy’ |
| A reduction in the distance between leaf veins and an enlargement in bundle sheath cells (BSC) (figure 1b) may evolve under dry environmental conditions to enhance leaf water status [19,71,72]. Since photosynthetic activity is limited in the BSC of most C3 leaves, increases in the number of chloroplasts may initially serve to maintain leaf light absorpance as the BSC occupy a larger fraction of the leaf. An increase in the numbers and asymmetric distribution of mitochondria in the BSC may establish a photorespiratory CO2 pump that shuttles glycine and refixes CO2within single BSC (see phase 2). This combination of traits has been termed ‘proto-Kranz anatomy’, and occurs in C3 species that are closely related to C3–C4 intermediates [70]. |
| phase 2. Evolution of a photorespiratory CO2pump |
| Photorespiration liberates CO2 via a decarboxylation reaction catalysed by the enzyme glycine decarboxylase (GDC). The increasing localization of this enzyme in BSC mitochondria requires glycine to be shuttled between the mesophyll cells (MC) and the BSC, liberating CO2 in the BSC and allowing its refixation by Rubisco in this compartment (figure 1b). Efficiency of the glycine shuttle increases greatly if BSC walls are resistant to CO2 diffusion, thereby concentrating CO2 in the BSC. This type of photorespiratory CO2 pump is typical of C3–C4 intermediates [19]. |
| phase 3. Evolution of the C4cycle |
| Increases in the PEPC activity of MC may occur initially to scavenge CO2 that leaks from the BSC, but eventually allows the fixation of CO2 from intercellular airspaces (figure 1a). Once this occurs, enhancement of decarboxylase enzyme activities in the BSC is needed to recover the acceptor molecule for carbon-fixation (figure 1a). As carbon-fixation by PEPC increases above that of Rubisco, the C3 cycle is increasingly confined to the BSC, and activities of the C4 and C3 cycles are coordinated. Finally, enzymes recruited into the C4 cycle adapt to their new catalytic environment via changes in turnover rate, substrate affinity and regulation. Changes in stomatal conductance occur during this phase [19]. |
We propose that atmospheric CO2 depletion and open environments select indirectly for C4 photosynthesis via plant–water relations at two points during the evolutionary sequence (table 1, phases 1 and 3). This mechanism acts in combination with the well-established effects of atmospheric CO2 depletion and open environments on photorespiration. A direct role of water relations provides a clear explanation for many of the anatomical changes in the early evolution of C3–C4 intermediacy; these are observed in other lineages of C3, C4 and CAM species, which indicates that these steps are not rare or extraordinary events, but that C4 evolution simply co-opts typical steps in adaptation to dry environments.
Evolution of ‘proto-Kranz anatomy’ (table 1, phase 1). Our hypothesized hydraulic mechanism is based on the vulnerability of C3 leaves to desiccation and hydraulic failure under conditions of high evaporative demand in hot, open environments. Indeed, as described above, the hydraulic vulnerability of leaves would have increased dramatically as C3 grass species migrated from the understorey of a tropical forest into more open tropical environments. If stomata close to protect the hydraulic system, the plant eventually faces carbon starvation through photorespiration and the depletion of carbon stores [73,74]. Carbon starvation is also exacerbated by atmospheric CO2 depletion [75]. These conditions would select for greater hydraulic capacity in C3 leaves, enabling greater gs to achieve rapid rates of photosynthesis in periods when water is abundant, and reducing both the requirement for stomatal closure and the risk of hydraulic failure as stomata partially close in a drying soil or under increasing VPD.
Notably, the anatomical preconditioning required for C4 is precisely that expected to evolve in C3 plants under selection for greater tolerance of dry soil, open environments, high VPD and/or low CO2. Previous studies of adaptation to these conditions within species or across closely related species within given lineages have shown increased vein densities, and thus shorter interveinal distances [76–79]. This higher vein density would permit greater gs and higher A during periods with high water availability, to compensate for low CO2. Indeed, the evolution of greater vein density in the early angiosperms may have allowed them to better cope with low CO2, while avoiding stomatal closure, contributing to or driving their contemporary dominance of world vegetation [78,80]. The higher vein densities in C4 than C3 species may indicate an extension of that trend, i.e. an adaptation to the water deficit that accompanies higher demand from transpiration to maintain photosynthetic rate in low CO2. In the same way that an increase in vein density may have enabled angiosperms to displace gymnosperms in dominating the world's forests, this trend in grasses may have provided a competitive advantage over grasses with lower vein densities, contributing to their dominance over large areas of the planet. The high vein densities in species with ‘proto-Kranz anatomy’ led to this feature being co-opted as an early part of the C4 syndrome, providing enough tissue to later serve as the locus of sequestered C3 metabolism (figure 1a,b) [19,71,72,81,82].
Similarly, the enlargement of bundle sheath during the early phase of C4 evolution may have contributed water storage capacitance to the tissue [19,71]. Indeed, the evolution of such tissue in species with ‘proto-Kranz anatomy’ would be an extension of a frequently observed trend in certain plant lineages in which the species adapted to saline and drier climates have more strongly developed achlorophyllous, large-celled tissue. This tissue serves an apparent function for water storage either in the bundle sheath, mesophyll or hypodermal layers, and this trend of greater water storage with aridity is apparent within C3 lineages with leaves [83,84] and phyllodes [85], but also within C4 and CAM lineages [86,87] (M. J. Sporck & L. Sack 2011, unpublished data).
Evolution of the C4 cycle (table 1, phase 3). The evolution of greater WUE is apparently an important step in C4 evolution. It may be achieved in certain C3–C4 intermediates operating a photorespiratory pump (table 1, phase 2) through the enhancement of A for a given gs (e.g. Heliotropium [88]). However, once the carboxylase activity of PEPC exceeds that of Rubisco (evolution of ‘C4-like’ plants during phase 3, table 1), further improvements in WUE may occur through reduced gs (e.g. Flaveria [89]). Thus, evolution towards C4 probably first involved a suppression of photorespiration (table 1, phase 2) and then increased PEPC activity (table 1, phase 3), but only this last step enabled a reduction of gs. These steps may even occur within C3 species: populations of the grass Phragmites australis in hot, arid and saline environments show increasing investment in elements required for the C4 cycle, including PEPC, decarboxylase enzymes and bundle sheath tissues, which may enhance both CO2-fixation and WUE [90]. Thus, the engagement of CO2-fixation via PEPC (table 1, phase 3) improves leaf–water relations, allowing a lower value of gs for a given rate of photosynthesis, and thus a smaller absolute response of gs to CO2 depletion in C4 leaves (figure 5b). This benefit would lead atmospheric CO2 depletion and open environments to select for the C4 cycle via plant–water relations, as well as via their effects on photosynthetic efficiency. Together, these physiological differences mean that leaves operating a fully integrated C4 cycle are less prone than C3 leaves to hydraulic failure or stomatal closure in hot, open environments. This contrast in sensitivity to water deficit increases dramatically under atmospheric CO2 depletion.
A greater hydraulic conductance relative to demand, as would arise from the adaptation described, would result in a greater ability to maintain open stomata during soil drying events in C4 than C3 leaves, and a lower sensitivity of stomata to increases in VPD and to decreases in CO2 (see §6). These improvements in water relations provide a plausible physiological basis for the greater likelihood for C4 than C3 grass lineages to invade xeric environments [42].
6. Hydraulic feedbacks on leaf physiology
Our hypothesis that C4 evolved not only to improve photosynthetic efficiency per se, but also to reduce water stress by enabling low gs, has additional implications that at first sight lead to inconsistency. While the C4 species can achieve higher A for a given gs, A declines more rapidly as gs decreases (figure 4a,b). Because of this greater sensitivity of A to gs, a C4 plant must keep stomata open to maintain its advantage in A over C3 plants. At first sight, this problem should render photosynthesis in C4 plants particularly sensitive to soil drying owing to stomatal closure. As an initial test of our hypothesis for a hydraulics-mediated advantage of C4 photosynthesis, and to further explore the physiological implications, we used a novel integrated model of leaf photosynthesis, stomatal and hydraulic systems to compare the physiological behaviour of C3 and C4 species (appendix B), with a particular focus on conditions of CO2 depletion (low CO2), atmospheric water vapour deficit (high VPD) and soil drying (low soil water potential). Given reasonable assumptions, these model simulations supported the hypothesis of a hydraulically based advantage of the C4 syndrome under high VPD and low soil water potential, enabling stomata to remain open, and maintaining A at higher levels than C3 species, with greatest differences at low CO2.
