Abstract
To investigate the impact of manipulating stomatal density, a collection of Arabidopsis epidermal patterning factor (EPF) mutants with an approximately 16-fold range of stomatal densities (approx. 20–325% of that of control plants) were grown at three atmospheric carbon dioxide (CO2) concentrations (200, 450 and 1000 ppm), and 30 per cent or 70 per cent soil water content. A strong negative correlation between stomatal size (S) and stomatal density (D) was observed, suggesting that factors that control D also affect S. Under some but not all conditions, mutant plants exhibited abnormal stomatal density responses to CO2 concentration, suggesting that the EPF signalling pathway may play a role in the environmental adjustment of D. In response to reduced water availability, maximal stomatal conductance was adjusted through reductions in S, rather than D. Plant size negatively correlated with D. For example, at 450 ppm CO2 EPF2-overexpressing plants, with reduced D, had larger leaves and increased dry weight in comparison with controls. The growth of these plants was also less adversely affected by reduced water availability than plants with higher D, indicating that plants with low D may be well suited to growth under predicted future atmospheric CO2 environments and/or water-scarce environments.
Keywords: stomatal density (D), stomatal size (S), carbon dioxide, signalling peptide, plant growth, epidermal patterning factor
1. Introduction
Microscopic stomatal pores on the aerial surfaces of higher plants regulate gas exchange between plants and their environment. Two guard cells surround and regulate each stomatal pore in response to a number of environmental cues. To optimize the trade-off between carbon gain and transpirational water loss, stomata sense and integrate a range of environmental signals, including atmospheric carbon dioxide (CO2) concentration and soil moisture [1–3]. Considerable progress has been made towards understanding these sensory and signalling mechanisms at the molecular level [3]. However, much less is known about the long-term response of stomata to changing environmental conditions, which involves the production of new leaves with altered stomatal size (S) and altered stomatal number per unit area, or density (D) [4].
When leaves are formed, their capacity for exchanging CO2 and water vapour across the epidermis is set by the maximum diffusive conductance of the stomatal pores to CO2, gc(max), and water vapour, gw(max), as well as being affected by photosynthetic capacity. These maximum conductances are determined by S and D [5]. Leaves can regulate stomatal aperture to control diffusive conductance at any point between zero and the maximum, but can only alter gc(max) and gw(max) by altered development. Adaptation of S and D is, therefore, central to the long-term response of leaf diffusive conductance to sustained shifts in environmental conditions. Here, we examine the interaction of S and D in response to two globally important and long-term environmental variables: atmospheric CO2 concentration (ca) and water availability.
(a). Stomatal size, density and carbon dioxide
Stomatal apertures reduce in response to an increase in ca, and increase in response to a decrease in ca. This short-term, reversible response is one of the several feedback mechanisms regulating leaf gas exchange [6]. For the majority of species, long-term exposure of plants to elevated ca results in leaves with reduced D [7–9] consistent with a more permanent downregulation of leaf diffusive conductance. The mature parts of the plant sense ca and transmit this environmental signal to the developing epidermis to regulate stomatal density [10,11]. We currently know little about how environmental factors, such as CO2, regulate D but as the transpiration rate of mature leaves correlates with D in developing leaves, a link between the short-term control of stomatal aperture and the long-term regulation of stomatal development has been suggested [12]. The molecular basis of this correlation is unknown. The only gene products known to modulate stomatal development in response to elevated CO2 are the carbonic anhydrases [13], and the high CO2 protein (HIC) believed to be involved in biosynthesis of the epicuticular waxes [14–16].
Recently, in addition to changes in stomatal aperture and D, a third stomatal response to atmospheric CO2 concentration has been suggested—a change in stomatal size (S). Periods of relatively low atmospheric CO2 concentration in prehistory are characterized by high densities of small stomata, whereas periods of CO2 concentration much higher than present levels are characterized by low densities of larger stomata [5]. Recent experimental evidence also supports this inverse correlation between CO2 concentration and S [17–19]. S determines two important physical dimensions contributing to gc(max) and gw(max): the maximum area of the open pore (amax), and the pore depth or diffusion path length (l). It is suggested that, because of their shorter aperture diffusion path, high densities of small stomata may represent the best way of achieving maximal gas exchange and preventing CO2 starvation in periods of low atmospheric CO2 concentrations. In addition, smaller stomata would be expected to have shorter opening and closing response times allowing for improved control of water loss [5]. On the other hand, a reduced number of, albeit larger, stomata may perhaps allow a metabolic cost-saving when CO2 is not scarce [20]. As yet, we do not know the mechanism by which guard cell size is determined, nor whether the signalling components that control guard cell size are shared with, or interact with, those that regulate stomatal apertures and/or stomatal densities. However, S has been shown to strongly correlate with genome size in extant plant specimens [21–24].
(b). Molecular basis of stomatal development
Our understanding of the molecular control of stomatal development has benefitted from studies of Arabidopsis thaliana mutants [24]. A family of secreted peptide signals known as the epidermal patterning factors (EPFs) are proposed to compete for a putative cell surface receptor, believed to comprise the receptor-like protein too many mouths (TMM) and a putative leucine-rich repeat receptor-like protein kinase [25,26]. Evidence suggests that receptor binding activates an intracellular mitogen-activated protein kinase cascade, which phosphorylates and destabilizes a basic helix–loop–helix transcription factor required, early in leaf development, for cells to enter the stomatal lineage [27].
