Abstract
Termite colonies are founded by a pair of primary reproductives. In many species, including subterranean termites (family Rhinotermitidae), the primary king and queen can be succeeded by neotenic reproductives that are produced from workers or nymphs within the colony. It is generally believed that these neotenics inbreed within the colony, sometimes for many generations. Here, we show that primary queens of the North American subterranean termite, Reticulitermes virginicus, are replaced by numerous parthenogenetically produced female neotenics. We collected functional female neotenics from five colonies of R. virginicus in North Carolina and Texas, USA. Genetic analysis at eight microsatellite loci showed that 91–100% of the neotenics present within a colony were homozygous at all loci, indicating that they were produced through automictic parthenogenesis with terminal fusion. In contrast, workers, soldiers and alates were almost exclusively sexually produced by mating between the female neotenics and a single king. This is the second termite species shown to undergo asexual queen succession, a system first described in the Japanese species, Reticulitermes speratus. Thus, the conditional use of sexual and asexual reproduction to produce members of different castes may be widespread within Reticulitermes and possibly other subterranean termites.
Keywords: parthenogenesis, inbreeding, caste differentiation, microsatellite, breeding system
1. Introduction
Subterranean termites have a complex life history (reviewed in [1,2]). Colonies are generally founded by a single primary king and a single primary queen who pair during nuptial flights, mate and produce the other colony members. When the king and/or queen die, they are often replaced by neotenics (wingless reproductive forms) that develop from nymphs (which produce brachypterous neotenics) or workers (which give rise to apterous neotenics) from within the colony. Neotenics, which can number from a few to many dozens within a colony, engage in inbreeding, possibly for several generations [1,2].
Owing to the cryptic nesting habits of subterranean termites, it is difficult to conduct extensive studies of their colony breeding structure through collection of functional reproductives in field colonies. Instead, researchers have increasingly turned to the application of molecular genetic markers to infer the numbers of reproductives within colonies and estimate levels of inbreeding [1]. These studies, which combine empirical genetic data with models of potential breeding systems, have greatly expanded our understanding of the diversity of breeding systems within subterranean termites, revealing extraordinary variation in this group [1,2]. Although this approach has led to important advances in our understanding of subterranean termite breeding structure, the mode of reproduction used by subterranean termites has always been assumed to be sexual. However, facultative parthenogenetic reproduction has been documented in seven termite species from four subfamilies (reviewed in [3]). In most cases, parthenogenetic reproduction has been reported from cases involving laboratory rearing of primary queens in isolation or paired with other queens after mating flights. Thus, this phenomenon was believed to be largely an artefact of rearing queens without access to a male, and the relevance of such facultative parthenogenesis in a natural context was not clear. However, Matsuura et al. [4] recently demonstrated that conditional parthenogenesis plays an integral role in the life cycle of the Japanese subterranean termite Reticulitermes speratus through the process of asexual queen succession (AQS). This species undergoes typical colony founding by a pair of primary reproductives. Relatively, early in the colony life cycle, the primary queen is replaced by numerous secondary queens (brachypterous female neotenics) that are produced asexually by the primary queen. These neotenic queens mate with the primary king and produce workers, soldiers and new primary reproductives through sexual reproduction. Collection of reproductives from field colonies was critical in uncovering this previously unknown breeding system since the genotypes of workers and other sexually produced individuals in such colonies cannot be distinguished from individuals produced in colonies containing a single king and a single queen. It is not known how widespread such a system of AQS is within subterranean termites.
The North American species, Reticulitermes virginicus, ranges throughout the eastern and central USA [5]. The breeding structure of 15 colonies of this species in North and South Carolina has been inferred from microsatellite genotypes of workers [6–10]. Altogether, results of these studies concluded that 11 of 15 colonies were simple families, i.e. headed by one king and one queen. However, in a study of colony founding in this species, Howard et al. [11] reported that paired primary female reproductives produced viable offspring in the absence of males suggesting that queens are capable of parthenogenetic reproduction. Therefore, our objective was to investigate the breeding structure of R. virginicus in greater detail to determine whether colonies of this species might also undergo AQS. To accomplish this, we collected functional reproductives from field colonies and genotyped them along with other caste members at eight microsatellite loci. Our results show that large colonies of R. virginicus are indeed headed by numerous neotenic females, nearly all of which are parthenogenetic daughters of the queen. These female neotenics mate with a single king to produce the other colony members through sexual reproduction. Thus, this is the second subterranean termite species demonstrated to have the remarkable AQS system.