We tested the role of hydraulic conductance in allowing gs and A to be maintained during drought under varying VPD and atmospheric CO2 in C3 and C4 species. We parametrized the model using data from the literature, and coupled this with photosynthesis models for C3 and C4 types (appendix B). Thus, we compared C3 and C4 species that differed in gs (0.23 versus 0.10 mol m−2 s−1, respectively) but assumed the same responsiveness of gs, and the same vulnerability of the hydraulic system, to declining leaf water potential. We tested three scenarios, with the C4 species having: the same whole-plant hydraulic conductance (Kplant) as the C3 species; double the Kplant; or half the Kplant (figures 7 and 8).
Figure 7.
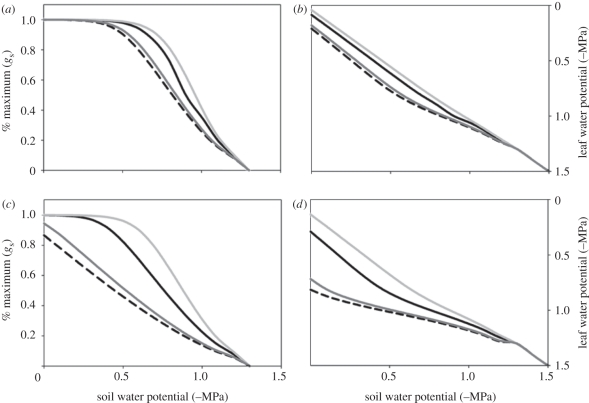
Simulated response of mid-day operating stomatal conductance (as a percentage of maximum for hydrated leaves) and leaf water potential to drying soil at VPD of (a,b) 1 kPa and (c,d) 3 kPa, for a C3 species and C4 species with identical plant hydraulic conductance (Kplant), for a simulated C4 species with double the Kplant, and for a simulated C4 species with half the Kplant (corresponding to its value of gs being approximately half that of the C3 species). Note that the C4 species maintains stomatal opening into drier soil and higher VPD than the C3. Increasing the Kplant improves this ability in the C4 species, whereas reducing the Kplant by half leads to very rapid decline of gs at high VPD or in a dry soil. (a–d) Dashed line, C3; black solid line, C4; light grey solid line, C4, 2 × Kplant; dark grey solid line, C4, 0.5 × Kplant.
Figure 8.
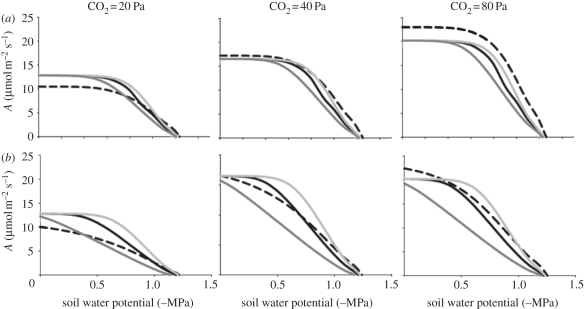
Simulated response of light-saturated photosynthetic rate to a drying soil at low (20 Pa), ambient (40 Pa) or high CO2 (80 Pa) and VPD of (a) 1 kPa or (b) 3 kPa, for a C3 species and C4 grass species with identical plant hydraulic conductance (Kplant), and for a simulated C4 species with double the Kplant, and a simulated C4 species with half the Kplant (corresponding to its value of gs being approximately half that of the C3 species). (a,b) Dashed line, C3; black solid line, C4; light grey solid line, C4, 2 × Kplant; dark grey solid line, C4, 0.5 × Kplant.
When the C4 species had the same Kplant as the C3, the C4 was able to maintain gs at a higher relative level (percentage of its maximum value) as the soil dried; this advantage over the C3 was especially strong at higher VPD (figure 7). This result is consistent with an experimental comparison of PACMAD grasses under drought, which showed a more sensitive response of gs to soil water deficits in C3 species than in closely related C4 species [57]. Model simulations also showed lower leaf water potential in the C3 than C4 species as soil dried, until the point of stomatal closure, where the leaves equilibrated with the soil (figure 7). This contrast is also broadly consistent with comparative experiments on closely related C3 and C4 PACMAD grasses, which showed a difference in leaf water potential under moist conditions that was diminished under chronic drought [57,68]. In our model simulations, this advantage was evidently owing to the higher Kplant relative to gs for the C4 over C3 species. When the C4 plant was given double the Kplant of the C3 species, its advantage in maintaining open stomata during soil and atmospheric drought was increased, and when the C4 plant was given half the Kplant of the C3 species, its advantage was diminished.
The ability of a C4 species with similar or higher Kplant to a C3 species to maintain open stomata during drought also translated into an ability to maintain A at a higher level. This advantage went beyond compensating for the tendency of A to decline more precipitously with declining gs in C4 than C3 species, described above (figure 4a,b), and typically provided C4 species with an ability to maintain higher A during mild drought, especially at higher VPD (notably, simulations at yet higher VPDs led to even stronger differences between photosynthetic types). When the C3 and C4 species had the same Kplant, A was higher in the C4 than the C3 under low CO2, but the rank was reversed under high CO2 (figure 8). Doubling the value of Kplant in C4 compared with C3 species gave an advantage in A, and the ability to maintain photosynthesis during drought. This finding is broadly consistent with an early demonstration that, during the dry season in the Negev desert, the C4 species Hammada scoparia showed a shallower decline of A with declining leaf water potential than three C3 species (Prunus armeniaca, Artemisia herba-alba and Zygophyllum dumosum). At a leaf water potential of −6 MPa, H. scoparia (C4) had twice the A of these C3 species [91]. In contrast, when the C4 species in our model simulations had reduced Kplant, this led to a much reduced value of A, and the C3 species showed an advantage of A across all CO2 levels as soil dried minimally (figure 8). These findings indicate that, theoretically, a reduced Kplant in C4 species would annul any advantage of the C4 pathway for photosynthesis under drought or low CO2 in open environments. However, an equal or higher Kplant in these conditions would provide an advantage not only in maintaining open stomata, but also in maintaining a high A, providing a strong benefit to C4 species.
What evidence is there that Kplant is high relative to gs in C4 compared with C3 species? To-date, no studies have made the necessary direct experimental comparisons. Nonetheless, the two comparative studies of five C3 and eight C4 lineages of PACMAD grasses presented in figure 4a (a total of 40 different species) found that C4 grasses generated a lower soil to leaf gradient in water potential (ΔΨ) during typical diurnal transpiration than closely related C3 species [57,58]. Similar patterns have been observed for the C3 and C4 subspecies of Alloteropsis semialata in common garden plots at high irradiance and temperature [92]. This finding is consistent with the hypothesis of a higher ratio of hydraulic supply to demand, i.e. a greater Kplant/gs in C4 than C3 grass species.
Notably, several studies of C4 woody eudicots have suggested the opposite situation, measuring reduced stem hydraulic conductance per supplied leaf area (Kstem) relative to C3 species [93–95]. This was hypothesized to evolve in the final stages of C4 evolution after WUE has increased (table 1, phase 3). A reduced Kstem would not necessitate a reduction of gs under well-watered conditions or mild drought. On the other hand, it would likely reduce xylem construction costs, and also possibly reduce the vulnerability to cavitation of stems, and thus their longevity [93–95]. Such adaptation would be especially important for the economics and protection of long-lived woody parts [93]. However, we note that a lower Kstem does not necessarily imply a lower Kplant; indeed, a higher leaf hydraulic conductance (Kleaf) can easily compensate. Recent work has shown the leaf is a critical bottleneck in the whole-plant hydraulic pathway, accounting for greater than 30 per cent of whole plant resistance, and that leaves have steeper hydraulic vulnerability curves than stem [96]. Consequently, Kleaf is a strong determinant of Kplant, especially when leaves begin to dehydrate during transpiration or incipient drought [97–101]. Such an importance of Kleaf in the whole plant pathway is thus consistent with a reduction of Kstem in woody dicot C4 species. It is also consistent with the evolution of high vein density in C3 species under drier climates (table 1, phase 1), which would serve to increase Kleaf and maintain or increase Kplant, while the evolution of higher water storage capacitance would buffer changes in leaf water potential during high VPD or drought [77,96,102,103]. These modifications would then be co-opted for further evolution of C3–C4 intermediates and C4 species (table 1).