The roles of three EPF signals have been described. Two act as inhibitors of stomatal development, and the third acts as an activator of stomatal development. The relative expression levels of these activator and inhibitor peptides during leaf development appear to determine D in mature leaves. EPF1 and EPF2 act to inhibit the formation of stomatal precursors by performing distinct but overlapping functions [28,29]. EPF1 is principally involved in orienting cell divisions and prevents stomata from forming in clusters or pairs. Thus, mature leaves of plants lacking EPF1 (epf1 knockout mutants) have increased numbers of stomata, and frequent stomatal pairs. EPF2 principally not only inhibits the formation of meristemoids but also promotes the formation of pavement cells. Thus, leaves of epf2 knockout plants have increased stomatal densities but also form small arrested stomatal lineage cells. The double mutant lacking both EPF1 and EPF2 has an additive phenotype. epf1epf2 plants exhibit greatly increased stomatal densities, and also have stomatal pairing and additional arrested cells. Plants manipulated to constitutively over-express EPF1 or EPF2 (EPF1OE and EPF2OE) have very few stomata [28–30].
The third peptide, EPFL9/STOMAGEN, has essentially the opposite function to EPF1 and EPF2, the promotion of stomatal development [31–33]. This activator peptide is secreted from mesophyll cells and is proposed to prevent inhibitory peptides such as EPF1 or EPF2 from binding their receptor and inhibiting stomatal development [26]. Plants manipulated to have reduced levels of EPFL9/STOMAGEN (EPFL9RNAi) have decreased D, whereas plants manipulated to over-express EPFL9/STOMAGEN (EPFL9OE) have increased D [32–34]. In line with this hypothesis, plants lacking EPF1 and EPF2, and overexpressing EPFL9/STOMAGEN would be expected to have exceptionally high D. We have created plants with this triple mutant epf1epf2EPFL9OE genotype for use in this study.
(c). Stomatal mutants as tools for studying S versus D
The availability of plants with, for the first time, a 16-fold range of D in the same genetic background has allowed us to investigate a number of outstanding questions: (i) Are changes in D accompanied by correlated changes in S ? (ii) Are increases or decreases in D beneficial at relatively low or high atmospheric CO2 concentrations, respectively, and particularly under water-stress? Here, we compare the phenotypes of Arabidopsis mutants ranging from EPF2OE, which has an approximately 80 per cent reduction in D, to epf1epf2EPFL9OE, which has approximately 225 per cent increase in D, following their growth in controlled environments at sub-ambient and elevated atmospheric CO2 concentrations. In a further experiment, we compare plants grown in well-watered and water-deficit conditions (70% and 30% soil water content) under these CO2 concentrations.
2. Materials and methods
(a). Plant material and growth conditions
Arabidopsis thaliana epf1-1, epf1-1epf2-1, EPF2OE (p35S:EPF2-CTAPi), p35S:EPFL9RNAi mutant seeds were as previously described [29,34]. epf1epf2EPFL9OE was generated by transforming epf1epf2 with p35S:EPFL9-CTAPi as previously described [34]. Gene accession numbers are EPF1 At2g20875, EPF2 At1g34245, EPFL9 At4g12970. T3 generation plants were used in these experiments following genotype confirmation by PCR. Mutant plants and their background ecotype Col-0 were stratified on wet M3 compost/perlite (4 : 1) at 4°C in dark for 72 h to synchronize germination. Plants were grown in three matched-controlled environment growth chambers (Conviron model BDR16) at 200, 450 and 1000 ppm CO2 at 22°C/16°C 9 L : 15 D cycle. CO2 levels were constantly monitored by VAISALA CO2 sensors. In one cabinet, CO2 was scrubbed from the air with a mixture of soda lime and charcoal, and CO2-free air mixed with ambient air to achieve 200 ppm CO2. In the other two cabinets, ambient air was supplemented with CO2 from a gas cylinder, derived from fossil carbon sources (BOC).
After two weeks of growth, plants were transferred to 100 ml volume plant pots and covered with clingfilm for the first 5 days. For the first experiment (presented in figure 1), plants were grown to maturity in these pots. When the plants were just beginning to initiate a floral bolt (stage 3.9 on Boyes scale [35]; at approx. 82 days, 74 days, and 68 days post germination for 200, 450 and 1000 ppm CO2,, respectively), fully expanded leaves were removed for stomatal analyses. Plants grown for the second experiment (presented in figures 2 and 3) were grown in 100 ml pots and well-watered for four to six weeks until the plants had developed 12–15 true leaves (41, 32 and 28 days post-germination (dpg) for 200, 450 and 1000 ppm CO2, respectively), at which point they were transplanted to larger 1000 ml volume pots filled with M5 compost/perlite (4 : 1). Pots with established plants and water-saturated soil were weighed and water was withheld from a subset of ‘water-restricted’ plants until the compost reached 30 per cent water saturation, whereupon the soil was watered to 30 per cent water saturation every 2–3 days. ‘Well-watered’ plants were grown in the same manner except compost was watered to 70 per cent water saturation every 2–3 days. The plants were maintained under well-watered and water-restricted conditions for a further 49, 49 or 42 days for 200, 450 and 1000 ppm CO2, respectively.
Figure 1.