2. Material and methods
(a). Sample collection
Over an 8 year period, we made numerous attempts to collect primary reproductives from dozens of field colonies of R. virginicus in North Carolina, Texas and Louisiana, USA. We focused our efforts on colonies inhabiting large downed trees and stumps. We used a chainsaw, hatchets and other tools to cut and split wood containing R. virginicus nests, concentrating our search on those chambers with eggs and small larvae. We managed to find functional reproductives in four colonies from North Carolina and one from Louisiana. We also collected workers and soldiers from these colonies, and, when present, we collected nymphs and alates. In all cases, reproductives were recovered either in the field or in the laboratory within a few hours of removal from the field. The locations and dates of collection as well as the colony compositions are shown in table 1.
Table 1.
Compositions of R. virginicus colonies sampled. All secondary queens collected were of the brachypterous (nymph-derived) form. −, not found or not present; +, present.
| colony | location | date collected | no. secondary queens | no. kings | nymphs present | alates present |
|---|---|---|---|---|---|---|
| 1 | Lake Johnson, Raleigh, NC, USA | 10 Oct 2008 | 22 | − | − | − |
| 2 | Schenck Forest, Raleigh, NC, USA | 16 Apr 2009 | 8 | − | + | − |
| 3 | Schenck Forest, Raleigh, NC, USA | 2 June 2009 | 11 | − | − | − |
| 4 | South Houston, TX, USA | 2 Apr 2003 | 17 | − | − | + |
| 5 | Schenck Forest, Raleigh, NC, USA | 28 Sep 2010 | 211 | 1, primary | − | − |
(b). Genetic analysis
All individuals were placed in vials containing 95 per cent ethanol and stored until DNA extraction. We used a modification of the Gentra PureGene kit (Gentra Systems, Inc., Minneapolis, MN, USA) for DNA extraction. Species identity was confirmed by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method of Szlanski et al. [12]. All collected secondary queens, or at least 20 individuals in the two colonies in which more than 20 neotenics were present (colonies 1 and 5), the one primary king present in colony 5, 20 workers and from 7 to 22 soldiers from each colony were genotyped at eight microsatellite loci: Rf 1-3, Rf 6-1, Rf 21-1, Rf 24-2 [13], and Rs 1, Rs 10, Rs 15 and Rs 76 [14]. We also genotyped 10 nymphs from colony 2, and 22 alates from colony 4. We used the PCR conditions described by Vargo [13] and Dronnet et al. [14]. The fluorescently labelled PCR products were loaded onto 6.5 per cent polyacrylamide gels onto which size standards were also loaded, and bands were detected using a Li-Cor 4300 automated DNA sequencer (Li-Cor, Inc., Lincoln, NE, USA). Allele sizes were determined using Gene Profiler v. 3.56 (Scanalytics, Inc., Fairfax, VA, USA).
We followed the methods of Matsuura et al. [4] in classifying individuals found to be homozygous at all loci as parthenogens, whereas individuals that were heterozygous at one or more loci were considered to be sexually produced. Observed and expected heterozygosities were determined using the program fstat v. 2.9.3.2 [15] based on all study colonies combined. The degree of relatedness among colony members was estimated using the program relatedness v. 5.0.8 [16] with all study colonies grouped into a single population (deme); standard errors were calculated by jack-knifing over loci.
3. Results
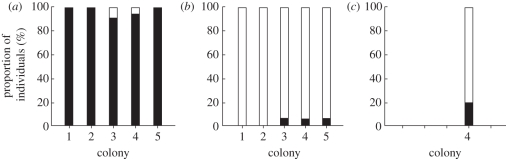
We found from 8 to 211 female nymphoid neotenics (i.e. brachypterous forms differentiated from nymphs) present in colonies (table 1; the electronic supplementary material). In only one case, colony 5, did we recover a male reproductive, a primary king. Out of a total of 76 secondary queens genotyped, 74 (97.4%) were determined to be parthenogens (figure 1; the electronic supplementary material). Within colony percentages varied from 91 per cent in colony 3 to 100 per cent in colonies 1, 2 and 5. Of the two individuals classified as parthenogens, one (from colony 3) was heterozygous at only a single locus (Rs 76), despite the original primary queen being heterozygous at five loci (based on the presence of two classes of homozygotes among secondary queens at these loci). Thus, it is possible that this secondary queen was actually parthenogenetically produced and was heterozygous owing to crossing over at this locus [17]. The other individual (from colony 4) was heterozygous at five loci and most probably was sexually produced. In contrast to the clear asexual origin of secondary queens, only four of the 92 (4.3%) workers (figure 1) and only one of 81 (1.2%) soldiers (table 2; the electronic supplementary material) were parthenogens. The frequency of parthenogens among alates and nymphs, the developmental stage giving rise to both alates and brachypterous neotenics, was intermediate (figure 1; the electronic supplementary material), reaching 18 per cent and 30 per cent, respectively. Correspondingly, the mean observed heterozygosity in secondary queens was 0.009 compared with a mean expected heterozygosity of 0.241. Observed and expected heterozygosities for workers and soldiers were much higher (He = 0.390, Ho = 0.500), whereas those of the nymphs and alates were intermediate (He = 0.185, Ho = 0.141).
