Abstract
In response to short-term fluctuations in atmospheric CO2 concentration, ca, plants adjust leaf diffusive conductance to CO2, gc, via feedback regulation of stomatal aperture as part of a mechanism for optimizing CO2 uptake with respect to water loss. The operational range of this elaborate control mechanism is determined by the maximum diffusive conductance to CO2, gc(max), which is set by the size (S) and density (number per unit area, D) of stomata on the leaf surface. Here, we show that, in response to long-term exposure to elevated or subambient ca, plants alter gc(max) in the direction of the short-term feedback response of gc to ca via adjustment of S and D. This adaptive feedback response to ca, consistent with long-term optimization of leaf gas exchange, was observed in four species spanning a diverse taxonomic range (the lycophyte Selaginella uncinata, the fern Osmunda regalis and the angiosperms Commelina communis and Vicia faba). Furthermore, using direct observation as well as flow cytometry, we observed correlated increases in S, guard cell nucleus size and average apparent 1C DNA amount in epidermal cell nuclei with increasing ca, suggesting that stomatal and leaf adaptation to ca is linked to genome scaling.
Keywords: leaf gas exchange, photosynthesis, plant evolution, gas exchange capacity, transpiration, vein density
1. Introduction
As an important greenhouse gas and the substrate for photosynthesis, atmospheric CO2 concentration, ca, is one of several important components in the feedback interaction between vegetation and the atmosphere [1,2]. Throughout the history of plants (approx. 400 Myr) on land, ca is estimated to have fluctuated from as high as several thousand ppm in the Devonian [3], to as low as around 180 ppm in the Last Glacial Maximum (LGM) [4]. Currently, it stands at approximately 390 ppm (National Oceanic and Atmospheric Administration data) but could exceed 1000 ppm by the end of this century according to Intergovernmental Panel on Climate Change estimates. Pre-historic CO2 fluctuations have been linked to alternating greenhouse/icehouse global climates [3,5] as well as major episodes of plant evolution and diversification [6,7]. Along with current concerns about the impact of future elevated atmospheric CO2 concentrations on global climate, the direct effects of the ongoing rapid rise in ca on plant photosynthetic productivity and water use is likely to be significant [1,8,9].
Changes in ca have a direct and immediate effect on the rate of CO2 assimilation [10–13]. To counteract or mitigate these effects in the short term (seconds to minutes), plants employ a physiological feedback mechanism involving interaction between the diffusive conductance of the leaf epidermis, governed by stomata, and the rate of photosynthesis occurring in the mesophyll (figure 1) [14–16]. This short-term feedback mechanism controls the operational stomatal conductance to CO2, gc(op), at a value between zero and the maximum, gc(max). However, it is the physical attributes of the stomata that determine gc(max) (figure 2). At any given average guard cell turgor pressure Pg, gc(op) will be a function of the average stomatal aperture, a, the depth of the stomatal pore, l, and the number of stomata per unit area (or density, D) [17,18]. Ultimately, as Pg approaches its maximum and the a versus Pg relationship saturates, average stomatal aperture will reach its maximum, amax (figure 2a), causing gc(op) to reach its maximum, gc(max) (figure 2b). Because the overall size of stomata determine both amax and l, gc(max) can be described conveniently as a function of stomatal size, S, and D [19,20]. When gc(max) adapts to new environmental conditions (figure 2c), it necessarily involves adaptation of S and/or D [19,20].
Figure 1.
A schematic of the physiological feedback loop for stomatal response to CO2. The arrows trace the feedback signal flow following a change in atmospheric CO2 concentration, Δca, showing the effect on CO2 assimilation rate, A, via the stomatal response. The sloped lines in boxes represent the sensitivities of the key variables involved, indicating a generally positive or negative relationship between them: A, CO2 assimilation rate; ci, leaf intercellular CO2 concentration; gc, stomatal conductance to CO2. Overall, the final change in assimilation rate, ΔA(final), is the sum of the initial change occurring without any stomatal response, ΔA(initial), plus the change due to stomatal response, ΔA(stomatal). The negative relationship between gc and ci (box with dashed line border in the feedback loop) indicates that ΔA(stomatal) is opposite in sign to ΔA(initial).
Figure 2.
Guard cell pressure drives stomatal aperture and stomatal conductance. (a) Stomatal aperture, a and, consequently, (b) stomatal conductance to CO2 diffusion, gc, increase with guard cell pressure Pg in a saturation fashion. Stomatal aperture control is achieved through osmotic regulation of Pg. The operational gc, gc(op), is typically regulated via Pg within the high sensitivity region of the curve (double arrow). Maximum conductance, gc(max), is determined by the number and size of stomata (see equation (2.1)). Data are for Tradescantia virginiana, adapted from Franks & Farquhar [17]. (c) Illustrating how altering gc(max) keeps gc(op) in the high sensitivity region of the Pg versus gc curve. Starting at the initial operating point (point a), a sustained change in ca from ambient (amb.; solid line) to elevated CO2 concentration results in adjustment of gc to a lower gc(op) (point b). The plant then changes stomatal size and density in new leaves, altering the gc versus Pg curve (dashed line, elevated CO2; elev.), to return gc(op) to its optimal position (point c). Similarly, following a sustained shift from ambient to subambient ca, the plant adjusts gc to a higher gc(op) (point d). New leaves are produced with altered stomatal size and density, shifting to a new gc versus Pg curve (dotted line, subambient CO2; sub.) and increasing gc(max) to return gc(op) to its optimal position (point e). Curves in (c) are representative, based on (b).