Stomatal properties of Arabidopsis epidermal patterning factor (EPF) family mutants grown at 200 (white), 450 (grey) or 1000 ppm (black) atmospheric CO2 concentration. (a) Mean stomatal densities (n = 3–8). (b) Mean stomatal sizes (n = 3–8). (c–h) Micrographs of epidermal peels of leaves from EPF2OE, EPFL9RNAi, Col-0, epf2, epf1epf2 and epf1epf2EPFL9OE, respectively, with red dots added to highlight stomatal pores. Scale bar, 25 µm. (i–n) False colour infrared images of EPF2OE, EPFL9RNAi, Col-0, epf2, epf1epf2 and epf1epf2 EFL9OE, respectively, grown at 1000 ppm CO2. Temperature scale bar on right. (o) Relationship between mean stomatal size and mean stomatal density. (p) Mean leaf temperatures of leaves relative to Col-0 grown at 200 ppm (n = 4–8). Error bars represent standard error. Asterisks indicate values that are significantly different from the same genotype grown at 450 ppm (p < 0.05; (a) and (b)) or values that are significantly different from Col-0 grown at the same CO2 concentration (p < 0.05; (p)).
Figure 2.
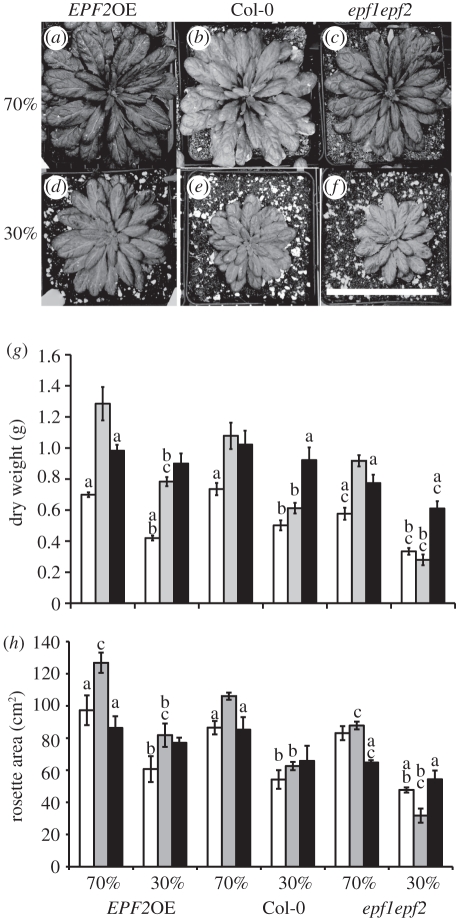
Size and dry weights of epidermal patterning factor (EPF) family mutants following growth at 200 (white bars), 450 (grey bars) or 1000 ppm (black bars) atmospheric CO2 concentration with either 30% or 70% relative soil water content. (a–f) Images of plants grown at 450 ppm CO2 at 70% (a–c) or 30% (d–f) soil water content at 70 dpg. Scale bar, 10 cm. (g) Mean dry weight and (h) mean leaf rosette area (n = 3–4). Error bars represent standard error. Letters indicate values that are significantly different from letter ‘a’, the same genotype grown at 450 ppm CO2, letter ‘b’, the same genotype grown at 70% soil water content and letter ‘c’, Col-0 grown in the same conditions (p < 0.05).
Figure 3.
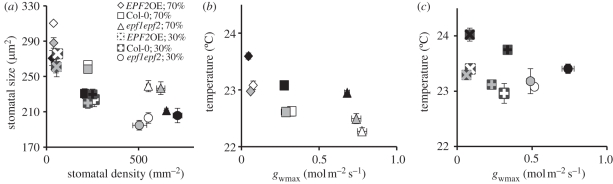
Stomatal characteristics of epidermal patterning factor (EPF) family mutants following growth at 200 (white), 450 (grey) or 1000 ppm (black) atmospheric CO2 concentration with either 30% or 70% relative soil water content. (a) Relationship between mean stomatal size and density across the CO2 conditions (n = 3–4). (b,c) Relationship between mean leaf temperature and mean maximum stomatal conductance to water vapour at (a) 70% and (b) 30% soil water conditions (n = 3–4). Error bars represent standard error.
(b). Stomatal density, index, size and maximum stomatal conductance measurements
Dental resin (Coltene Whaledent, Switzerland) was applied to both surfaces of fully expanded leaves and nail varnish peels were taken from set resin. Cell counts were taken from the widest area of two leaves each from at least four plants of each genotype at each growth condition. S was calculated from measurements of guard cell length and width following incubation of leaf samples with the fungal toxin fusicoccin to open pores. Abaxial leaf epidermal peels of mature leaves were removed 2–3 h into the photoperiod and floated on opening buffer (10 mM KCl, 10 mM MES, pH 6.2, supplemented with 500 nM fusicoccin, Sigma-Aldrich) and incubated at 22°C for 2 h [36,37]. Stomatal measurements and maximum apertures were determined by light microscopy (Olympus BX51), using a fitted camera (Olympus DP70), and measured using ImageJ software v. 1.43u. Maximum stomatal aperture was estimated from
| 2.1 |
where Wa is aperture width and La is aperture length. Stomatal size (S) was estimated from
| 2.2 |
where Ws is the width of the fully inflated guard cell pair and Ls is stomatal length.
Maximum leaf conductance to water vapour was calculated from
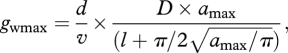 |
2.3 |
where d is the diffusivity of water vapour in air at 22°C (m2 s−1), v is the molar volume of air at 22°C and 1 atm (m3 mol−1), D is stomatal density (m−2) and l is pore depth defined as guard cell width (m) [17].
Leaf size was estimated from digital photos using ImageJ. Mean leaf size was used to calculate the number of stomata per leaf. Rosette leaf area was calculated as the sum of the areas of all mature leaves on a plant.