In §5, we argued that the low values of gs in C4 species save the hydraulic system from embolism, especially as leaves are heated in open environments, and as stomata are stimulated to open under low CO2. Our model findings further show that the low values of gs in C4 species also reduce stomatal sensitivity to hydraulic feedbacks, allowing stomata to remain open during drought, and photosynthesis to continue, but only if C4 species have similar or higher Kplant than C3 species, and thus a high hydraulic supply relative to demand. A lower Kplant would serve to make C4 species more sensitive to soil drought, especially under high VPD and low CO2. The circumstantial evidence we have presented for grass species is consistent with our hypothesis for a high hydraulic supply relative to demand in C4 species. However, to our knowledge, there are no published data comparing Kleaf for C3 and C4 plants in general, or for grasses specifically. Such data are essential to test our hypothesis.
7. Metabolic limitation of photosynthesis during severe drought
We hypothesized that the evolution of the C4 pathway provides significant physiological benefits for carbon-fixation and water conservation in well-watered soil, during the early stages of soil drought, or during transient drought events. However, there is no a priori reason to expect a greater tolerance of severe desiccation in C4 than C3 species [104]. In fact, recent comparative analyses of closely related C3 and C4 PACMAD grasses suggests the converse; that the C4 photosynthetic system may be more prone to greater metabolic inhibition under chronic and severe drought events. Experimental evidence from controlled environment, common garden and field investigations all indicate that photosynthetic capacity declines to a greater extent with leaf water potential in C4 than C3 grass species after weeks of soil drying, or at very high VPD [57,92,105,106]. Chronic or severe drought may thus diminish, eliminate or even reverse the WUE advantage of C4 over C3 species. In the extreme case, photosynthetic capacity is significantly slower to recover after the end of the drought event [106]. Experimental evidence therefore suggests that metabolic limitation of photosynthesis will eventually offset hydraulic benefits of the C4 syndrome during acute or prolonged drought events. For tolerance of severe drought, tight stomatal control and tissue water storage, as is strongly developed in CAM species, or desiccation-tolerant tissue, are far superior to C4 metabolism. However, for tolerance of repeated transient droughts in the growing season, C4 metabolism carries strong advantages, particularly under low CO2.
8. Conclusions
We hypothesized that atmospheric CO2 depletion coupled with high temperatures, open habitat and seasonally dry subtropical environments caused excessive demand for water transport, and selected for C4 photosynthesis to enable lower stomatal conductance as a water-conserving mechanism. C4 photosynthesis allowed high rates of carbon-fixation to be maintained at low stomatal conductance, and reduced stomatal opening in response to low atmospheric CO2. These mechanisms served to reduce strain on the hydraulic system. Maintaining a high hydraulic conductance enabled stomatal conductance and photosynthesis to be sustained for longer during drought events. The evolution of C4 photosynthesis, therefore, rebalanced the fundamental trade-off between plant carbon and water relations, as atmospheric CO2 declined in the geological past, and as ecological transitions drove increasing demand for water.
Our hypothesis adds the evolution of C4 photosynthesis to the list of exceptional innovations based on the plant hydraulic system that arose during periods of low CO2 that have impacted on plant life history, biogeography and the distribution of ecosystems in the deep past, present and future [107]. Previously hypothesized modifications of the water transport system and associated plant features driven by low CO2 include the evolution of xylem vessels and stomata [108], and the planate leaf [109,110] with high vein density [78]. These innovations permitted more rapid growth and diversification, leading to the succession of dominance from pteridophytes to gymnosperms to angiosperms [78,80,111,112]. The evolution of C4 photosynthesis, for improved performance in exposed, seasonally dry habitats in low CO2, thus joins a long line of hydraulic innovations driven by low CO2 that changed plants and the world.
Acknowledgements
We thank Nate McDowell, Brad Ripley, Jessica Pasquet-Kok and Christine Scoffoni for stimulating discussions on the role of photosynthetic pathway in plant hydraulics, Sam Taylor, Hui Liu and Mark Rees for help with the data analysis and presentation, and Rowan Sage and an anonymous reviewer for their insights and critical reviews of this paper. This work was inspired by the International Scientific Seminar ‘Atmospheric CO2 as a driver of plant evolution’ organized by David Beerling at the Kavli Royal Society International Centre in September 2010, and we thank The Royal Society for funding and the opportunity to attend this meeting (C.P.O.). We also gratefully acknowledge funding for this work from a Royal Society University Research Fellowship (C.P.O.), NERC standard grant number NE/DO13062/1 (C.P.O.), and National Science Foundation Grant no. 0546784 (L.S.).
Appendix A
(a). Model of evaporation from a leaf in a forest understorey or in open pasture
The net radiation balance at the leaf surface (Φn) is a function of shortwave (S) and longwave (L) radiation fluxes (both W m−2):
| A1 |
where 2Ll represents longwave radiation emitted upwards and downwards from the leaf, Ls the upwards emission from soil and Ld the downwards emission from the sky or forest canopy, depending on tree cover. Values of L were calculated according to Stefan's Law, tracking the temperatures of leaf, soil, tree canopy and sky, by assuming that the lowest layer of leaves in the forest canopy is approximately at air temperature, and the apparent temperature of the clear sky is 20 K lower than that of surface air [113,114]. Heat storage by the leaf was assumed to be negligible, such that Φn is dissipated entirely via latent and sensible heat fluxes (λE and H, respectively)
| A2 |
Evaporation from the leaf surface (E, mol H2O m−2 s−1) was modelled using two variants of the Penman–Monteith equation [115,116]. For the simulations shown in figure 3, we were interested in the potential for evapotranspiration in the absence of any limitation imposed by stomata, and used the original form [115]
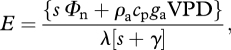 |
A3a |
where Φn is net radiation at the leaf surface (W m−2), VPD is the atmospheric VPD (Pa), ga is the leaf boundary layer conductance (m s−1) and the remaining parameters are physical properties of air and water: s, the rate of change of saturation vapour pressure with temperature (Pa K−1), ρa, the density of dry air (kg m−3), cp, the specific heat capacity of water (J kg−1 K−1), γ, the psychrometer constant (Pa K−1) and λ, the latent heat of evaporation for water (J mol−1). The values of s, γ, ρa and λ were corrected for temperature following Friend [117]. We made the simplifying assumption that the leaf boundary layer conductance is approximately equal for heat and water.
For the simulations shown in figure 6, we were interested in the regulation of E by stomata, and used the modification by Monteith [116]:
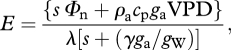 |
A3b |
where gW is the total leaf conductance for water:
| A4 |
and ga is a function of leaf width (d = 0.01 m), and wind speed (u, m s−1):
| A5 |
Values of gs were prescribed for these simulations as a function of atmospheric CO2, using the equations derived for C3 and C4 leaves in figure 5.
The sensible heat flux is given by:
| A6 |
where Tl is the leaf temperature and Ta the air temperature (both K). To solve the leaf energy balance, equation (A 2) must be rearranged to give H, and equation (A 6) to give Tl:
| A7 |
and
| A8 |
Equations (A 1), (A 3), (A 7) and (A 8) are then solved simultaneously using iteration in R (The R foundation for Statistical Computing) to yield values for Φn, E, H and Tl.
Appendix B
(a). Modelling of hydraulic-stomatal limitation on photosynthesis in C3 and C4 species
A model based on a few simplifying assumptions was used to determine the response of operating gs and leaf water potential (Ψleaf) to declining soil water potential (Ψsoil) and increasing vapour pressure deficit (VPD). The gs was then used to estimate photosynthesis from a diffusion-biochemistry model (see appendix B). The hydraulic-stomatal model determines Ψleaf, plant hydraulic conductance (Kplant) and gs at a given Ψsoil and VPD. First, Ψleaf is determined based on steady-state water transport according to the Ohm's Law analogy [118,119]:
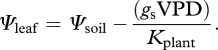 |
B1 |
Secondly, a hydraulic response function modelled the vulnerability of Kplant to declining Ψleaf. This used a linear function to approximate observations between full hydration and turgor loss, as reported for the leaves of grasses and several other taxa [69,120,121]:
| B2 |
where Kmax and a are constants for given species. We assumed that Kplant showed a similar vulnerability response to leaves [69,99], calculating Kplant = 80% × leaf hydraulic conductance, based on the range shown in previous work on grasses (65% to over 80% of plant resistance in the leaf [69,101,118]). Changing these assumptions would not affect the comparative findings of our simulations.