(a). Stomatal conductance and CO2
Sustained exposure to elevated or subambient ca invokes feedback regulation of the biochemical capacity for CO2 assimilation [21–26]. Over a gradient of growth ca treatments from subambient to elevated CO2 concentration, the maximum rate of carboxylation, Vcmax, typically declines [24,26–28], consistent with a mechanism of adaptive feedback regulation of photosynthesis via adjustment of the amount of key photosynthetic enzymes [21,23]. Regulation of CO2 assimilation rate under changing ca therefore involves stomatal and non-stomatal feedbacks operating over several timescales. However, despite significant advances in understanding the non-stomatal feedbacks, much less is known about the long-term response of stomata to CO2 (weeks to millennia).
Our hypothesis is that over the long term, the leaf epidermis adapts to keep gc(op) and gc(max) aligned optimally with the leaf's biochemical capacity for carbon assimilation under the prevailing ca. Taking the short-term feedback response to ca as an analogue for the long term, we predict that following sustained exposure to a significant change in ca, new leaves or plants will exhibit altered gc(max) in the direction of the short-term gc(op) response, through adjustment of S and/or D. This adjustment is a practical necessity, ensuring that normal gc(op) is within the high sensitivity region of the gc(op) versus Pg curve (figure 2b). Furthermore, there is a strong body of evidence suggesting that both across and within species, S versus D forms a negative linear log–log relationship, such that higher gc(max) is achieved through smaller S and higher D [19,20,29,30]. We predict, therefore, that in plants grown under low ca, gc(max) will be higher, and this will be attributed to smaller S and higher D. If our hypothesis is correct, then over evolutionary timescales (millions of years), sustained shifts in ca could select for gc(max) on the basis of adaptation of S and D. Reconstructions of gc(max) from S and D in fossil leaves show a significant negative correlation with reconstructed ca over geological time [20], suggesting that gc(max) and ca have covaried in the long term. However, the pattern of adaptation of S, D and gc(max) has not been examined closely under independent control of ca.
(b). Stomatal size and genome size
Although S and D can be modified in many different combinations to alter gc(max), there are constraints. For example, allocation of more epidermal space to stomata may be at the expense of other important epidermal structures [20]. The tradeoffs involved in altering S and D may therefore be complex. There is, however, a readily apparent constraint with regard to changing S. The size of the guard cell nucleus is proportional to the width of the guard cell (figure 3a) resulting in a correlation between the two across species [31]. Because guard cell nuclei are typically diploid [32], this scaling of the size of a guard cell and its nucleus leads to a correlation between stomatal size and plant genome size (or C-value, measured as haploid or 1C DNA amount) [31,33,34]. This suggests that the large changes in S over geological time were accompanied by changes in plant genome size, and that both S and plant genome size co-evolved with ca. This of course would not mean that ca alone drove the evolution of S and/or plant genome size. Other environmental factors, including drought and light intensity, are known to affect S [19,35,36]. However, if S, gc(max) and 1C DNA respond to independent control of ca, then a firmer physiological framework can be established for the adaptation, natural selection and evolution of plants in relation to past and future changes in global ca. The objective of this study was to test for sensitivity, in the form predicted earlier, of gc(max), S and 1C DNA amount to controlled, long-term changes in ca, from a subambient concentration (180 ppm) approximating that in the LGM, to an elevated concentration (1000 ppm) predicted for the end of this century. The timescale of sensitivity in this case is that of plant growth and development, i.e. in the order of several weeks.
Figure 3.
Stomatal guard cells. (a) Guard cell nuclei. Fluorescing guard cell nuclei of V. faba after preparation with a DNA fluorphore (see Materials and Methods). Suspensions of similarly stained nuclei isolated from bulk leaf tissue were used to measure genome size (1C DNA amount). Scale bar, 20 µm. (b) Maximum stomatal aperture, amax. Fully inflated guard cells of C. communis forming maximum stomatal apertures approximating circular pores. This is common in angiosperms but less so in non-angiosperms. Scale bar, 50 µm.
2. Materials and methods
(a). Plant material and treatments
Plants were grown in controlled environment chambers under standard conditions: 1000 µmol m−2 s−1 photosynthetically active radiation (500 µmol m−2 s−1 for Selaginella uncinata); 10 h photoperiod; 25°C; and well-watered, commercial compost soil. Growth chamber CO2 concentration (ca) was controlled at 180 or 240 ppm for ‘subambient CO2’, representing glacial atmospheric conditions, 450 ppm for ‘ambient CO2’, representing current day conditions, and 1000 ppm for ‘elevated CO2’, projected to occur this century. Commelina communis and Vicia faba were grown from seed sown directly into pots in their respective growth chambers; Osmunda regalis was grown from spores and S. uncinata cloned by layering and dividing parent plants. All measurements are reported as the mean ± s.e. Significance of difference between means, at the 0.05 level, was determined with one-way analysis of variance (ANOVA) and post hoc means comparison tests (Tukey test) using standard statistical software (OriginPro v. 8.0, OriginLab Corporation, Northampton).