(c). Infrared thermal imaging
Infrared images were taken using a FLIR SC660 camera (FLIR systems) vertically positioned approximately 1 m above the leaf rosette. Infrared images of plants in the first experiment (presented in figure 1) were taken immediately after their removal from the growth cabinet. Sixty infrared images of all plants involved in the second experiment (presented in figures 2 and 3) were taken within the growth cabinets over a period of 1 h, beginning 2 h after the start of the photoperiod. Plants (3–4) of each genotype were imaged and average temperature data were recorded from the widest uncovered regions of three leaves per plants. Images were analysed using ThermaCAM Researcher v. 2.9 Professional (FLIR systems) and corrected for small variations in growth cabinet temperatures in comparison with background temperatures recorded using the same camera system (less than 0.5°C).
(d). Statistical analysis
Two- and three-way analysis of variance was calculated using the R programming language available from the Comprehensive R Archive Network family of Internet sites. Variance was considered significant for p < 0.05. Individual Student's t-tests were conducted with Microsoft Excel.
3. Results
(a). D versus S across epidermal patterning factor mutants
Mean stomatal densities of mature leaves on plants grown at 450 ppm CO2 ranged from approximately 40 for EPF2OE, up to 650 mm−2 for epf1epf2EPFL9 OE (figure 1a,c–h) and, with the exception of the epf1epf2EPFL9OE plants which were created for this study, were consistent with their previously published values of D [29–34]. There was a significant effect of genotype and CO2 conditions, as well as a significant interaction between genotype and CO2 conditions on D (see the electronic supplementary material, table S1 for analysis of variance results). Four out of six genotypes exhibited a significant reduction in D following growth at elevated CO2 (1000 ppm CO2) as would be expected from previous studies of Arabidopsis [10]. However, none of the EPF mutant genotypes showed increases in D at the low CO2 concentration (200 ppm CO2), perhaps indicating that without an intact EPF signalling pathway plants are unable to respond to sub-ambient CO2 concentrations. Non-stomatal cell densities were also recorded and stomatal index (the number of stomata divided by the total number of stomata and other epidermal cells) was calculated (see electronic supplementary material, figure S1 and table S1). Stomatal indices were in line with the EPF mutant phenotypes described previously [29–34] but in contrast to D, stomatal indices were generally not affected by growth at either the higher or lower CO2 environments (200 or 1000 ppm CO2).
There was significant effect of genotype on S. The mean S of EPF2OE and EPFL9RNAi open stomata was significantly larger, and epf1epf2 and epf1epf2EPFL9OE were significantly smaller than the Col-0 control S (figure 1b and the electronic supplementary material, table S1). Thus, a strong inverse correlation between S and D was apparent in this experiment (figure 1o) and for individual plants (electronic supplementary material, figure S2).
(b). Effect of growth carbon dioxide concentration on leaf temperature
We used infrared thermal imaging to record the temperature of plant leaf rosette surfaces. This technique measures the level of leaf evaporative cooling owing to transpiration, and hence provides a proxy measurement for transpiration [38–40]. There were significant effects of genotype and CO2 concentration on the mean rosette leaf temperatures. Mean temperatures for each genotype grown at 1000 ppm CO2 were higher with respect to those grown at 450 ppm CO2 which were in turn higher than those grown at 200 ppm CO2 (figure 1p), indicating that stomata of the EPF family mutants are functional in transpiration, and that their stomatal aperture opening/closing response to CO2 is intact. Plants with high D (epf1epf2 and epf1epf2EPFL9OE) transpired more than those with lower D (EPFL9RNAi, Col-0 and epf2) under all CO2 conditions (figure 1i–n,p). The difference between mean leaf temperatures of genotypes with the most extreme D (EPFL9RNAi and epf1epf2EPFL9OE) was 0.64°C, 0.56°C and 0.67°C for plants grown at 200, 450 and 1000 ppm CO2, respectively. Thus, a clear correlation between D and the rate of leaf transpiration was observed, which was remarkably consistent between the different CO2 conditions. Furthermore, plants with high D, such as epf1epf2, had significantly smaller stomata (figure 1o,p) and the highest transpiration rates, confirming expectations from physical diffusion theory [5].
(c). Interaction of growth, carbon dioxide and water restriction
In our second experiment, we compared the growth and stomatal characteristics of plants with differing D grown under well-watered or water-restricted conditions (70% or 30% relative soil water content) in matched chambers at the same three CO2 levels as above: 200, 450 and 1000 ppm CO2. Three genotypes were used for this experiment; EPF2OE with low D, epf1epf2 with high D and Col-0 control plants. Plants with reduced D (EPF2OE) were larger, and those with increased D (epf1epf2) smaller, than Col-0 control plants at each CO2 level (figure 2a–c), and in comparison with plants grown at 70 per cent soil water content (figure 2a–c), plants of all genotypes were smaller when grown at 30 per cent (figure 2d–f). We investigated this further by measuring mean dry weight (figure 2g) and mean rosette area (figure 2h). There were significant effects of genotype, CO2 concentration and water availability on rosette area and dry weight, as well as significant effects of the interaction between genotype and CO2 concentration and between water availability and CO2 concentration on both rosette area and dry weight (electronic supplementary material, table S1). Biomass was most severely restricted in the plants with high D (epf1epf2) at 200 and 450 ppm CO2 when transpiration would be expected to be highest. In addition, epf1epf2 plants showed the largest decrease in dry weight and rosette diameter following growth under water restriction at both 200 and 450 ppm CO2 conditions (figure 2g). However, the restriction of water availability had no significant effect on the dry weight of any genotype at 1000 ppm CO2 when transpiration would be expected to be low (figure 2g,h). Interestingly, EPF2OE plants, which have low stomatal densities, grown at 450 ppm CO2 acquired a significantly larger biomass and rosette area than Col-0 plants under both watering regimes (figure 2g,h). Thus, at 450 ppm CO2 plants with the least stomata appeared to be the most successful, growing larger even under restricted water availability. In contrast, plants with increased D (epf1epf2) did not appear to show any growth advantage even under sub-ambient CO2 conditions and epf1epf2 dry weights were significantly lower than Col-0 at all CO2 conditions when water was restricted.