Figure 1.
Proportions of individuals of different castes and developmental stages produced sexually and parthenogenetically in five colonies of R. virginicus based on their genotypes at eight microsatellite loci. Individuals homozygous at all loci were considered to be parthenogens, whereas those that were heterozygous at one or more loci were considered to be sexually produced. Open bars, sexually produced; filled bars, parthenogemetically produced. (a) Secondary queens; (b) workers; (c) alates.
Table 2.
Genotypes and their frequencies (n) found in the various castes in colony 5. The genotype of the primary queen (PQ) was inferred from the genotypes of the secondary queens (SQ). The genotypes of the primary king (PK) were determined directly from the collected king. Eighteen of the workers (W) were sexually produced (S) and one worker was parthenogenetic (P) in origin. All of the 20 soldiers (So) genotyped were sexually produced. In some cases, genotypes are missing owing to failed PCRs.
| caste | Rf 6-1 | n | Rs 15 | n | Rs 10 | n | Rf 24-2 | n | Rf 21-1 | n | Rs 76 | n | Rs 1 | n | Rf 1-3 | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PK | 185/179 | (1) | 252/252 | (1) | 146/146 | (1) | 078/078 | (1) | 193/193 | (1) | 179/175 | (1) | 276/264 | (1) | 210/195 | (1) |
| PQ | 179/173 | (1) | 267/252 | (1) | 146/143 | (1) | 105/081 | (1) | 208/208 | (1) | 179/167 | (1) | 270/264 | (1) | 210/192 | (1) |
| SQ | 179/179 | (10) | 267/267 | (17) | 146/146 | (7) | 105/105 | (9) | 208/208 | (20) | 179/179 | (7) | 270/270 | (9) | 210/210 | (12) |
| 173/173 | (10) | 252/252 | (3) | 143/143 | (13) | 081/081 | (11) | 167/167 | (13) | 264/264 | (11) | 192/192 | (8) | |||
| W (S) | 185/179 | (3) | 267/252 | (10) | 146/146 | (11) | 105/078 | (8) | 208/193 | (16) | 179/179 | (7) | 276/270 | (5) | 210/210 | (4) |
| 185/173 | (4) | 252/252 | (8) | 146/143 | (7) | 081/078 | (9) | 179/175 | (2) | 276/264 | (4) | 210/195 | (4) | |||
| 179/179 | (5) | 179/167 | (6) | 270/264 | (6) | 210/192 | (3) | |||||||||
| 179/173 | (6) | 175/167 | (3) | 264/264 | (3) | 195/192 | (7) | |||||||||
| W (P) | 179/179 | (1) | 267/267 | (1) | 143/143 | (1) | 081/081 | (1) | 208/208 | (1) | 179/179 | (1) | 264/264 | (1) | 192/192 | (1) |
| So | 185/179 | (5) | 267/252 | (8) | 146/146 | (9) | 105/078 | (13) | 208/193 | (18) | 179/179 | (6) | 276/270 | (3) | 210/210 | (6) |
| 185/173 | (4) | 252/252 | (10) | 146/143 | (10) | 081/078 | (6) | 179/175 | (4) | 276/264 | (3) | 210/195 | (3) | |||
| 179/179 | (5) | 179/167 | (4) | 270/264 | (7) | 210/192 | (4) | |||||||||
| 179/173 | (5) | 175/167 | (6) | 264/264 | (7) | 195/192 | (3) |
In all cases, there were no more than two homozygous classes among putative parthenogens within a colony, and these never contained alleles unique to the king. This is illustrated clearly in the genotypes present in colony 5 in which the primary king was collected (table 2). In this colony, all of the secondary queens were homozygous at all loci, but in no cases did they contain an allele found only in the king; in contrast, 18 of the 19 workers and all 20 soldiers were heterozygotes containing one of the king's alleles. The exceptional worker appeared to be a parthenogen, produced either by the original primary queen or by one of the female neotenics. These results are consistent with parthenogenesis through either automixis with terminal fusion as reported in R. speratus [4,17], or gamete fusion [18].