(b). Stomatal size and density
Epidermal peels were dissected from leaves and mounted on glass slides for viewing with a light microscope. Digital images of stomata were recorded at 200× and 630× magnification. Guard cell length and width were measured using image analysis software (ImageJ), and stomatal size (S) was calculated as guard cell length multiplied by the width of the guard cell pair (not including aperture) [20]. Guard cell dimensions were measured on 20 stomata from three epidermal peels from three plants per CO2 treatment (n = 60). Stomatal density (D) was calculated as the number of stomata per square millimetre of epidermis, measured in ten 0.09 mm2 fields on epidermis from three plants (n = 30). The volume of a single guard cell was approximated as that of a cylinder the same width as the guard cell, with hemispherical ends. A simplified volumetric measure of guard cell size, guard cell width cubed, is also reported.
(c). Maximum stomatal conductance, gc(max)
Maximum stomatal conductance to CO2 (gc(max), mol m−2 s−1) was estimated using a modified version of the Brown and Escombe equation [17,18,20]:
 |
2.1 |
where dc is the diffusivity of CO2 in air (m2 s−1), D is the stomatal density (m−2), amax is the mean maximum stomatal pore area (m2), v is the molar volume of air (m3 mol−1), l is the depth of the stomatal pore (m, approximated as the width of a single guard cell [37]) and π is the mathematical constant (typically rounded to 3.142).
Note that amax relates to fully inflated guard cells that, for many species, can form stomatal pores approximating circular dimensions (figure 3b). It is therefore convenient in some cases to estimate amax as the area of a circle, π(p/2)2, where p is the stomatal pore length [20]. Alternatively, where images of stomata with fully inflated guard cells are available, either for the species of interest or for a species with similar guard cell geometry, amax can be estimated as a fraction α of S, i.e. amax = αS [19,20]. We adopted the latter approach here, estimating amax as 0.08S for S. uncinata (based on the lycophyte Huperzia prolifera [37]), 0.1S for O. regalis (based on the fern Nephrolepis exaltata [37]) and 0.33S for both C. communis and V. faba (based on the herbaceous angiosperm Tradescantia virginiana [37]). Importantly, amax and hence gc(max) occur only under the somewhat artificial conditions that promote fully inflated guard cells, such as the combination of low atmospheric CO2 concentration (below that at which plants grew), high (photosynthesis-saturating) light intensity and 100 per cent humidity, or for certain in vitro preparations using epidermal peels [37]. Under more typical conditions, the highest operating stomatal conductance, gc(op), is likely to be around half of gc(max) [19]. Owing to the infinite combination of natural conditions under which gc could be operating, gc(max) is a more informative standard measure of the capacity of the leaf epidermis to support the diffusion of CO2 from the atmosphere to the leaf interior, as it is determined solely on the basis of static anatomical dimensions.
(d). DNA flow cytometry
Plant nuclear genome size was estimated for bulk epidermal tissue (in epidermal peels) using flow cytometry following the method described in Doležel et al. [38], using LB01 buffer and the DNA fluorophore propidium iodide. To minimize potential error due to variations in the standard, the same tissue was used as the standard across treatments for each species. For O. regalis and V. faba, the standard was Allium cepa cv. ‘Ailsa Craig’, and for C. communis it was Solanum lycopersicum cv. ‘Gardener's Delight’, propagated from seed at the Royal Botanic Gardens, Kew. The mean 1C-value includes both epidermal and guard cell nuclei. Guard cell and nucleus size were measured in fresh epidermal peels of O. regalis, C. communis and V. faba using standard light and fluorescence microscopy techniques (excited with 488 nm wavelength light, and detected with a 562–588 nm band pass filter), after treating peels with the same fluorescent nuclear stain and buffer as in the flow cytometry. Guard cell nuclei of S. uncinata could not be viewed clearly with this method and were not examined.