The mean abaxial stomatal densities of plants grown with reduced water availability were not significantly different from those of well-watered plants, except for a reduction in epf1epf2 stomatal density at 450 ppm CO2 and a small increase in EPF2OE stomatal densities at 200 and 1000 ppm CO2 (electronic supplementary material, figure S3 and table S1). The mean adaxial values of D were similar to the abaxial D although the D of Col-0 grown at 1000 ppm CO2 was significantly lower than the D of Col-0 grown at 450 and 200 ppm CO2 (electronic supplementary material, figure S4 and table S1). epf1epf2 plants did not respond to increasing CO2 by reducing D on either the abaxial or adaxial side of the leaf in this experiment. It is possible that the breakdown of the stomatal density response to CO2 in these mutants is owing to the lack of EPF1 and EPF2 inhibitory peptides; however, this is not consistent with the results of our first experiment (figure 1a). All three genotypes showed a significant reduction in mean stomatal size following growth under drought conditions at 200 and 450 but not at 1000 ppm CO2 (electronic supplementary material, figure S5 and table S1). Thus, the inverse correlation between stomatal density and stomatal size that we observed in our first experiment appeared to be modulated by water availability but remained consistent across the genotypes within those conditions (figure 3a). When plants were grown under water restriction, total stomatal aperture area appeared to be reduced by changes in S rather than reductions in D (electronic supplementary material, figures S4, S5 and table S1).
Although values of D were largely unaffected by water restriction, plants of all genotypes were significantly smaller following growth at 30 per cent soil water content, at 450 and 200 ppm CO2 as described above (figure 2d–f). In the light of the dramatic differences in leaf size, we calculated the mean total number of abaxial stomata per leaf (electronic supplementary material, figure S6). The number of stomata per leaf remained stable for EPF2OE and Col-0 at all three CO2 conditions and also under water restriction, except for Col-0 following growth under water restriction at 450 ppm CO2. The epf1epf2 plants, however, which have high D, had reduced numbers of stomata per leaf following growth under water restriction at 200 and 450 ppm CO2. Our results indicate that Arabidopsis plants respond to restricted water availability primarily by producing smaller leaves with smaller stomata. Plants with the highest D (epf1epf2) responded by a more extreme restriction in leaf size while D remained relatively consistent, resulting in fewer stomata per leaf under water restriction (electronic supplementary material, figures S3, S4 and S6).
To assess transpiration, mean rosette leaf temperatures of Arabidopsis plants were taken by infrared thermography. Leaf temperatures ranged from 22.27°C for well-watered plants with high D grown at sub-ambient CO2 (epf1epf2, 70% soil water content, 200 ppm CO2), to 24.02°C for plants with reduced D, grown with restricted water availability at elevated CO2 (EPF2OE, 30% soil water content, 1000 ppm CO2; figure 3b,c, electronic supplementary material, figure S7 and table S1). There were significant effects of genotype, CO2 concentration and water availability on leaf temperature as well as a significant interaction between genotype and water availability on mean leaf temperature (electronic supplementary material, table S1). At 70 per cent soil water content, plants with low D (EPF2OE) had significantly warmer leaves than either Col-0 or epf1epf2 across all CO2 conditions, and plants with high D (epf1epf2) were significantly cooler than Col-0 at 200 ppm CO2 (figure S5b, electronic supplementary material, figure S7 and table S1). Thus, a negative correlation between maximum stomatal conductance and leaf temperature was observed (figure 3b). At 1000 ppm CO2, the temperatures of all plants were significantly higher than those grown at 200 and 450 ppm CO2, probably owing to reductions in stomatal aperture. However, no significant difference in temperature between well-watered plants grown at 200 and 450 ppm CO2 was observed, suggesting that transpiration rate does not differ greatly between leaves acclimatized to these CO2 levels. Following growth under water restriction, all plants had significantly higher leaf temperatures (electronic supplementary material, figure S7 and table S1) and a negative correlation between stomatal density and leaf temperature was observed in plants grown at 1000 ppm CO2 (figure 3c). Plants with reduced stomatal density (EPF2OE) had higher leaf temperature (implying reduced transpiration rate) under water restriction at 200 ppm CO2 in comparison with Col-0. However, mean leaf temperatures of epf1epf2 plants grown under water restriction at 200 or 450 ppm CO2 were not significantly different from Col-0 controls, perhaps because these plants with high D had begun to wilt under these conditions. Despite this the plants with increased stomatal density (epf1epf2) had a larger difference in temperature between growth at 70 per cent soil water content and 30 per cent soil water content and appeared more able to increase transpiration when well-watered, than plants with normal or reduced stomatal density (Col-0 and EPF2OE).