Thirdly, we simulated the decline in gs with more negative Ψleaf as a sigmoidal function:
| B3 |
where g* is the maximum value of gs at Ψleaf = 0, and b is the Ψleaf at 50 per cent stomatal closure. The constant c defines the shape of the sigmoidal curve.
For given Ψsoil and VPD, equations (B 1–B 3) were solved simultaneously, minimizing the implicit forms by iteration [122] (Microsoft Visual Basic; Microsoft, Redmond, WA, USA). Using this model, we simulated the response of gs to Ψsoil for C3 and C4 species, from 0 to −2 MPa, and at VPD of 1 and 3 kPa, using parameters for equations (B 1–B 3) from the literature, as available (table 2). We also tested scenarios for C4 species with double the Kplant and half the Kplant of the C3 species.
Table 2.
Parameters used for hydraulic-stomatal-photosynthesis modelling.
| parameter | units | C3, C4 values | source |
|---|---|---|---|
| leaf Kmax | mmol m−2 s−1 MPa−1 | 15, 15 | [69] |
| a | mmol m−2 s−1 MPa−2 | −7.5, −7.5 | [69] |
| g* | mol m−2 s−1 | 0.25, 0.10 | [58] |
| b | MPa | −1.0, −1.0 | [69] |
| c | MPa | 0.1, 0.1 | [69,123] |
| Vc, max | µmol m−2 s−1 | 83, 39 | [124] |
| Γ* | µmol mol−1 | 46, 10 | [124] |
| Kc | µmol mol−1 | 302, 302 | [124] |
| Ko | µmol mol−1 | 256, 256 | [124] |
| O | mol mol−1 | 0.210, 0.210 | [124] |
| Jmax | µmol m−2 s−1 | 132, 180 | [124] |
| Rd | µmol m−2 s−1 | 1.66, 0.78 | [124] |
| Vp,max | µmol m−2 s−1 | n.a., 120 | [124] |
| Kp | µmol mol−1 | n.a., 80 | [124] |
| Vpr | µmol m−2 s−1 | n.a., 80 | [124] |
(b). Modelling of photosynthetic rate from diffusion-biochemical limitations for C3 and C4 species during drought under varying vapour pressure deficit and atmospheric CO2
We simulated photosynthetic rate (A) and its response to CO2, by first modelling a direct response of gs to CO2, and then inputting the adjusted gs values into equations for C3 and C4 photosynthesis.
First, for low and high CO2 we multiplied the gs values by a factor corresponding to 20 or 80 Pa (1.72 and 0.58, respectively) based on the data and fitted equations of figure 5. This multiplicative adjustment of gs for CO2 level was applied independently of the gs response to water status (from which gs was determined from equations (B 1–B 3) above). Thus, the model considers a simplified scenario in which the response of stomata to VPD and soil drought on one hand, and to CO2 on the other, is independent and multiplicative, with no hydraulic or hormonal feedback on gs. The assumption is consistent with previous work measuring the effects of CO2 and VPD on gs in C3 and C4 species [68]. This independence of the responses would put plants in low CO2 at risk of severe leaf dehydration if stomata open strongly when VPD is low, and, indeed, such dehydration has been observed [125]. However, there is evidence that declining soil water availability can interact with the CO2 response, resulting in greater sensitivity of stomata to CO2, to a varying degree across different C3 and C4 species, in part modulated by abscisic acid responsiveness [126,127]. More work is needed to resolve and to explicitly model the potential interactions of stomatal responses to different factors in C3 and C4 species. For example, the interaction with falling soil water potential during drought would be expected to produce a stronger decline of gs and of A.
In our model, the gs, now adjusted for CO2, was used to predict A based on the equations for C3 and C4 photosynthesis provided by von Caemmerer [29], using parameters from Vico & Porporato [124] (table 2). We assumed that the impact of Ψleaf on A was mediated by gs without any separate, direct impacts on mesophyll conductance or on metabolism itself; these impacts could be added but, without detailed information of differential impacts on C3 and C4 species, would not change the outcome of our scenarios [124].
Thus, for C3 species, A was determined as
| B4 |
where Ac is the Rubisco-limited rate of photosynthesis, Aj is the rate of RuBP-limited CO2 assimilation and Rd is the total mitochondrial rate of respiration. In turn,
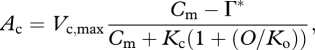 |
B5 |
where Vc,max is the maximum catalytic activity of Rubisco at current leaf temperature (here considered as optimal); Cm is the CO2 concentration at the site of photosynthesis in the mesophyll cell (MC); Γ* is the equilibrium CO2 compensation point for gross photosynthesis; Kc and Ko are the coefficients for CO2 and O2 of the Michaelis–Menten kinetics, accounting for competitive inhibition by O2; and O is the O2 concentration at the site of photosynthesis.
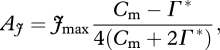 |
B6 |
where Jmax is the maximum potential rate of electron transport. To determine A, the equations (B 5) and (B 6) were each equated separately with the diffusion equation:
| B7 |
where Ca is the atmospheric CO2 concentration. The value of gt was determined as the conductance to CO2 from the atmosphere to the intercellular space (stomatal conductance to CO2, gs,CO2) and from the intercellular space to the chloroplast (mesophyll conductance, gm) in series:
| B8 |
where gs,CO2 was determined as gs/1.6 and gm as gs × 1.65 [124]. In each case (equations B 6 = B 8, and equations B 7 = B 8), the equations were solved for a given gs and Ca, to determine Cm using the quadratic equation. The values of Cm were inserted into equations (B 5) and (B 6), respectively, to determine Ac and Aj, before applying equation (B 4) to determine A.
For the C4 species, a similar approach was used, but the first step involved determining the PEP carboxylation rate (Vp):
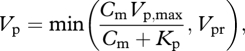 |
B9 |
where Vp,max is the maximum rate of PEP carboxylation, Vpr is an upper bound set by PEP regeneration rate and Kp is the Michaelis–Menten coefficient of PEPC. The Cm was determined by equating equation (B 9) with equation (B 7) for a given Ca and gs. We then used the Cm and Vp to determine the A, by combining two equations:
| B10 |
where Lbs is bundle sheath leakage, given by:
| B11 |
and Cbs is the CO2 concentration in the bundle sheath. Substituting equation (B 11) into equation (B 10), and making this equation equal to each of equations (B 5) and (B 6) separately (substituting Cbs for Cm in those equations), allowed them to be solved for Cbs. Equations (B 5) and (B 6) were then used to determine Ac and Aj, and equation (B 4) to determine A.