3. Results and discussion
(a). Stomatal size, density and gc(max) response to CO2
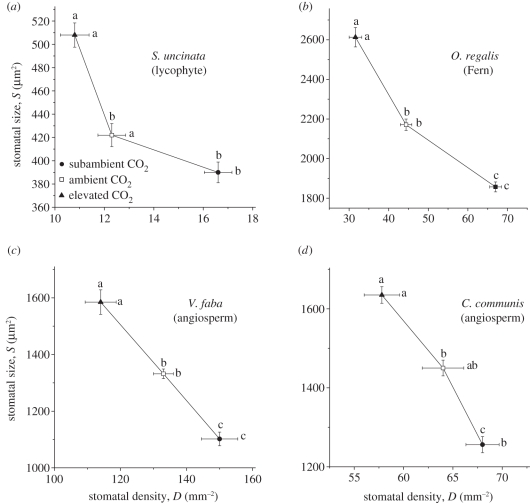
The results show a consistent pattern of correlated adaptation of S and D to ca that may serve as a physiological framework for adaptation, selection and evolution of gc(max) under changing global ca regimes. In response to growth across a gradient of increasing ca, all four species developed leaves with increasingly large guard cells and lower stomatal density (figure 4). This translated into lower gc(max) with increasing ca, as calculated from S and D measurements using equation (2.1) (figure 5). In all cases, gc(max) and D were significantly lower, and S significantly larger, at elevated relative to subambient ca. The consistent response across these four species, representing a broad taxonomic range (lycophyte, fern and angiosperm), suggests that this mode of adaptation to ca is widespread in vascular plants and its genetic basis may therefore be highly conserved through plant lineages.
Figure 4.
Stomatal size and density covary with CO2. (a–d) Negative relationship between stomatal size (S) and density (D) in (a) S. uncinata (lycophyte), (b) O. regalis (Fern), (c) V. faba (angiosperm) and (d) C. communis (angiosperm), respectively, when grown at three different atmospheric CO2 concentrations. Treating ca as a continuous variable, each datum point (mean ± s.e.m.) is connected by lines in the order of highest to lowest ca treatment. (a–d) Stomata were significantly smaller and more numerous at subambient compared with elevated atmospheric CO2 concentration. Means with different adjacent letters, along respective axes, are significantly different. Filled circles, subambient CO2; unfilled squares, ambient CO2; filled triangles, elevated CO2.
Figure 5.
Maximum stomatal conductance, gc(max), is altered by CO2. The observed changes in stomatal size and density (figure 4) translate to significant changes in gc(max) when calculated from stomatal dimensions using equation (2.1). (a–d) gc(max) decreases with atmospheric CO2 concentration in S. uncinata, O. regalis, V. faba and C. communis, respectively. (a–d) gc(max) in plants grown at elevated atmospheric CO2 concentration is significantly lower than in plants grown at subambient CO2 concentration. Symbols are the same as for figure 4; mean ± s.e.m.
The CO2-induced shift in gc(max) is in the direction of the short-term feedback adjustment of operational stomatal conductance resulting from stomatal aperture changes in response to CO2 [14]. This correction of gc(max) via anatomical adjustment of S and D indicates a realignment of the gc versus Pg relationship (figure 2b) that keeps the new operational gc in the same region of the curve as before the CO2 change. This adaptive feedback response is consistent with a general mechanism of feedback adaptation of operational stomatal conductance and gc(max) to environmental changes. For example, in response to growth under treatment with the drought hormone abscisic acid, Tradescantia virginiana leaves altered S and D, which lowered gc(max) and increased water-use efficiency, while maintaining operational gc in the same region of the gc versus Pg curve [17].
Although the response to ca was significant, the change in gc(max) resulting from adaptation of S and D was less than 10 per cent in three of the four species (S. uncinata, V. faba and C. communis) over the subambient to elevated CO2 gradient. In O. regalis, it was closer to 40 per cent. The sensitivity of gc(max) to growth at different ca is therefore variable and may, in some cases, be small or negligible. This is reflected in a survey of stomatal density response [26], where the change from ambient (mean 363 ppm) to elevated (mean 571 ppm) ca was a mean reduction of 5 per cent, with the most frequent response being a reduction of less than 10 per cent. The same survey reported a reduction in operating stomatal conductance of 22 per cent, suggesting partial stomatal closure in addition to a reduction in D. However, the magnitude of the ca treatment or change should be considered when assessing the sensitivity of S, D and gc(max) to ca. The survey by Ainsworth & Rogers [26] spanned a 1.5-fold range of ca, averaging −2.4 per cent change in D per 100 ppm; in this study the ca treatment spanned a fivefold range, averaging –3.2 per cent change in D per 100 ppm; and for the survey of fossils in Franks & Beerling [20], where ca spanned a greater than eightfold range over 400 Myr, there was an average −4.2 per cent change in D per 100 ppm increase in ca (noting that the response over large ranges of ca appears to be nonlinear [20]). The sensitivity of D to ca is therefore typically in the order of minus a few per cent per 100 ppm increase in CO2, but this may be difficult to detect over smaller ranges of CO2, both in studies of the geological record and in experimental treatments. Furthermore, the response appears to be nonlinear over a large range in ca [20,39], with greater sensitivity at low ca. The study of herbarium samples by Woodward [40], spanning the approximately 100 ppm rise in ca over the last 200 years (i.e. subambient conditions), reports a −41 per cent change in D and a −61 per cent change for growth chamber experiments simulating similar conditions. This is supported by a recent study of leaf material preserved in peat deposits, together with herbarium samples, for the same period [30], which shows an average −28 per cent change in D.