(d). Effect of manipulating epidermal patterning factor signalling on non-stomatal leaf cells
Stomatal density is influenced by direct effects on stomatal development but also via indirect effects on the size and number of non-stomatal cells. These indirect effects are also likely to be influenced by changes in atmospheric CO2 concentration and water availability. Our analysis of stomatal indices, leaf size and calculation of the number of stomata per leaf showed that plants lacking EPF2 and EPFL9 had a higher proportion of non-stomatal epidermal cells (electronic supplementary material, figure S1 and table S1), and that plants manipulated to have low or high D (EPFL9OE, epf1epf2) developed larger or smaller leaves, respectively (electronic supplementary material, figure S8). Although manipulation of EPF levels also altered leaf size, EPFL9OE still had significantly decreased, and epf1epf2 still had significantly increased, numbers of stomata per leaf (electronic supplementary material, figure S5 and table S1). All genotypes showed a dramatic reduction in leaf size following growth under restricted water availability under all three CO2 conditions, with the exception of epf1epf2 at 1000 ppm (electronic supplementary material, figure S8).
4. Discussion
It has been observed recently from the study of fossilized plants that, over the past 400 Myr, changes in stomatal density have been accompanied by large changes in stomatal size [17]. Our results show that this inverse relationship between stomatal size and stomatal density holds true across the Arabidopsis genotypes characterized in this study. Altering EPF family expression levels to increase or decrease D caused an opposite effect in S. Thus, the pathways controlling D and S appear to be linked but whether the EPF signalling pathway impacts directly, or indirectly, on S remains to be explored.
Current atmospheric CO2 concentration is approximately 390 ppm (2010 average at Mauna Loa Observatory was 389.78), and if it continues to rise at the current rate then it is expected to reach 450 ppm in the next 25–30 years [41]. Our results suggest that plants with reduced D and increased S may have advantageous growth characteristics in such future CO2 environments. At 450 ppm CO2, Arabidopsis plants manipulated to have lower D and large S had reduced transpiration, a larger biomass, and increased growth tolerance to limited water availability. The improved growth rate of plants with substantially decreased stomatal density perhaps reflects a combination of improved tissue water status from lower transpiration rate, improved CO2 assimilation rate from favourably higher leaf temperature, and lower metabolic cost from developing and operating less guard cells [20]. However, why this trait, which can be brought about by changing the expression level of just one gene (EPF2), has not evolved in the natural population is not clear. It is possible that, under the current rapidly rising atmospheric CO2 concentration, plants have not yet fully adapted their stomatal densities to be optimal for growth. Alternatively, there may be unknown adverse effects associated with developing lower D when plants are growing in a competitive natural environment. Despite the apparent benefits of reduced D on Arabidopsis leaves in the highly controlled growth conditions that we used, it is not known whether the same beneficial effects would be observed by reducing D of economically useful plants in harsher or fluctuating environments. Indeed, decreased transpirational cooling resulting from lower levels of conductance has been associated with lower agronomic yields of irrigated pima cotton (Gossypium barbadense), as well as bread wheat (Triticum aestivum), in hot environments [42]. Nevertheless, in marked contrast to the enhanced growth characteristics of plants with reduced D, we did not identify any growth conditions in which plants manipulated to have increased D (e.g. epf1epf2) grew larger. However, we did not attempt to assess seed yield in our experiments and it is possible that such plants may partition biomass differently into their reproductive structures.
Although in previous studies Arabidopsis leaves of another stomatal density mutant were apparently able to compensate for altered D by adjusting stomatal aperture size [43], our results suggest that the genotypes in the current study were unable to completely compensate for altered D, by adjusting either S or aperture. Under two different watering regimes (70% and 30% soil water content) and across three atmospheric CO2 conditions (200, 450 and 1000 ppm), EPF2OE leaves with reduced D transpired less than controls, as evidenced by increased leaf temperature. In addition, epf1epf2 and epf1epf2EPFL9OE plants, both with increased D, generally had higher transpiration rates than their counterpart controls at all three CO2 conditions. Thus, our results indicate a strong correlation between D and transpiration rate. Given the widespread conservation of the EPF family across different plant species [44], we consider genetic manipulation of this peptide family a potential route for altering leaf conductance without disrupting stomatal aperture control.
In our experiments, plants lacking inhibitors of stomatal development EPF1 and EPF2 were unable to increase D at sub-ambient atmospheric CO2 concentrations, or to decrease D at elevated CO2 concentrations when grown in larger pots (figure 1a and electronic supplementary material, figures S3 and S4). Thus, it could be concluded that an intact EPF signalling pathway is required for the adjustment of D in response to atmospheric CO2 concentration. However, as D is responsive to many environmental signals and some results were inconsistent between our two experiments this possibility requires further study before firm conclusions can be drawn.
In summary, genetic manipulation of the EPF signalling pathway not only produced plants with dramatically altered stomatal densities but it also affected stomatal size, plant transpiration, rosette growth and tolerance to restricted water availability across a range of atmospheric CO2 environments.
Acknowledgements
We thank David Mentlak, Antje Amand, Aaron Thierry and Weihao Zhong for their assistance with stomatal and statistical analysis. This work was funded by a BBSRC grant to J.E.G. and studentship to T.D-A. P.J.F. gratefully acknowledges support from the ARC Future Fellowships scheme. J.E.G., D.J.B. and P.J.F. are grateful to the Royal Society for hosting ‘The role of atmospheric CO2 in orchestrating green evolution’ meeting.