Glossary
- A
net rate of leaf photosynthetic CO2 assimilation
- BSC
bundle sheath cells
- CA
carbonic anhydrase
- CAM
crassulacean acid metabolism
- CCM
carbon-concentrating mechanism
- DC
decarboxylase enzymes
- E
actual rate of leaf transpiration
- gs
leaf stomatal conductance to water vapour
- Kleaf
hydraulic conductance of leaves
- Kplant
whole-plant hydraulic conductance
- Kstem
stem hydraulic conductance
- MC
mesophyll cells
- PACMAD
acronym for the lineage of grasses comprising the subfamilies Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae and Danthonioideae
- PAR
photosynthetically active radiation
- PEPC
phosphoenolpyruvate carboxylase
- PET
potential evapotranspiration
- PPFD
photosynthetic photon flux density
- RH
relative humidity
- Rubisco
ribulose-1,5-bisphosphate carboxylase/oxygenase
- VPD
vapour pressure deficit
- WUE
leaf water-use efficiency (A/E)
- Ψleaf
leaf water potential
References
- 1.Hohmann-Marriott M. F., Blankenship R. E. 2011. Evolution of photosynthesis. Annu. Rev. Plant Biol. 441, 940–941 10.1146/annurev-arplant-042110-103811 (doi:10.1146/annurev-arplant-042110-103811) [DOI] [PubMed] [Google Scholar]
- 2.Crayn D. M., Winter K., Smith J. A. C. 2004. Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae. Proc. Natl Acad. Sci. USA 101, 3703–3708 10.1073/pnas.0400366101 (doi:10.1073/pnas.0400366101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards E. J., Osborne C. P., Strömberg C. A. E., Smith S. A. & C4 Grasses Consortium 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591 10.1126/science.1177216 (doi:10.1126/science.1177216) [DOI] [PubMed] [Google Scholar]
- 4.Beerling D. J., Royer D. L. 2011. Convergent cenozoic CO2 history. Nat. Geosci. 4, 418–420 10.1038/ngeo1186 (doi:10.1038/ngeo1186) [DOI] [Google Scholar]
- 5.Young J. N., Rickaby R. E. M., Kapralov M. V., Filatov D. A. 2012. Adaptive signals in algal Rubisco reveal a history of ancient atmospheric carbon dioxide. Phil Trans. R. Soc. B 367, 483–492 10.1098/rstb.2011.0145 (doi:10.1098/rstb.2011.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osmond C. B., Troughton J. H., Goodchild D. J. 1969. Physiological, biochemical and structural studies of photosynthesis and photorespiration in two species of Atriplex. Zeit. Pflanzenphysiol. 61, 218–237 [Google Scholar]
- 7.Berry J. A., Downton W. J. S., Tregunna E. B. 1970. The photosynthetic carbon metabolism of Zea mays and Gomphrena globosa: the location of the CO2 fixation and carboxyl transfer reactions. Can. J. Bot. 48, 777–786 10.1139/b70-106 (doi:10.1139/b70-106) [DOI] [Google Scholar]
- 8.Voznesenskaya E. V., Franceschi V. R., Kiirats O., Freitag H., Edwards G. E. 2001. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543–546 10.1038/35107073 (doi:10.1038/35107073) [DOI] [PubMed] [Google Scholar]
- 9.Watson L., Dallwitz M. J. 1992. The grass genera of the world: descriptions, illustrations, identification, and information retrieval; including synonyms, morphology, anatomy, physiology, phytochemistry, cytology, classification, pathogens, world and local distribution, and references. Version: 25th November 2008. See http://delta-intkey.com
- 10.Cerling T. E., Harris J. M., MacFadden B. J., Leakey M. G., Quade J., Eisenmann V., Ehleringer J. R. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158 10.1038/38229 (doi:10.1038/38229) [DOI] [Google Scholar]
- 11.Ehleringer J. R., Cerling T. E., Helliker B. R. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299 10.1007/s004420050311 (doi:10.1007/s004420050311) [DOI] [PubMed] [Google Scholar]
- 12.Hatch M. D., Osmond C. B., Slatyer R. O. (eds) 1971. Photosynthesis and photorespiration. New York, NY: Wiley-Interscience [Google Scholar]
- 13.Bräutigam A., et al. 2011. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol. 155, 142–156 10.1104/pp.110.159442 (doi:10.1104/pp.110.159442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown N. J., Newell C. A., Stanley S., Chen J. E., Perrin A. J., Kajala K., Hibberd J. M. 2011. Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331, 1436–1439 10.1126/science.1201248 (doi:10.1126/science.1201248) [DOI] [PubMed] [Google Scholar]
- 15.Christin P. A., Salamin N., Savolainen V., Duvall M. R., Besnard G. 2007. C4 photosynthesis evolved in grasses via parallel adaptive genetic changes. Curr. Biol. 17, 1241–1247 10.1016/j.cub.2007.06.036 (doi:10.1016/j.cub.2007.06.036) [DOI] [PubMed] [Google Scholar]
- 16.Sage R. F., Christin P. A., Edwards E. J. 2011. The C4 lineages of planet Earth. J. Exp. Bot. 62, 3155–3169. (doi:10.1093/jxb/err048) [DOI] [PubMed] [Google Scholar]
- 17.Westhoff P., Govik U. 2010. Evolution of C4 photosynthesis—looking for the master switch. Plant Physiol. 154, 598–601 10.1104/pp.110.161729 (doi:10.1104/pp.110.161729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibberd J. M., Quick W. P. 2002. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415, 451–454 10.1038/415451a (doi:10.1038/415451a) [DOI] [PubMed] [Google Scholar]
- 19.Sage R. F. 2004. The evolution of C4 photosynthesis. New Phytol. 161, 341–370 10.1111/j.1469-8137.2004.00974.x (doi:10.1111/j.1469-8137.2004.00974.x) [DOI] [PubMed] [Google Scholar]
- 20.Hibberd J. M., Covshoff S. 2010. The regulation of gene expression required for C4 photosynthesis. Annu. Rev. Plant Biol. 61, 181–207 10.1146/annurev-arplant-042809-112238 (doi:10.1146/annurev-arplant-042809-112238) [DOI] [PubMed] [Google Scholar]
- 21.Christin P. A., Freckleton R. P., Osborne C. P. 2010. Can phylogenetics identify C4 origins and reversals? Trends Ecol. Evol. 25, 403–409 10.1016/j.tree.2010.04.007 (doi:10.1016/j.tree.2010.04.007) [DOI] [PubMed] [Google Scholar]
- 22.Ehleringer J. R., Sage R. F., Flanagan L. B., Pearcy R. W. 1991. Climate change and the evolution of C4 photosynthesis. Trends Ecol. Evol. 6, 95–99 10.1016/0169-5347(91)90183-X (doi:10.1016/0169-5347(91)90183-X) [DOI] [PubMed] [Google Scholar]
- 23.Griffiths H. 2006. Plant biology: designs on Rubisco. Nature 441, 940–941 10.1038/441940a (doi:10.1038/441940a) [DOI] [PubMed] [Google Scholar]
- 24.Tcherkez G. G. B., Farquhar G. D., Andrews T. J. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl Acad. Sci. USA 103, 7246–7251 10.1073/pnas.0600605103 (doi:10.1073/pnas.0600605103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long S. P. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant Cell Environ. 14, 729–739 10.1111/j.1365-3040.1991.tb01439.x (doi:10.1111/j.1365-3040.1991.tb01439.x) [DOI] [Google Scholar]
- 26.Furbank R. T., Hatch M. D. 1987. Mechanism of C4 photosynthesis. The size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiol. 85, 958–964 10.1104/pp.85.4.958 (doi:10.1104/pp.85.4.958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehleringer J., Björkman O. 1977. Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2, and O2 concentration. Plant Physiol. 59, 86–90 10.1104/pp.59.1.86 (doi:10.1104/pp.59.1.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehleringer J., Pearcy R. W. 1983. Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiol. 73, 555–559 10.1104/pp.73.3.555 (doi:10.1104/pp.73.3.555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Victoria, Australia: CSIRO Publishing [Google Scholar]
- 30.Cunniff J., Osborne C. P., Ripley B. S., Charles M., Jones G. 2008. Response of wild C4 crop progenitors to sub-ambient CO2 highlights a possible role in the origin of agriculture. Glob. Change Biol. 14, 576–587 10.1111/j.1365-2486.2007.01515.x (doi:10.1111/j.1365-2486.2007.01515.x) [DOI] [Google Scholar]