A negative relationship between S and D appears to characterize the general adaptation, plasticity or variability in gc(max) in response to various environmental variables. It is expressed within the canopy of individual species as part of the natural variability in gc(max) [19] and holds for the pooled data of hundreds of fossil species across the geological record and the present [20]. Adaptation or selection of gc(max) by large global changes in ca appears to operate within a general framework of down- or upregulation along a negative S versus D relationship in order to optimize plant carbon/water balance.
(b). Stomatal size and nucleus response to CO2
Direct observations of guard cell nucleus size, made following fluorescent DNA staining, indicate significant increases in nucleus volume at elevated relative to subambient CO2 concentration in all three species (figure 6a–c). Accompanying these changes were correlative increases in the volume of the guard cells (figure 6d), with guard cell volume and nucleus volume both significantly larger at elevated compared with subambient ca. This pattern is reflected as an increase in the apparent 1C DNA amount in epidermal tissue with increasing growth ca (figure 7a–c). In all three species, 1C DNA amount in epidermal tissue was significantly greater at elevated relative to subambient ca. This suggests that the physiological adaptation of stomatal size to ca involves a coordinated change in guard cell nucleus size and genome size.
Figure 6.
Atmospheric CO2 influences the size of guard cell nuclei. Guard cell nuclei are significantly larger in plants grown under elevated compared with subambient atmospheric CO2 concentration for (a) O. regalis, (b) C. comunis and (c) V. faba. (d) Guard cell nucleus volume increasing with guard cell volume for all three species grown under the different atmospheric CO2 concentrations. Symbols are as given for figure 4; in (d) the solid line is O. regalis, dashed line is C. comunis and dotted line is V. faba; mean ± s.e.m.
Figure 7.
Effect of growth CO2 on genome size. 1C DNA amount in epidermal tissue is significantly larger in plants grown at elevated compared with subambient atmospheric CO2 concentration for (a) O. regalis, (b) C. communis and (c) V. faba. To illustrate the relationship to guard cell size, 1C DNA amount is plotted against guard cell width cubed, a simplified volumetric measure of guard cell size. Symbols are as given for figure 4; mean ± s.e.m.
In contrast to the clear physiological consequences of adaptation of S, D and gc(max) to ca, the physiological benefit of an accompanying change in nucleus size or genome size is unclear. The minimum size of guard cells is of course constrained physically by the size of the guard cell nucleus (small guard cells have small nuclei), but the close coupling between guard cell size, nucleus size and apparent genome size in figures 6 and 7 appears to relate to subcellular scaling processes rather than the diffusion physics of leaf gas exchange. It has long been observed that there is a fundamental positive correlation between cell size (specifically the cytoplasm volume) and the size of the cell nucleus, corresponding broadly to a correlation between cell size and genome size [41–43]. The underlying mechanism for this has been the subject of considerable debate [43,44], but one model with an advanced biophysical basis is the ‘skeletal DNA theory’ [43]. The principle of the skeletal DNA theory is that the correlation of cell cytoplasm and nucleus volume maintains a balance between the rate of protein synthesis (cytoplasm-based) and the rate of RNA synthesis and processing (nucleus-based). It is possible that this energetic balancing of cell size and genome size applies to small, plastic changes in cell size within species, as observed in this study with guard cells. However, considerably more work is required to prove this. Until recently, even the possibility of variation in genome size within species (variation between populations and within individuals, as observed in this study) was hotly debated, but there is now overwhelming evidence to show that variability and plasticity in genome size is common within species [45]. Assuming that within-species differences in genome size are linked to the fitness of the phenotype, as shown here in relation to the feedback adjustment of gc(max) involving guard cell size, then it is possible for natural selection to act on these individuals, i.e. for ca to select for genome size [31].
The size of the nucleus comprises the total amount of DNA in the nucleus (i.e. genome size, or ‘C-value’) and the degree of chromatin packing, and our results suggest that ca may play a role in influencing either one or both of these. Over the short time frames of the CO2 experiments (up to three months), the observed increase in guard cell nuclear volume at elevated ca (figure 6a–c) is likely mediated through changes in the states of euchromatin and/or heterochromatin, which are known to be dynamic and responsive to developmental and environmental cues [46,47]. The results may indicate an epigenetic response [48], whereby adaptation of guard cell size to ca to facilitate the optimization of leaf gas exchange invokes chromatin repackaging possibly mediated via RNA interference [49]. The qualitatively consistent results across species suggest at least that ca is an external cue to which the plant nucleus responds, either directly or as a correlated character with guard cell size. An alternative possibility is that the differences in the amount of DNA detected result from altered binding of the fluorochrome to the DNA owing to changes in the condensation state of chromatin [50], rather than absolute changes in genome size. However, it is noteworthy that the same result, as predicted from physiological and biophysical theory, was observed across three species.