The coefficient of relatedness among nest-mates is shown in table 3. Of particular interest, the reigning kings and original primary queens (based on inferred genotypes) were significantly related (r = 0.411, p < 0.001, one-sample t-test), suggesting that some kings may have been neotenics that were the sons of the primary queen. In support of this conclusion, the coefficient of relatedness between the presumptive king and the primary queen within individual colonies varied from −0.061 to 0.791 (figure 2). The inferred king and primary queen were unrelated in two of the five colonies, including the colony in which the primary king was collected (colony 5), whereas kings and queens were highly related in the three remaining colonies. Similarly, the inferred kings and nest-mate secondary queens showed elevated levels of relatedness (r = 0.208), although this value was not significant (p > 0.30, one-sample t-test). Inferred primary queens were highly related to secondary queens within the same colony (r = 0.595), a value not significantly different from the value of 0.50 expected for mother–daughter relationships (p > 0.07, one-sample t-test). Both kings and primary queens were highly related to workers (r ≥ 0.665), significantly more so than the normal parent to offspring value of one-half (both p < 0.013, one-sample t-test). By contrast, secondary queens were related to workers by the expected value of 0.50 (r = 0.509) for mother–offspring relationships.
Table 3.
Coefficients of relatedness among nest-mates within five colonies of R. virginicus. Shown are means ± s.e. The genotypes of the primary queens were inferred from the genotypes of the secondary queens. In four cases, the genotypes of the kings were inferred from the genotypes of their presumed worker offspring. It was not known whether these kings were primary kings or secondary kings. In one colony, the primary king was collected and genotyped directly.
| king | primary queen | secondary queens | |
|---|---|---|---|
| primary queen | 0.411 ± 0.055 | — | — |
| secondary queens | 0.208 ± 0.389 | 0.595 ± 0.059 | 0.609 ± 0.073 |
| workers | 0.667 ± 0.053 | 0.665 ± 0.058 | 0.509 ± 0.061 |
Figure 2.
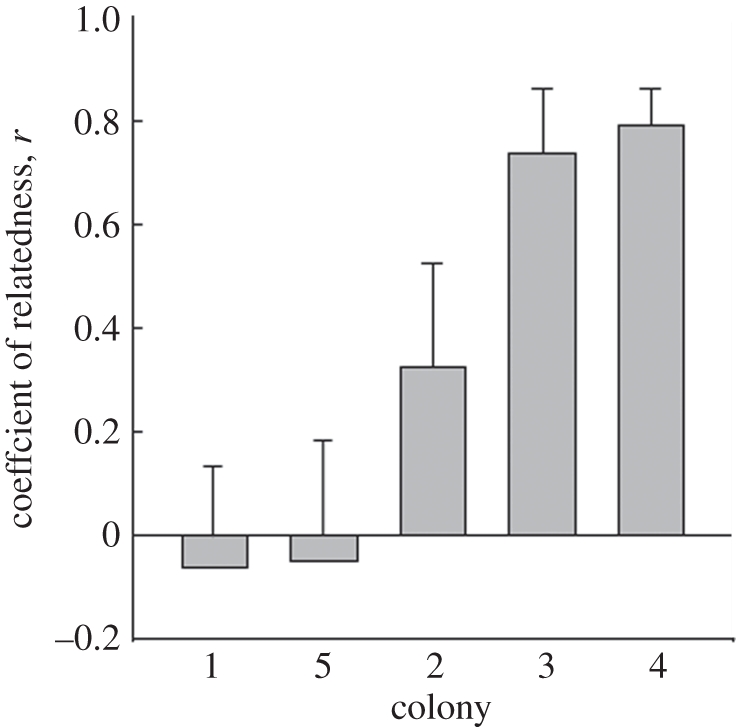
Degree of relatedness between the presumed primary queen founding a colony and the reigning king in five colonies of R. virginicus estimated from eight microsatellite loci. The genotypes of the primary queens were inferred from the genotypes of the parthenogenetically produced secondary queens within colonies. Genotypes of kings were inferred from a combination of the genotypes of their secondary queen mates and the genotypes of their worker offspring, except in colony 5, where the primary king was genotyped directly. Standard error bars were estimated by jack-knifing over loci.