- 31.Long S. P. 1999. Environmental responses. In C4 plant biology (eds Sage R. F., Monson R. K.), pp. 215–249 San Diego, CA: Academic Press [Google Scholar]
- 32.Ehleringer J. 1978. Implications of quantum yield differences to the distributions of C3 and C4 plants. Oecologia 31, 255–267 10.1007/BF00346246 (doi:10.1007/BF00346246) [DOI] [PubMed] [Google Scholar]
- 33.Robichaux R. H., Pearcy R. W. 1984. Evolution of C3 and C4 plants along an environmental moisture gradient: patterns of photosynthetic differentiation in Hawaiian Scaevola and Euphorbia species. Am. J. Bot. 71, 121–129 10.2307/2443631 (doi:10.2307/2443631) [DOI] [Google Scholar]
- 34.Ehleringer J. R., Monson R. K. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 24, 411–439 10.1146/annurev.es.24.110193.002211 (doi:10.1146/annurev.es.24.110193.002211) [DOI] [Google Scholar]
- 35.Christin P. A., Osborne C. P., Sage R. F., Arakaki M., Edwards E. J. 2011. C4 eudicots are not younger than C4 monocots. J. Exp. Bot. 62, 3171–3181 10.1093/jxb/err041 (doi:10.1093/jxb/err041) [DOI] [PubMed] [Google Scholar]
- 36.Christin P. A., Besnard G., Samaritani E., Duvall M. R., Hodkinson T. R., Savolainen V., Salamin N. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr. Biol. 18, 37–43 10.1016/j.cub.2007.11.058 (doi:10.1016/j.cub.2007.11.058) [DOI] [PubMed] [Google Scholar]
- 37.Vicentini A., Barber J. C., Aliscioni A. S., Giussani A. M., Kellogg E. A. 2008. The age of the grasses and clusters of origins of C4 photosynthesis. Glob. Change Biol. 14, 2963–2977 10.1111/j.1365-2486.2008.01688.x (doi:10.1111/j.1365-2486.2008.01688.x) [DOI] [Google Scholar]
- 38.Teeri J. A., Stowe L. G. 1976. Climatic patterns and the distribution of C4 grasses in North America. Oecologia 23, 1–12 10.1007/BF00351210 (doi:10.1007/BF00351210) [DOI] [PubMed] [Google Scholar]
- 39.Rundel P. W. 1980. The ecological distribution of C4 and C3 grasses in the Hawaiian Islands. Oecologia 45, 354–359 10.1007/BF00540205 (doi:10.1007/BF00540205) [DOI] [PubMed] [Google Scholar]
- 40.Edwards E. J., Still C. J. 2008. Climate, phylogeny and the ecological distribution of C4 grasses. Ecol. Lett. 11, 266–276 10.1111/j.1461-0248.2007.01144.x (doi:10.1111/j.1461-0248.2007.01144.x) [DOI] [PubMed] [Google Scholar]
- 41.Edwards E. J., Smith S. A. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proc. Natl Acad. Sci. USA 107, 2532–2537 10.1073/pnas.0909672107 (doi:10.1073/pnas.0909672107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osborne C. P., Freckleton R. P. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proc. R. Soc. B 276, 1753–1760 10.1098/rspb.2008.1762 (doi:10.1098/rspb.2008.1762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellogg E. A. 2001. Evolutionary history of the grasses. Plant Physiol. 125, 1198–1205 10.1104/pp.125.3.1198 (doi:10.1104/pp.125.3.1198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze E.-D., Ellis R., Schulze W., Trimborn P., Ziegler H. 1996. Diversity, metabolic types and 13C carbon isotope ratios in the grass flora of Namibia in relation to growth form, precipitation and habitat conditions. Oecologia 106, 352–369 10.1007/BF00334563 (doi:10.1007/BF00334563) [DOI] [PubMed] [Google Scholar]
- 45.Ruddiman W. J. 2007. Earth's Climate: past and future, 2nd edn. New York, NY: W. H. Freeman [Google Scholar]
- 46.Pound M. J., Haywood A. M., Salzmann U., Riding J. B., Lunt D. J., Hunter S. J. 2011. A Tortonian (Late Miocene, 11.61–7.25 Ma) global vegetation reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 300, 29–45 10.1016/j.palaeo.2010.11.029 (doi:10.1016/j.palaeo.2010.11.029) [DOI] [Google Scholar]
- 47.Sankaran M., et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849 10.1038/nature04070 (doi:10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 48.Tipple B. J., Pagani M. 2007. The early origins of terrestrial C4 photosynthesis. Annu. Rev. Earth Planet Sci. 35, 435–461 10.1146/annurev.earth.35.031306.140150 (doi:10.1146/annurev.earth.35.031306.140150) [DOI] [Google Scholar]
- 49.Lehmann C. E. R., Archibald S. A., Hoffmann W. A., Bond W. J. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209 10.1111/j.1469-8137.2011.03689.x (doi:10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 50.Beerling D. J. 1999. New estimates of carbon transfer to terrestrial ecosystems between the last glacial maximum and the Holocene. Terra Nova 11, 162–167 10.1046/j.1365-3121.1999.00240.x (doi:10.1046/j.1365-3121.1999.00240.x) [DOI] [Google Scholar]
- 51.Bond W. J., Midgley G. F. 2000. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Change Biol. 6, 865–869 10.1046/j.1365-2486.2000.00365.x (doi:10.1046/j.1365-2486.2000.00365.x) [DOI] [Google Scholar]
- 52.Beerling D. J., Osborne C. P. 2006. The origin of the savannah biome. Glob. Change Biol. 12, 2023–2031 10.1111/j.1365-2486.2006.01239.x (doi:10.1111/j.1365-2486.2006.01239.x) [DOI] [Google Scholar]
- 53.Bond W. J., Midgley G. F. 2012. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Phil Trans. R. Soc. B 367, 601–612 10.1098/rstb.2011.0182 (doi:10.1098/rstb.2011.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams-Linera G., Domínguez-Gastelú V., García-Zurita M. E. 1998. Microenvironment and floristics of different edges in a fragmented tropical rainforest. Conserv. Biol. 12, 1091–1102 10.1046/j.1523-1739.1998.97262.x (doi:10.1046/j.1523-1739.1998.97262.x) [DOI] [Google Scholar]
- 55.Davies-Colley R. J., Payne G. W., van Elswijk M. 2000. Microclimate gradients across a forest edge. New Zeal. J. Ecol. 24, 111–121 [Google Scholar]
- 56.Buckley T. N. 2005. The control of stomata by water balance. New Phytol. 168, 275–292 10.1111/j.1469-8137.2005.01543.x (doi:10.1111/j.1469-8137.2005.01543.x) [DOI] [PubMed] [Google Scholar]
- 57.Taylor S. H., Ripley B. S., Woodward F. I., Osborne C. P. 2011. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell Environ. 34, 65–75 10.1111/j.1365-3040.2010.02226.x (doi:10.1111/j.1365-3040.2010.02226.x) [DOI] [PubMed] [Google Scholar]
- 58.Taylor S. H., Hulme S. P., Rees M., Ripley B. S., Woodward F. I., Osborne C. P. 2010. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytol. 185, 780–791 10.1111/j.1469-8137.2009.03102.x (doi:10.1111/j.1469-8137.2009.03102.x) [DOI] [PubMed] [Google Scholar]
- 59.Farquhar G. D., von Caemmerer S., Berry J. A. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 10.1007/BF00386231 (doi:10.1007/BF00386231) [DOI] [PubMed] [Google Scholar]
- 60.Downes R. W. 1969. Differences in transpiration rates between tropical and temperate grasses under controlled conditions. Planta 88, 261–273 10.1007/BF00385069 (doi:10.1007/BF00385069) [DOI] [PubMed] [Google Scholar]
- 61.Pearcy R. W., Ehleringer J. 1983. Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ. 7, 1–13 10.1111/j.1365-3040.1984.tb01194.x (doi:10.1111/j.1365-3040.1984.tb01194.x) [DOI] [Google Scholar]
- 62.Ward J. K., Tissue D. T., Thomas R. B., Strain B. R. 1999. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Glob. Change Biol. 5, 857–867 10.1046/j.1365-2486.1999.00270.x (doi:10.1046/j.1365-2486.1999.00270.x) [DOI] [Google Scholar]
- 63.Cowling S. A., Sage R. F. 1998. Interactive effects of low atmospheric CO2 and elevated temperature on growth, photosynthesis and respiration in Phasaeolus vulgaris. Plant Cell Environ. 21, 427–435 10.1046/j.1365-3040.1998.00290.x (doi:10.1046/j.1365-3040.1998.00290.x) [DOI] [Google Scholar]