References
- 1.Berry J. A., Beerling D. J., Franks P. J. 2010. Stomata: key players in the Earth system, past and present. Curr. Opin. Plant Biol. 13, 233–240 10.1016/j.pbi.2010.04.013 (doi:10.1016/j.pbi.2010.04.013) [DOI] [PubMed] [Google Scholar]
- 2.Farquhar G. D., Sharkey T. D. 1982. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33, 317–345 10.1146/annurev.pp.33.060182.001533 (doi:10.1146/annurev.pp.33.060182.001533) [DOI] [Google Scholar]
- 3.Kim T., Böhmer M., Hu H., Nishimura N., Schroeder J. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant. Biol. 61, 561–591 10.1146/annurev-arplant-042809-112226 (doi:10.1146/annurev-arplant-042809-112226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetherington A. M., Woodward F. I. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 10.1038/nature01843 (doi:10.1038/nature01843) [DOI] [PubMed] [Google Scholar]
- 5.Franks P. J., Beerling D. J. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geological time. Proc. Natl Acad. Sci. USA 106, 10 343–10 347 10.1073/pnas.0904209106 (doi:10.1073/pnas.0904209106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farquhar G. D., Dubbe D. R., Raschke K. 1978. Gain of the feedback loop involving carbon dioxide and stomata: theory and measurement. Plant Physiol. 62, 406–412 10.1104/pp.62.3.406 (doi:10.1104/pp.62.3.406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward F. I. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327, 617–618 10.1038/327617a0 (doi:10.1038/327617a0) [DOI] [Google Scholar]
- 8.Beerling D. J., Chaloner W. G., Huntley B., Pearson J. A., Tooley M. J. 1993. Stomatal density responds to the glacial cycle of environmental-change. Proc. R. Soc. Lond. B 251, 133–138 10.1098/rspb.1993.0019 (doi:10.1098/rspb.1993.0019) [DOI] [Google Scholar]
- 9.Beerling D. J., Woodward F. I. 1997. Changes in land plant function over the Phanerozoic: reconstructions based on the fossil record. Bot. J. Linn. Soc. 124, 137–153 10.1111/j.1095-8339.1997.tb01787.x (doi:10.1111/j.1095-8339.1997.tb01787.x) [DOI] [Google Scholar]
- 10.Lake J. A., Quick W. P., Beerling D. J., Woodward F. I. 2001. Plant development: signals from mature to new leaves. Nature 411, 154. 10.1038/35075660 (doi:10.1038/35075660) [DOI] [PubMed] [Google Scholar]
- 11.Coupe S. A., Palmer B. G., Lake J. A., Overy S. A., Oxborough K., Woodward F. I., Gray J. E., Quick W. P. 2006. Systemic signalling of environmental cues in Arabidopsis leaves. J. Exp. Bot. 57, 329–341 10.1093/jxb/erj033 (doi:10.1093/jxb/erj033) [DOI] [PubMed] [Google Scholar]
- 12.Lake J. A., Woodward F. I. 2008. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytol. 179, 397–404 10.1111/j.1469-8137.2008.02485.x (doi:10.1111/j.1469-8137.2008.02485.x) [DOI] [PubMed] [Google Scholar]
- 13.Hu H., Boisson-Dernier A., Israelsson-Nordström M., Böhmer M., Xue S., Ries A., Godoski J., Kuhn J. M., Schroeder J. I. 2010. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12, 87–93 10.1038/ncb2009 (doi:10.1038/ncb2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray J. E., Holroyd G. H., van der Lee F. M., Bahrami A. R., Sijmons P. C., Woodward F. I., Schuch W., Hetherington A. M. 2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408, 713–716 10.1038/35042663 (doi:10.1038/35042663) [DOI] [PubMed] [Google Scholar]
- 15.Bird S. M., Gray J. E. 2003. Signals from the cuticle affect epidermal cell differentiation. New Phytol. 157, 9–23 10.1046/j.1469-8137.2003.00543.x (doi:10.1046/j.1469-8137.2003.00543.x) [DOI] [PubMed] [Google Scholar]
- 16.Casson S., Gray J. E. 2008. Influence of environmental factors on stomatal development. New Phytol. 178, 9–23 10.1111/j.1469-8137.2007.02351.x (doi:10.1111/j.1469-8137.2007.02351.x) [DOI] [PubMed] [Google Scholar]
- 17.Franks P. J., Beerling D. J. 2009. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7, 227–236 10.1111/j.1472-4669.2009.00193.x (doi:10.1111/j.1472-4669.2009.00193.x) [DOI] [PubMed] [Google Scholar]
- 18.Uprety D. C., Dwivedi N., Jain V., Mohan R. 2002. Effect of elevated carbon dioxide concentration on the stomatal parameters of rice cultivars. Photosynthetica 40, 315–319 10.1023/A:1021322513770 (doi:10.1023/A:1021322513770) [DOI] [Google Scholar]
- 19.Driscoll S. P., Prins A., Olmos E., Kunert K. J., Foyer C. H. 2006. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. J. Exp. Bot. 57, 381–390 10.1093/jxb/erj030 (doi:10.1093/jxb/erj030) [DOI] [PubMed] [Google Scholar]
- 20.Franks P. J., Drake P. L., Beerling D. J. 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant Cell Environ. 12, 1737–1748 10.1111/j.1365-3040.2009.002031.x (doi:10.1111/j.1365-3040.2009.002031.x) [DOI] [PubMed] [Google Scholar]
- 21.Lomax B. H., Woodward F. I., Leitch I. J., Knight C. A., Lake J. A. 2009. Genome size as a predictor of guard cell length in Arabidopsis thaliana is independent of environmental conditions. New Phytol. 181, 311–314 10.1111/j.1469-8137.2008.02700.x (doi:10.1111/j.1469-8137.2008.02700.x) [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu J. M., Leitch I. J., Patel S., Pendharkar A., Knight C. A. 2008. Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol. 179, 975–986 10.1111/j.1469-8137.2008.02528.x (doi:10.1111/j.1469-8137.2008.02528.x) [DOI] [PubMed] [Google Scholar]