4. Discussion
Our results show that colonies of R. virginicus undergo AQS as described previously in the Japanese subterranean termite, R. speratus [4]. As is typical of subterranean termites, colonies of both R. speratus and R. virginicus are founded by monogamous pairs of primary reproductives [1,19,20], but at some point, relatively early in the colony life cycle, the primary queen is replaced by her parthenogenetically produced neotenic daughters. These female neotenics then mate with either a primary or a secondary king to produce workers, soldier and alates through sexual reproduction. The fact that our colonies of R. virginicus came from opposite ends of its distribution (North Carolina and Texas) indicates that such a system is widespread in this species.
As described previously [4], there are many advantages of AQS over a system of queen replacement involving normal sexual production of female neotenics. First, by producing numerous secondary replacement queens, the queen is able to increase her reproductive output, allowing the colony to grow faster and larger because the combined egg production of dozens to hundreds of queens far exceeds the capacity of a single queen [2,21]. Second, AQS reduces inbreeding among workers, soldiers and alates within colonies compared with queen succession through sexual reproduction because there is no king–daughter inbreeding in the former [3,4]. Thus, under AQS, the worker force, soldiers and new alates, all of which have to deal with many environmental contingencies, remain genetically diverse, while the secondary queens, who are completely homozygous, are always cared for and protected by the workers. Third, because secondary queens only inherit the queen's genes, the founding primary queen does not suffer any loss in her genetic contribution to future generations [3,4]. In R. speratus, the parthenogenetically produced secondary queens can also produce neotenic daughters parthenogenetically [22], ensuring the queen's continued full genetic contribution to sexual offspring even after multiple rounds of queen replacement. It is not yet known whether neotenic queens of R. virginicus can also reproduce parthenogenetically. Fourth, the production of homozygous parthenogens could be an effective means to purge recessive deleterious alleles preventing their transmission to sexual offspring in the next generation [3], much like the genetic purging in Hymenoptera through the exposure of recessive deleterious alleles in haploid males.
Other social insects have been shown to also use conditional parthenogenesis to determine the caste of female offspring. In some ant species, new queens are produced asexually, whereas workers develop through normal sexual reproduction, including the myrmicines Wasmannia auropunctata [23] and Vollenhovia emeryi [24] and the formicine, Cataglyphis cursor [25]. Similar to the system of AQS in termites, the genetic diversity among the worker force in these ants is maintained through sexual reproduction. New queens in these species, which are produced parthenogenetically, found colonies dependently, i.e. accompanied by workers, and are thus cared for and protected their entire lives. The secondary queens of termites are analogous to the dependant founding queens of ants as they develop in large, established colonies where they are under the protective care of workers. Under AQS, the primary queens of termites, which leave the nest on mating flights and found colonies independently, are produced sexually. Thus, in species of both termites and ants that use conditional parthenogenesis to determine caste, genetic diversity is retained in the individuals faced with the greatest environmental uncertainty (workers in ants, workers and alates in termites), whereas asexual reproduction is reserved for the production of the next generation of reproductives, but only those buffered from environmental vagaries by a supporting worker force.
The mode of parthenogenesis underlying AQS in R. virginicus is likely to be automixis with terminal fusion, as has been shown to occur in R. speratus [17], although we cannot rule out the possibility of gamete duplication. Under automixis with terminal fusion, the first meiotic division proceeds normally, but the polar body formed in the second division fuses with the oocyte nucleus to restore diploidy [26]. This results in offspring being homozygous for all maternal alleles that did not crossover. In cases where crossover occurs, offspring will have the same genotype as the mother. Thus, parthenogenetic offspring produced by automixis with terminal fusion are largely homozygous, and, in the absence of crossover, are essentially half clones of the mother. In this system, heterozygote offspring should occur at the same frequency as the rate of crossover. This mode of parthenogenesis has been shown in R. speratus among the parthenogenetic worker offspring produced by virgin female primary reproductives in incipient colonies [17]. However, a recent study comparing the genotypes of workers and secondary queens produced in experimental colonies of this species showed that individuals that were homozygous at two microsatellite loci (Rf 21-1 and Rf 24-2) were more likely to develop into neotenics than individuals that were heterozygous at one or both of these loci [27]. Such a developmental bias results in neotenics that are more homozygous than the parthenogenetically produced offspring at large; heterozygous individuals develop instead into workers or die at a young age. The results of the present study are consistent with a similar system operating in R. virginicus. Excluding the one secondary queen that was heterozygous at five loci and presumably sexually produced, we found that 74 of 75 presumed parthenogenetically produced secondary queens were completely homozygous at eight microsatellite loci despite being produced by queens that were presumed to be heterozygous at between one and seven loci (mean = 4.2 loci). Thus, if parthenogenesis also occurs through automixis with terminal fusion in R. virginicus, then the near lack of heterozygous neotenics observed here could be owing to a developmental bias similar to that observed in R. speratus. Alternatively, parthenogenesis through gamete duplication would likewise enforce homozygosity in neotenics (and all parthenogens) as diploidy in this mechanism is restored through replication of the resulting gamete. Definitive determination of the mode of parthenogenesis in R. virginicus will require more detailed investigation, including cytological studies and the possibility of developmental biasing of homozygotes.