- 64.Anderson L. J., Maherali H., Johnson H. B., Polley H. W., Jackson R. B. 2001. Gas exchange and photosynthetic acclimation over subambient to elevated CO2 in a C3–C4 grassland. Glob. Change Biol. 7, 693–707 10.1046/j.1354-1013.2001.00438.x (doi:10.1046/j.1354-1013.2001.00438.x) [DOI] [Google Scholar]
- 65.Pinto H., Tissue D. T., Ghannoum O. 2011. Panicum milioides (C3–C4) does not have improved water or nitrogen economies relative to C3 and C4 congeners exposed to industrial-age climate change. J. Exp. Bot. 62, 3223–3234 (doi:10.1093/jxb/err005) [DOI] [PubMed] [Google Scholar]
- 66.Wand S. J. E., Midgley G. F., Jones M. H., Curtis P. S. 1999. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Glob. Change Biol. 5, 723–741 10.1046/j.1365-2486.1999.00265.x (doi:10.1046/j.1365-2486.1999.00265.x) [DOI] [Google Scholar]
- 67.Ainsworth E. A., Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 10.1111/j.1365-3040.2007.01641.x (doi:10.1111/j.1365-3040.2007.01641.x) [DOI] [PubMed] [Google Scholar]
- 68.Morison J. I. L., Gifford R. M. 1983. Stomatal sensitivity to carbon dioxide and humidity: a comparison of two C3 and two C4 grass species. Plant Physiol. 71, 789–796 10.1104/pp.71.4.789 (doi:10.1104/pp.71.4.789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holloway-Phillips M.-M., Brodribb T. J. 2011. Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant Cell Environ. 34, 302–313 10.1111/j.1365-3040.2010.02244.x (doi:10.1111/j.1365-3040.2010.02244.x) [DOI] [PubMed] [Google Scholar]
- 70.Muhaidat R., Sage T. L., Frohlich M. W., Dengler N. G., Sage R. F. 2011. Characterization of C3-C4 intermediate species in the genus Heliotropium L. (Boraginaceae): anatomy, ultrastructure and enzyme activity. Plant Cell Environ. 34, 1723–1736. (doi:10.1111/j.1365-3040.2011.02367.x) [DOI] [PubMed] [Google Scholar]
- 71.Sage R. F. 2001. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Ecol. 3, 202–213 10.1055/s-2001-15206 (doi:10.1055/s-2001-15206) [DOI] [Google Scholar]
- 72.McKown A. D., Dengler N. G. 2007. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am. J. Bot. 94, 382–399 10.3732/ajb.94.3.382 (doi:10.3732/ajb.94.3.382) [DOI] [PubMed] [Google Scholar]
- 73.McDowell N. G., et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive whilst others succumb to drought? New Phytol. 178, 719–739 10.1111/j.1469-8137.2008.02436.x (doi:10.1111/j.1469-8137.2008.02436.x) [DOI] [PubMed] [Google Scholar]
- 74.McDowell N. G. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 155, 1051–1059 10.1104/pp.110.170704 (doi:10.1104/pp.110.170704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell C. D., Sage R. F., Kocacinar F., Way D. A. 2005. Estimation of the whole-plant CO2 compensation point of tobacco (Nicotiana tabacum L.). Glob. Change Biol. 11, 1956–1967 10.1111/j.1365-2486.2005.01045.x (doi:10.1111/j.1365-2486.2005.01045.x) [DOI] [Google Scholar]
- 76.Uhl D., Mosbrugger V. 1999. Leaf venation density as a climate and environmental proxy: a critical review and new data. Paleogeogr. Paleoclimatol. Paleoecol. 149, 15–26 10.1016/S0031-0182(98)00189-8 (doi:10.1016/S0031-0182(98)00189-8) [DOI] [Google Scholar]
- 77.Sack L., Frole K. 2006. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87, 483–491 10.1890/05-0710 (doi:10.1890/05-0710) [DOI] [PubMed] [Google Scholar]
- 78.Brodribb T. J., Feild T. S. 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175–183 10.1111/j.1461-0248.2009.01410.x (doi:10.1111/j.1461-0248.2009.01410.x) [DOI] [PubMed] [Google Scholar]
- 79.Dunbar-Co S., Sporck M. J., Sack L. 2009. Leaf trait diversification and design in seven rare taxa of the Hawaiian Plantago radiation. Int. J. Plant Sci. 170, 61–75 10.1086/593111 (doi:10.1086/593111) [DOI] [Google Scholar]
- 80.Feild T. S., et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl Acad. Sci. USA 108, 8363–8366 10.1073/pnas.1014456108 (doi:10.1073/pnas.1014456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogle K. 2003. Implications of interveinal distance for quantum yield in C4 grasses: a modeling and meta-analysis. Oecologia 136, 532–542 10.1007/s00442-003-1308-2 (doi:10.1007/s00442-003-1308-2) [DOI] [PubMed] [Google Scholar]
- 82.McKown A. D., Dengler N. G. 2010. Vein patterning and evolution in C4 plants. Botany-Botanique 88, 775–786 10.1139/B10-055 (doi:10.1139/B10-055) [DOI] [Google Scholar]
- 83.Philpott J. 1953. A blade tissue study of leaves of 47 species of Ficus. Bot. Gaz. 115, 15–35 10.1086/335794 (doi:10.1086/335794) [DOI] [Google Scholar]
- 84.Fisher D. D., Schenk H. J., Thorsch J. A., Ferren W. R. 1997. Leaf anatomy and subgeneric affiliations of C3 and C4 species of Suaeda (Chenopodiaceae) in North America. Am. J. Bot. 84, 1198–1210 10.2307/2446043 (doi:10.2307/2446043) [DOI] [PubMed] [Google Scholar]
- 85.Boughton V. H. 1986. Phyllode structure, taxonomy and distribution in some Australian Acacias. Aust. J. Bot. 34, 663–674 10.1071/BT9860663 (doi:10.1071/BT9860663) [DOI] [Google Scholar]
- 86.Voznesenskaya E. V., Koteyeva N. K., Edwards G. E., Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3-C4 intermediate in genus Portulaca L. (Portulacaceae). J. Exp. Bot. 61, 3647–3662 10.1093/jxb/erq178 (doi:10.1093/jxb/erq178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arakaki M., Christin P.-A., Nyffeler R., Lendel A., Eggli U., Ogburn R. M., Spriggs E., Moore M. J., Edwards E. J. 2011. Contemporaneous and recent radiations of the world's major succulent plant lineages. Proc. Natl Acad. Sci. USA 108, 8379–8384 10.1073/pnas.1100628108 (doi:10.1073/pnas.1100628108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vogan P. J., Frohlich M. W., Sage R. F. 2007. The functional significance of C3-C4 intermediate traits in Heliotropium L. (Boraginaeae): gas exchange perspectives. Plant Cell Environ. 10, 1337–1345 10.1111/j.1365-3040.2007.01706.x (doi:10.1111/j.1365-3040.2007.01706.x) [DOI] [PubMed] [Google Scholar]
- 89.Monson R. K., Schuster W. S., Ku M. S. B. 1987. Photosynthesis in Flaveria brownii A.M. Powell. A C4-like C3-C4 intermediate. Plant Physiol. 85, 1063–1067 10.1104/pp.85.4.1063 (doi:10.1104/pp.85.4.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gong C. M., Bai J., Deng J. M., Wang G. X., Liu X. P. 2011. Leaf anatomy and photosynthetic carbon metabolic characteristics in Phragmites communis in different soil water availability. Plant Ecol. 212, 675–687 10.1007/s11258-010-9854-2 (doi:10.1007/s11258-010-9854-2) [DOI] [Google Scholar]
- 91.Schulze E.-D., Hall A. E. 1982. Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In Encyclopedia of plant physiology, vol. 12B: physiological plant ecology II: Water relations and carbon asssimilation (eds Lange O. L., Nobel P. S., Osmond C. B., Ziegler H.), Berlin, Germany: Springer [Google Scholar]
- 92.Ibrahim D. G., Gilbert M. E., Ripley B. S., Osborne C. P. 2008. Seasonal differences in photosynthesis between the C3 and C4 subspecies of Alloteropsis semialata are offset by frost and drought. Plant Cell Environ. 31, 1038–1050 10.1111/j.1365-3040.2008.01815.x (doi:10.1111/j.1365-3040.2008.01815.x) [DOI] [PubMed] [Google Scholar]
- 93.Kocacinar F., Sage R. F. 2003. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant Cell Environ. 26, 2015–2026 10.1111/j.1365-2478.2003.01119.x (doi:10.1111/j.1365-2478.2003.01119.x) [DOI] [PubMed] [Google Scholar]