- 23.Hodgson J. G., et al. 2010. Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog? Ann. Bot. 105, 573–584 10.1093/aob/mcq011 (doi:10.1093/aob/mcq011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franks P. J., Freckleton R. P., Beaulieu J. M., Leitch I. J., Beerling D. J. 2012. Megacycles of atmospheric carbon dioxide concentration correlate with fossil plant genome size. Phil. Trans. R. Soc. B 367, 556–564 10.1098/rstb.2011.0269 (doi:10.1098/rstb.2011.0269). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmann D. C., Sack F. 2007. Stomatal development. Annu. Rev. Plant Biol. 58, 163–181 10.1146/annurev.arplant.58.032806.104023 (doi:10.1146/annurev.arplant.58.032806.104023) [DOI] [PubMed] [Google Scholar]
- 26.Rowe M. H., Bergmann D. C. 2010. Complex signals for simple cells: the expanding ranks of signals and receptors guiding stomatal development. Curr. Opin. Plant Biol. 13, 548–555 10.1016/j.pbi.2010.06.002 (doi:10.1016/j.pbi.2010.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rychel A. L., Peterson K. M., Torii K. U. 2010. Plant twitter: ligands under 140 amino acids enforcing stomatal patterning. J. Plant Res. 123, 275–280 10.1007/s10265-010-0330-9 (doi:10.1007/s10265-010-0330-9) [DOI] [PubMed] [Google Scholar]
- 28.Lampard G. R., Macalister C. A., Bergmann D. C. 2008. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322, 1113–1116 10.1126/science.1162263 (doi:10.1126/science.1162263) [DOI] [PubMed] [Google Scholar]
- 29.Hara K., Yokoo T., Kajita R., Onishi T., Yahata S., Peterson K. M., Torii K. U., Kakimoto T. 2009. Epidermal cell density is autoregulated via a secretory peptide, epidermal patterning factor 2 in Arabidopsis leaves. Plant Cell Physiol. 50, 1019–1031 10.1093/pcp/pcp068 (doi:10.1093/pcp/pcp068) [DOI] [PubMed] [Google Scholar]
- 30.Hunt L., Gray J. E. 2009. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr. Biol. 19, 864–869 10.1016/j.cub.2009.03.069 (doi:10.1016/j.cub.2009.03.069) [DOI] [PubMed] [Google Scholar]
- 31.Hara K., Kajita R., Torii K. U., Bergmann D. C., Kakimoto T. 2007. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 21, 1720–1725 10.1101/gad.1550707 (doi:10.1101/gad.1550707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugano S. S., Shimada T., Imai Y., Okawa K., Tamai A., Mori M., Hara-Nishimura I. 2009. Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–244 10.1038/nature08682 (doi:10.1038/nature08682) [DOI] [PubMed] [Google Scholar]
- 33.Kondo T., et al. 2010. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 51, 1–8 10.1093/pcp/pcp180 (doi:10.1093/pcp/pcp180) [DOI] [PubMed] [Google Scholar]
- 34.Hunt L., Bailey K. J., Gray J. E. 2010. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 186, 609–614 10.1111/j.1469-8137.2010.03200.x (doi:10.1111/j.1469-8137.2010.03200.x) [DOI] [PubMed] [Google Scholar]
- 35.Boyes D. C., Zayed A. M., Ascenzi R., McCaskill A. J., Hoffman N. E., Davis K. R., Görlach J. 2001. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13, 1499–1510 10.1105/tpc.13.7.1499 (doi:10.1105/tpc.13.7.1499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb A. A. R., Hetherington A. M. 1997. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol. 114, 1557–1560 10.1104/pp.114.4.1557 (doi:10.1104/pp.114.4.1557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stout R. G. 1988. Fusicoccin activity and binding in Arabidopsis thaliana. Plant Physiol. 88, 999–1001 10.1104/pp.88.4.999 (doi:10.1104/pp.88.4.999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merlot S., Mustilli A. C., Genty B., North H., Lefebvre V., Sotta B., Vavasseur A., Giraudat J. 2002. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609 10.1046/j.1365-313X.2002.01322.x (doi:10.1046/j.1365-313X.2002.01322.x) [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto M., Negi J., Young J., Israelsson M., Schroeder J. I., Iba K. 2006. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 8, 391–397 10.1038/ncb1387 (doi:10.1038/ncb1387) [DOI] [PubMed] [Google Scholar]
- 40.Xie X., et al. 2006. The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr. Biol. 16, 882–887 10.1016/j.cub.2006.03.028 (doi:10.1016/j.cub.2006.03.028) [DOI] [PubMed] [Google Scholar]
- 41.IPCC 2007. Climate Change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 129–234 Cambridge, UK: Cambridge University Press [Google Scholar]
- 42.Lu Z. M., Percy R. G., Qualset C. O., Zeiger E. 1998. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J. Exp. Bot. 49, 453–460 10.1093/jexbot/49.suppl_1.453 (doi:10.1093/jexbot/49.suppl_1.453) [DOI] [Google Scholar]
- 43.Büssis D., von Groll U., Fisahn J., Altmann T. 2006. Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol. 33, 1037–1043 10.1071/FP06078 (doi:10.1071/FP06078) [DOI] [PubMed] [Google Scholar]
- 44.Peterson K. M., Rychel A. L., Torii K. U. 2010. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. Plant Cell 22, 296–306 10.1105/tpc.109.072777 (doi:10.1105/tpc.109.072777) [DOI] [PMC free article] [PubMed] [Google Scholar]