Compared with R. speratus, the frequency of colonies containing secondary kings in R. virginicus appears to be higher. In R. speratus, secondary kings have been found in only 6 per cent of colonies (3 of 50 colonies; [3,4]; K. Matsuura 2011, unpublished data). Although we did not collect any secondary kings of R. virginicus in the present study, three of our five colonies appeared to be headed by a king who was closely related to the original primary queen, most probably her son or grandson. A sexually produced male neotenic, whether mothered by her or one of her parthenogenetically produced daughters, would bear half the queen's genes. Any sexual offspring produced by this male neotenic through mating with either the primary or secondary queens, would bear three-quarters of the primary queen's genes. Thus, second generation male neotenics produced in such colonies are expected to be related to the queen by r = 0.75. Such high levels of relatedness between the primary queen and the reigning male reproductive are consistent with the coefficients of relatedness observed in colonies 3 and 4 (figure 2), suggesting that the male neotenics in these two colonies were the grandsons of the primary queen. Alternatively, the high degree of relatedness between the primary queen and reigning king could result from closely related kings and queens pairing during colony founding. However, in a study of R. virginicus in Raleigh, NC, USA, the location of four of the five study colonies in the present investigation, DeHeer & Vargo [20] showed that male and female alates forming tandem pairs in the field were unrelated to each other. Thus, it is unlikely that the high degree of relatedness between primary queens and reigning kings in the present study was owing to close relatives pairing during colony founding. Rather it was likely owing to the presence of male neotenics that were the sons or grandsons of the primary queen. In support of this conclusion, the one primary king that we recovered was unrelated to the presumptive primary queen (colony 5).
Our results suggest that most if not all mature colonies of R. virginicus are headed by a single king and multiple female neotenics. These findings have important implications for the results of earlier work on the breeding system and population genetic structure of this species. Previous studies of the breeding system of this species have largely used the genotypes of workers to infer the numbers of reproductives within colonies [6–10]. These studies have concluded that 11 of the 15 (73.3%) R. virginicus colonies characterized in North and South Carolina were headed single monogamous pairs; the remaining colonies all had the genotypes expected for simple families, but the frequencies of some genotypes differed significantly from those expected for a single pair of parents and were therefore classified as extended families. However, the genotypes of workers produced in colonies headed by a single king and multiple parthenogenetic daughters of the primary queen may be indistinguishable from those produced by a monogamous pair; therefore many, if not all, of the previously studied colonies may have been misclassified as simple families. This seems particularly true of the very expansive colonies reported from North Carolina [9] and South Carolina [10], with linear foraging distances of greater than 120 m. The large size of these colonies makes it likely that they had multiple queens present even though the worker genotypes were consistent with the presence of a single king and a queen. In a previous study, DeHeer & Vargo [20] genotyped male and female alates of R. virginicus during mating flights and found that female alates were significantly inbred, whereas male alates were not (FIS = 0.106 and 0.018, for females and males, respectively). These authors concluded that female alates were more likely to originate from inbred colonies than were male alates. However, a re-examination of these data indicates this slightly elevated level of inbreeding in females was due to the presence of a small percentage of individuals that were parthenogens (six of 157; 4%). When these are removed from the analysis, the FIS-value for females is no longer significant.
AQS in termites has so far been reported only in R. speratus and R. virginicus. This raises an important question concerning the evolutionary origin of this unusual breeding system. In a phylogenetic analysis of the family Rhinotermitidae, Austin et al. [28] found that R. speratus and R. virginicus were not close relatives within the genus Reticulitermes, belonging to distinctly different clades that probably branched early in the history of the genus. Thus, either AQS arose early in the genus and is therefore widespread throughout Reticulitermes (and possibly other subterranean termite genera), or it evolved independently in these two species. Distinguishing between these two possibilities will require extensive studies of the breeding systems in this genus and in closely related genera such as Coptotermes and Heterotermes.