4. Conclusions
The results provide a physiological framework for analysing and predicting the adaptation of stomata and maximum leaf diffusive conductance to atmospheric CO2 concentrations ranging from glacial to the elevated levels forecast for this century. The results suggest that for many species gc(max) exhibits developmental plasticity through a negative relationship between S and D that shifts the operational range of leaf diffusive conductance in the direction of the short-term feedback response of stomatal aperture to ca. Stomata therefore appear to exhibit both short-term dynamic and long-term adaptive feedback responses to ca. The plasticity in S appears also to be linked to correlated plasticity in the size of the guard cell nucleus as well as the apparent 1C DNA amount in the cells of epidermal tissue. The results also suggest that the potential productivity of plants, as governed by ca, S, D and consequently gc(max), is tightly coupled to plant genome size. However, the mechanism underlying this coupling remains unclear. Over the much longer time frame of millions of years, in which natural selection and evolution can operate [44,51–53], our results suggest that large changes in ca may, in selecting for the phenotypic benefits of optimal gc(max), select also for correlated changes in S, D and genome size.
Acknowledgements
We thank T. Cavalier-Smith and M. W. Chase for helpful comments and discussion on plant genome size, and S. Pearce, G. Nicholson and B. Palmer for technical assistance with the plant growth chambers. The work was supported by funding from the Australian Research Council and the University of Sheffield (P.J.F. and D.J.B.). D.J.B. gratefully acknowledges a Royal Society–Wolfson Research Merit Award. E.M.R. is grateful to the Gatsby Charitable Foundation for the Award of a Sainsbury post-graduate studentship.
References
- 1.Sellers P. J., et al. 1996. Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science 271, 1402–1406 10.1126/science.271.5254.1402 (doi:10.1126/science.271.5254.1402) [DOI] [Google Scholar]
- 2.Hetherington A. M., Woodward F. I. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 10.1038/nature01843 (doi:10.1038/nature01843) [DOI] [PubMed] [Google Scholar]
- 3.Berner R. A. 2006. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664 10.1016/j.gca.2005.11.032 (doi:10.1016/j.gca.2005.11.032) [DOI] [Google Scholar]
- 4.Monnin E., et al. 2004. EPICA Dome C Ice Core High Resolution Holocene and Transition CO2 Data. IGBP PAGES/World Data Center for Paleoclimatology. Data Contribution Series #2004-055. Boulder, CO: NOAA/NGDC Paleoclimatology Program
- 5.Fischer A. G. 1986. Climatic rhythms recorded in strata. Annu. Rev. Earth Planet. Sci. 14, 351–376 10.1146/annurev.ea.14.050186.002031 (doi:10.1146/annurev.ea.14.050186.002031) [DOI] [Google Scholar]
- 6.Knoll A. H., Niklas K. J., Tiffney B. H. 1979. Phanerozoic land plant diversity in North America. Science 206, 1400–1402 10.1126/science.206.4425.1400 (doi:10.1126/science.206.4425.1400) [DOI] [PubMed] [Google Scholar]
- 7.Osborne C. P., Beerling D. J., Lomax B. H., Chaloner W. G. 2004. Biophysical constraints on the origin of leaves inferred from the fossil record. Proc. Natl Acad. Sci. USA 101, 10 360–10 362 10.1073/pnas.0402787101 (doi:10.1073/pnas.0402787101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward F. I., Lomas M. R., Betts R. A. 1998. Vegetation–climate feedbacks in a greenhouse world. Phil. Trans. R. Soc. Lond. B 353, 29–38 10.1098/rstb.1998.0188 (doi:10.1098/rstb.1998.0188) [DOI] [Google Scholar]
- 9.Betts R. A., et al. 2007. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448, 1037–1041 10.1038/nature06045 (doi:10.1038/nature06045) [DOI] [PubMed] [Google Scholar]
- 10.Raschke K. 1972. Saturation kinetics of velocity of stomatal closing in response to CO2. Plant Physiol. 49, 229–234 10.1104/pp.49.2.229 (doi:10.1104/pp.49.2.229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farquhar G. D., von Caemmerer S., Berry J. A. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 plants. Planta 149, 78–90 10.1007/BF00386231 (doi:10.1007/BF00386231) [DOI] [PubMed] [Google Scholar]
- 12.Morison J. I. L., Gifford R. M. 1983. Stomatal sensitivity to carbon dioxide and humidity: a comparison of two C3 and two C4 grass species. Plant Physiol. 71, 789–796 10.1104/pp.71.4.789 (doi:10.1104/pp.71.4.789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxman T. E., Monson R. 2003. Stomatal responses of C3, C3–C4 and C4 Flaveria species to light and intercellular CO2 concentration: implications for the evolution of stomatal behaviour. Plant Cell Environ. 26, 313–322 10.1046/j.1365-3040.2003.00964.x (doi:10.1046/j.1365-3040.2003.00964.x) [DOI] [Google Scholar]