- 94.Kocacinar F., Sage R. F. 2004. Photosynthetic pathway alters hydraulic structure and function in woody plants. Oecologia 139, 214–223 10.1007/s00442-004-1517-3 (doi:10.1007/s00442-004-1517-3) [DOI] [PubMed] [Google Scholar]
- 95.Kocacinar F., McKown A. D., Sage T. L., Sage R. F. 2008. Photosynthetic pathway influences xylem structure and function in Flaveria (Asteraceae). Plant Cell Environ. 31, 1363–1376 10.1111/j.1365-3040.2008.01847.x (doi:10.1111/j.1365-3040.2008.01847.x) [DOI] [PubMed] [Google Scholar]
- 96.Sack L., Cowan P. D., Jaikumar N., Holbrook N. M. 2003. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ. 26, 1343–1356 10.1046/j.0016-8025.2003.01058.x (doi:10.1046/j.0016-8025.2003.01058.x) [DOI] [Google Scholar]
- 97.Sack L., Holbrook N. M. 2006. Leaf hydraulics. Annu. Rev. Plant Biol. 57, 361–381 10.1146/annurev.arplant.56.032604.144141 (doi:10.1146/annurev.arplant.56.032604.144141) [DOI] [PubMed] [Google Scholar]
- 98.Brodribb T. J. 2009. Xylem hydraulic physiology: the functional backbone of terrestrial plant productivity. Plant Sci. 177, 245–251 10.1016/j.plantsci.2009.06.001 (doi:10.1016/j.plantsci.2009.06.001) [DOI] [Google Scholar]
- 99.Brodribb T. J., Cochard H. 2009. Hydraulic failure defines the recovery and point of death in water stressed conifers. Plant Physiol. 149, 575–584 10.1104/pp.108.129783 (doi:10.1104/pp.108.129783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson D. M., McCulloh K. A., Meinzer F. C., Woodruff D. R., Eissenstat D. M. 2011. Hydraulic patterns and safety margins, from stem to stomata, in three eastern US tree species. Tree Physiol. 31, 659–668 10.1093/treephys/tpr050 (doi:10.1093/treephys/tpr050) [DOI] [PubMed] [Google Scholar]
- 101.Martre P., Cochard H., Durand J. L. 2001. Hydraulic architecture and water flow in growing grass tillers (Festuca arundinacea Schreb.). Plant Cell Environ. 24, 65–76 10.1046/j.1365-3040.2001.00657.x (doi:10.1046/j.1365-3040.2001.00657.x) [DOI] [Google Scholar]
- 102.Brodribb T. J., Feild T. S., Jordan G. J. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144, 1890–1898 10.1104/pp.107.101352 (doi:10.1104/pp.107.101352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKown A. D., Cochard H., Sack L. 2010. Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am. Nat. 175, 447–460 10.1086/650721 (doi:10.1086/650721) [DOI] [PubMed] [Google Scholar]
- 104.Osmond C. B., Winter K., Ziegler H. 1982. Functional significance of different pathways of CO2 fixation in photosynthesis. In Encyclopedia of plant physiology: new series (eds Lange O. L., Nobel P. S., Osmond C. B., Ziegler H.), pp. 479–547 Berlin, Germany: Springer [Google Scholar]
- 105.Ripley B. S., Gilbert M. E., Ibrahim D. G., Osborne C. P. 2007. Drought constraints on C4 photosynthesis: stomatal and metabolic limitations in C3 and C4 subspecies of Alloteropsis semialata. J. Exp. Bot. 58, 1351–1363 10.1093/jxb/erl302 (doi:10.1093/jxb/erl302) [DOI] [PubMed] [Google Scholar]
- 106.Ripley B., Frole K., Gilbert M. E. 2010. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Ann. Bot. 105, 493–503 10.1093/aob/mcp307 (doi:10.1093/aob/mcp307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leakey A. D. B., Lau J. A. 2012. Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric [CO2]. Phil Trans. R. Soc. B 367, 613–629 10.1098/rstb.2011.0248 (doi:10.1098/rstb.2011.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sperry J. S. 2003. Evolution of water transport and xylem structure. Int. J. Plant Sci. 164, S115–S127 10.1086/368398 (doi:10.1086/368398) [DOI] [Google Scholar]
- 109.Beerling D. J., Osborne C. P., Chaloner W. G. 2001. Evolution of leaves in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410, 352–354 10.1038/35066546 (doi:10.1038/35066546) [DOI] [PubMed] [Google Scholar]
- 110.Osborne C. P., Beerling D. J., Lomax B. H., Chaloner W. G. 2004. Biophysical constraints on the origin of leaves inferred from the fossil record. Proc. Natl Acad. Sci. USA 101, 10 360–10 362 10.1073/pnas.0402787101 (doi:10.1073/pnas.0402787101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bond W. J. 1989. The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 36, 227–249 10.1111/j.1095-8312.1989.tb00492.x (doi:10.1111/j.1095-8312.1989.tb00492.x) [DOI] [Google Scholar]
- 112.Haworth M., Elliott-Kingston C., McElwain J. C. 2011. Stomatal control as a driver of plant evolution. J. Exp. Bot. 62, 2419–2423 10.1093/jxb/err086 (doi:10.1093/jxb/err086) [DOI] [PubMed] [Google Scholar]
- 113.Monteith J. L., Unsworth M. 1990. Principles of environmental physics, p. 291 London, UK: Edward Arnold [Google Scholar]
- 114.Jones H. G. 1992. Plants and microclimate, p. 428, 2nd edn. Cambridge, UK: Cambridge University Press [Google Scholar]
- 115.Penman H. L. 1948. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. Lond. A 194, 120–145 10.1098/rspa.1948.0037 (doi:10.1098/rspa.1948.0037) [DOI] [PubMed] [Google Scholar]
- 116.Monteith J. L. 1965. Evaporation and environment. Symp. Soc. Exp. Biol. 19, 205–234 [PubMed] [Google Scholar]
- 117.Friend A. D. 1995. PGEN - an integrated model of leaf photosynthesis, transpiration, and conductance. Ecol. Model. 77, 233–255 10.1016/0304-3800(93)E0082-E (doi:10.1016/0304-3800(93)E0082-E) [DOI] [Google Scholar]
- 118.Wei C. F., Tyree M. T., Steudle E. 1999. Direct measurement of xylem pressure in leaves of intact maize plants. A test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol. 121, 1191–1205 10.1104/pp.121.4.1191 (doi:10.1104/pp.121.4.1191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tyree M. T., Zimmermann M. H. 2002. Xylem structure and the ascent of sap. Berlin, Germany: Springer [Google Scholar]
- 120.Brodribb T. J., McAdam S. A. M. 2011. Passive origins of stomatal control in vascular plants. Science 331, 582–585 10.1126/science.1197985 (doi:10.1126/science.1197985) [DOI] [PubMed] [Google Scholar]
- 121.Scoffoni C., Rawls M., McKown A. D., Cochard H., Sack L. 2011. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156, 832–843 10.1104/pp.111.173856 (doi:10.1104/pp.111.173856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wittwer J. W. 2011. Excel solver examples. See http://www.vertex42.com/ExcelArticles/excel-solver-examples.html
- 123.Pasquet-Kok J., Creese C., Sack L. 2010. Turning over a new ‘leaf’: multiple functional significances of leaves versus phyllodes in Hawaiian Acacia koa. Plant Cell Environ. 33, 2084–2100 10.1111/j.1365-3040.2010.02207.x (doi:10.1111/j.1365-3040.2010.02207.x) [DOI] [PubMed] [Google Scholar]
- 124.Vico G., Porporato A. 2008. Modelling C3 and C4 photosynthesis under water-stressed conditions. Plant Soil. 313, 187–203 10.1007/s11104-008-9691-4 (doi:10.1007/s11104-008-9691-4) [DOI] [Google Scholar]
- 125.Meidner H., Mansfield T. A. 1968. Physiology of stomata. Maidenhead, UK: McGraw-Hill [Google Scholar]
- 126.Dubbe D. R., Farquhar G. D., Raschke K. 1978. Effect of abscisic acid on the gain of the feedback loop involving carbon dioxide and stomata. Plant Physiol. 62, 413–417 10.1104/pp.62.3.413 (doi:10.1104/pp.62.3.413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aasamaa K., Sõber S. 2011. Stomatal sensitivities to changes in leaf water potential, air humidity, CO2 concentration and light intensity, and the effect of abscisic acid on the sensitivities in six temperate deciduous tree species. Environ. Exp. Bot. 71, 72–78 10.1016/j.envexpbot.2010.10.013 (doi:10.1016/j.envexpbot.2010.10.013) [DOI] [Google Scholar]