Parthenogenetic reproduction has been observed in five other termite species belonging to three families: the Termopsids, Zootermopsis angusticollis and Z. nevadensis [29]; the Kalotermitids, Kalotermes flavicollis [30] and Bifiditermes beesoni [31,32]; and the Termitid, Velocitermes sp. [33]. In all cases, it was reported anecdotally, usually in the context of pairing females in laboratory studies of colony founding. Thus, the biological relevance of parthenogenesis in the natural life cycle of these species was not known. Parthenogenetic reproduction had been previously reported in R. virginicus [11] and R. speratus [17], but it was not until thorough studies of the reproductive composition and colony genetic structure of these species were undertaken, as in the present study, that the importance of conditional parthenogenesis as an integral part of the breeding system was revealed. Thus, the other species in which parthenogenesis occurs are prime candidates for also having AQS.
Another clue that species may have a system of AQS is a female-biased sex investment ratio among the alates. This is because of the relatedness asymmetries that emerge in colonies where the primary queen has been replaced by asexually produced female neotenics, and the primary king has been replaced by a sexually produced male neotenic who is the son of the primary queen. In such colonies, workers will be more closely related to the primary queen than to the primary king, and are therefore expected to invest more resources into the production of primary queens, as found in both R. speratus and R. virginicus (K. Kobayashi, E. Hasegawa, Y. Yamamoto, E. L. Vargo, J. Yoshimura & K. Matsuura 2011, unpublished data). Therefore, species exhibiting female-biased investment in alates could also be good candidates for replacement of queens through parthenogenetic reproduction.
In conclusion, this work extends the previous discovery of a novel breeding system in termites involving the conditional use of sexual and asexual reproduction by showing it occurs in a second species of Reticulitermes. Given the large number of subterranean termite species, and how few have been the subject of detailed studies of their breeding system, it would not be surprising to see that such a system is more widespread within the Rhinotermitidae and possibly beyond. Detecting AQS requires both the genetic analysis of colony members and the excavation of functional reproductives. Future studies focusing on species that are capable of parthenogenetic reproduction as well as species with a female-biased sex investment ratio may prove particularly fruitful.
Acknowledgements
This work was supported by USDA NRI grant (no. 2008-35302-04565) to E.L.V. and C. Schal, and by a grant from the Japan Society for the Promotion of Science (no. 09001407) to K.M.
References
- 1.Vargo E. L., Husseneder C. 2009. Biology of subterranean termites: insights from molecular studies of Reticulitermes and Coptotermes. Ann. Rev. Entomol. 54, 379–403 10.1146/annurev.ento.54.110807.090443 (doi:10.1146/annurev.ento.54.110807.090443) [DOI] [PubMed] [Google Scholar]
- 2.Thorne B. L., Traniello J. F. A., Adams E. S., Bulmer M. 1999. Reproductive dynamics and colony structure of subterranean termites of the genus Reticulitermes (Isoptera Rhinotermitidae): a review of the evidence from behavioral, ecological and genetic studies. Ethol. Ecol. Evol. 11, 149–169 10.1080/08927014.1999.9522833 (doi:10.1080/08927014.1999.9522833) [DOI] [Google Scholar]
- 3.Matsuura K. 2011. Sexual and asexual reproduction in termites. In Biology of termites: a modern synthesis (eds Bignell D. E., Roisin Y., Lo N.), pp. 255–277 Dordrecht, The Netherlands: Springer Science+Business Media B.V [Google Scholar]
- 4.Matsuura K., Vargo E. L., Kawatsu K., Labadie P. E., Nakano H., Yashiro T., Tsuji K. 2009. Queen succession through asexual reproduction in termites. Science 323, 1687. 10.1126/science.1169702 (doi:10.1126/science.1169702) [DOI] [PubMed] [Google Scholar]
- 5.Survey N. T. 2003. City of New Orleans: mosquito & termite control board; See http://www.termitesurvey.com/distribution/reticulitermes_virginicus.shtml [Google Scholar]