- 14.Farquhar G. D., Dubbe D. R., Raschke K. 1978. Gain of feedback loop involving carbon-dioxide and stomata: theory and measurement. Plant Physiol. 62, 406–412 10.1104/pp.62.3.406 (doi:10.1104/pp.62.3.406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messinger S. M., Buckley T. N., Mott K. A. 2006. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 140, 771–778 10.1104/pp.105.073676 (doi:10.1104/pp.105.073676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mott K. A., Sibbernsen E. D., Shope J. C. 2008. The role of mesophyll in stomatal response to light and CO2. Plant Cell Environ. 31, 1299–1306 10.1111/j.1365-3040.2008.01845.x (doi:10.1111/j.1365-3040.2008.01845.x) [DOI] [PubMed] [Google Scholar]
- 17.Franks P. J., Farquhar G. D. 2001. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 125, 935–942 10.1104/pp.125.2.935 (doi:10.1104/pp.125.2.935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown H. T., Escombe F. 1900. Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Phil. Trans. R. Soc. Lond. B 193, 223–291 10.1098/rstb.1900.0014 (doi:10.1098/rstb.1900.0014) [DOI] [Google Scholar]
- 19.Franks P. J., Drake P. L., Beerling D. J. 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ. 32, 1737–1748 10.1111/j.1365-3040.2009.002031.x (doi:10.1111/j.1365-3040.2009.002031.x) [DOI] [PubMed] [Google Scholar]
- 20.Franks P. J., Beerling D. J. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl Acad. Sci. USA 106, 10 343–10 347 10.1073/pnas.0904209106 (doi:10.1073/pnas.0904209106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stitt M. 1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 14, 741–762 10.1111/j.1365-3040.1991.tb01440.x (doi:10.1111/j.1365-3040.1991.tb01440.x) [DOI] [Google Scholar]
- 22.Sage R. F. 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth. Res. 39, 351–368 10.1007/BF00014591 (doi:10.1007/BF00014591) [DOI] [PubMed] [Google Scholar]
- 23.Woodrow I. E. 1994. Optimal acclimation of the C3 photosynthetic system under enhanced CO2. Photosynth. Res. 39, 401–412 10.1007/BF00014594 (doi:10.1007/BF00014594) [DOI] [PubMed] [Google Scholar]
- 24.Anderson L. J., Maherali H., Johnson H. B., Polley H. W., Jackson R. B. 2001. Gas exchange and photosynthetic acclimation over subambient to elevated CO2 in a C3–C4 grassland. Global Change Biol. 7, 693–707 10.1046/j.1354-1013.2001.00438.x (doi:10.1046/j.1354-1013.2001.00438.x) [DOI] [Google Scholar]
- 25.Sholtis J. D., Gunderson C. A., Norby R. J., Tissue D. T. 2004. Persistent stimulation of photosynthesis by elevated CO2 in a sweetgum (Liquidambar styraciflua) forest stand. New Phytol. 162, 343–354 10.1111/j.1469-8137.2004.01028.x (doi:10.1111/j.1469-8137.2004.01028.x) [DOI] [Google Scholar]
- 26.Ainsworth E. A., Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 10.1111/j.1365-3040.2007.01641.x (doi:10.1111/j.1365-3040.2007.01641.x) [DOI] [PubMed] [Google Scholar]
- 27.Maherali H., Johnson H. B., Jackson R. B. 2003. Stomatal sensitivity to vapour pressure difference over a subambient to elevated CO2 gradient in a C-3/C-4 grassland. Plant Cell Environ. 26, 1297–1306 10.1046/j.1365-3040.2003.01054.x (doi:10.1046/j.1365-3040.2003.01054.x) [DOI] [Google Scholar]
- 28.Tissue D. T., Lewis J. D. 2010. Photosynthetic responses of cottonwood seedlings grown in glacial through future atmospheric [CO2] vary with phosphorus supply. Tree Physiol. 30, 1361–1372 10.1093/treephys/tpq077 (doi:10.1093/treephys/tpq077) [DOI] [PubMed] [Google Scholar]
- 29.Franks P. J., Beerling D. J. 2009. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7, 227–236 10.1111/j.1472-4669.2009.00193.x (doi:10.1111/j.1472-4669.2009.00193.x) [DOI] [PubMed] [Google Scholar]
- 30.Lammertsma E. I., de Boer H. J., Dekker S. C., Dilcher D. L., Lotter A. F., Wagner-Cremer F. 2011. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl Acad. Sci. USA 108, 4035–4040 10.1073/pnas.1100371108 (doi:10.1073/pnas.1100371108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franks P. J., Freckleton R. P., Beaulieu J. M., Leitch I. J., Beerling D. J. 2012. Megacycles of atmospheric carbon dioxide concentration correlate with fossil plant genome size. Phil. Trans. R. Soc. B 367, 556–564 10.1098/rstb.2011.0269 (doi:10.1098/rstb.2011.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melarango J. E., Mehrotra B., Coleman A. W. 1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5, 1661–1668 10.1105/tpc.5.11.1661 (doi:10.1105/tpc.5.11.1661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulieu J. M., Leitch I. J., Patel S., Pendharkar A., Knight C. A. 2008. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 179, 975–986 10.1111/j.1469-8137.2008.02528.x (doi:10.1111/j.1469-8137.2008.02528.x) [DOI] [PubMed] [Google Scholar]
- 34.Knight C. A., Beaulieu J. M. 2008. Genome size scaling through phenotype space. Ann. Bot. 101, 759–766 10.1093/aob/mcm321 (doi:10.1093/aob/mcm321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spence R. D., Wu H., Sharpe P. J. H., Clark K. G. 1986. Water stress effects on guard cell anatomy and the mechanical advantage of the epidermal cells. Plant Cell Environ. 9, 197–202 10.1111/1365-3040.ep11611639 (doi:10.1111/1365-3040.ep11611639) [DOI] [Google Scholar]
- 36.Xia H. 1994. Effects of soil drought during the generative development phase of faba bean (Vicia faba) on photosynthetic characters and biomass production. J. Agric. Sci. 122, 67–72 10.1017/S0021859600065813 (doi:10.1017/S0021859600065813) [DOI] [Google Scholar]
- 37.Franks P. J., Farquhar G. D. 2007. The mechanical diversity of stomata and its significance in gas exchange control. Plant Physiol. 143, 78–87 10.1104/pp.106.089367 (doi:10.1104/pp.106.089367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolež J., Greilhuber J., Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2, 2233–2244 10.1038/nprot.2007.310 (doi:10.1038/nprot.2007.310) [DOI] [PubMed] [Google Scholar]
- 39.Beerling D. J., Woodward F. I. 1997. Changes in land plant function over the Phanerozoic: reconstructions based on the fossil record. Bot. J. Linn. Soc. 124, 137–153 10.1111/j.1095-8339.1997.tb01787.x (doi:10.1111/j.1095-8339.1997.tb01787.x) [DOI] [Google Scholar]
- 40.Woodward F. I. 1987. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature 327, 617–618 10.1038/327617a0 (doi:10.1038/327617a0) [DOI] [Google Scholar]
- 41.Mirsky A. E., Ris H. 1951. The desoxyribonucleic acid content of animal cells and its evolutionary significance. J. Gen. Physiol. 34, 451–462 10.1085/jgp.34.4.451 (doi:10.1085/jgp.34.4.451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavalier-Smith T. 1985. Cell volume and the evolution of genome size. In The evolution of genome size (ed. Cavalier-Smith T.), pp. 105–184 Chichester, UK: Wiley [Google Scholar]
- 43.Cavalier-Smith T. 2005. Economy, speed and size matter: evolutionary forces driving nuclear genome miniaturization and expansion. Ann. Bot. 95, 147–175 10.1093/aob/mci010 (doi:10.1093/aob/mci010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregory T. R. 2001. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol. Rev. 76, 65–101 10.1017/S1464793100005595 (doi:10.1017/S1464793100005595) [DOI] [PubMed] [Google Scholar]
- 45.Biémont C. 2008. Within-species variation in genome size. Heredity 101, 297–298 10.1038/hdy.2008.80 (doi:10.1038/hdy.2008.80) [DOI] [PubMed] [Google Scholar]
- 46.Exner V., Henning L. 2008. Chromatin rearrangement in development. Curr. Opin. Plant Biol. 11, 64–69 10.1016/j.pbi.2007.10.004 (doi:10.1016/j.pbi.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 47.Tessadori F., et al. 2009. PHYTOCHROME B and HISTONE DEACTYLASE 6 control light-induced chromatin compaction in Arabidopsis thaliana. PLoS Genet. 5, e1000638. 10.1371/journal.pgen.1000638 (doi:10.1371/journal.pgen.1000638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson I. R., Jacobson E. 2007. Epigenetic inheritance in plants. Nature 447, 418–424 10.1038/nature05917 (doi:10.1038/nature05917) [DOI] [PubMed] [Google Scholar]
- 49.Kloc A., Martienssen R. 2008. RNAi, heterochromatin and the cell cycle. Trends Genet. 24, 511–517 10.1016/j.tig.2008.08.002 (doi:10.1016/j.tig.2008.08.002) [DOI] [PubMed] [Google Scholar]
- 50.Suda J., Kron P., Husband B. C., Travnicek P. 2007. Flow cytometry and ploidy: applications in plant systematics, ecology and evolutionary biology. In Flow cytometry with plant cells (eds Dolež J., Greilhuber J., Suda J.), pp. 103–130 Weinheim, Germany: Wiley-VCH [Google Scholar]
- 51.Petrov D. A. 2001. Evolution of genome size: a new approach to an old problem. Trends Genet. 17, 23–28 10.1016/S0168-9525(00)02157-0 (doi:10.1016/S0168-9525(00)02157-0) [DOI] [PubMed] [Google Scholar]
- 52.Baucom R. S., Estill J. C., Chaparro C., Upshaw N., Jogi A., Deragon J.-M., Westerman R. P., SanMiguel P. J., Bennetzen J. L. 2009. Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. PLoS Genet. 5, e100732. 10.1371/journal.pgen.1000732 (doi:10.1371/journal.pgen.1000732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitte C., Bennetzen J. L. 2006. Analysis of retrotransposon structural diversity uncovers properties and propensities in angiosperm genome evolution. Proc. Natl Acad. Sci. USA 103, 17 638–17 643 10.1073/pnas.0605618103 (doi:10.1073/pnas.0605618103) [DOI] [PMC free article] [PubMed] [Google Scholar]