- 6.DeHeer C. J., Vargo E. L. 2004. Colony genetic organization and colony fusion in the termite Reticulitermes flavipes as revealed by foraging patterns over time and space. Mol. Ecol. 13, 431–441 10.1046/j.1365-294X.2003.02065.x (doi:10.1046/j.1365-294X.2003.02065.x) [DOI] [PubMed] [Google Scholar]
- 7.Parman V., Vargo E. L. 2008. Population density, species abundance, and breeding structure of subterranean termite colonies in and around infested houses in central North Carolina. J. Econ. Entomol. 101, 1349–1359 10.1603/0022-0493(2008)101[1349:PDSAAB]2.0.CO;2 (doi:10.1603/0022-0493(2008)101[1349:PDSAAB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 8.Vargo E. L. 2003. Genetic structure of Reticulitermes flavipes and R. virginicus (Isoptera: Rhinotermitidae) colonies in an urban habitat and tracking of colonies following treatment with hexaflumuron bait. Environ. Entomol. 32, 1271–1282 10.1603/0046-225X-32.5.1271 (doi:10.1603/0046-225X-32.5.1271) [DOI] [Google Scholar]
- 9.Vargo E. L., Carlson J. C. 2006. Comparative study of breeding systems of sympatric subterranean termites (Reticulitermes flavipes and R. hageni) in central North Carolina using two classes of molecular genetic markers. Environ. Entomol. 35, 173–187 10.1603/0046-225X-35.1.173 (doi:10.1603/0046-225X-35.1.173) [DOI] [Google Scholar]
- 10.Vargo E. L., Juba T. R., DeHeer C. J. 2006. Relative abundance and comparative breeding structure of subterranean termite colonies (Reticulitermes flavipes, R. hageni, R. virginicus, and Coptotermes formosanus) in a South Carolina lowcountry site as revealed by molecular markers. Ann. Entomol. Soc. Am. 99, 1101–1109 10.1603/0013-8746(2006)99[1101:RAACBS]2.0.CO;2 (doi:10.1603/0013-8746(2006)99[1101:RAACBS]2.0.CO;2) [DOI] [Google Scholar]
- 11.Howard R. W., Mallett E. J., Haverty M. I., Smythe R. V. 1981. Laboratory evaluation of within-species, between-species, and parthenogenetic reproduction in Reticulitermes flavipes and Reticulitermes virginicus. Psyche 88, 75–87 10.1155/1981/36030 (doi:10.1155/1981/36030) [DOI] [Google Scholar]
- 12.Szalanski A. L., Austin J. W., Owens C. B. 2003. Identification of Reticulitermes spp. (Isoptera: Reticulitermatidae [sic]) from south central United States by PCR-RFLP. J. Econ. Entomol. 96, 1514–1519 10.1603/0022-0493-96.5.1514 (doi:10.1603/0022-0493-96.5.1514) [DOI] [PubMed] [Google Scholar]
- 13.Vargo E. L. 2000. Polymorphism at trinucleotide microsatellite loci in the subterranean termite Reticulitermes flavipes. Mol. Ecol. 9, 817–820 10.1046/j.1365-294x.2000.00915.x (doi:10.1046/j.1365-294x.2000.00915.x) [DOI] [PubMed] [Google Scholar]
- 14.Dronnet S., Bagnères A.-G., Juba T. R., Vargo E. L. 2004. Polymorphic microsatellite loci in the European subterranean termite, Reticulitermes santonensis Feytaud. Mol. Ecol. Notes 4, 127–129 10.1111/j.1471-8286.2004.00600.x (doi:10.1111/j.1471-8286.2004.00600.x) [DOI] [Google Scholar]
- 15.Goudet J. 1995. fstat (version 1.2): a computer program to calculate F-statistics. J. Hered. 86, 485–486 [Google Scholar]
- 16.Queller D. C., Goodnight K. F. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 17.Matsuura K., Fujimoto M., Goka K. 2004. Sexual and asexual colony foundation and the mechanism of facultative parthenogenesis in the termite Reticulitermes speratus (Isoptera, Rhinotermitidae). Insectes Sociaux 51, 325–332 10.1007/s00040-004-0746-0 (doi:10.1007/s00040-004-0746-0) [DOI] [Google Scholar]
- 18.Lamb R. Y., Willey R. B. 1987. Cytological mechanisms of thelytocous parthenogenesis in insects. Genome 29, 367–369 10.1139/g87-062 (doi:10.1139/g87-062) [DOI] [Google Scholar]
- 19.Matsuura K., Nishida T. 2002. Mechanism, induction factors, and adaptive significance of dealation in the subterranean termite Reticulitermes speratus (Isoptera, Rhinotermitidae). Insectes Sociaux 49, 241–244 10.1007/s00040-002-8308-9 (doi:10.1007/s00040-002-8308-9) [DOI] [Google Scholar]
- 20.DeHeer C. J., Vargo E. L. 2006. An indirect test of inbreeding depression in the termites Reticulitermes flavipes and Reticulitermes virginicus. Behav. Ecol. Sociobiol. 59, 753–761 10.1007/s00265-005-0105-9 (doi:10.1007/s00265-005-0105-9) [DOI] [Google Scholar]
- 21.Grube S., Forschler B. T. 2004. Census of monogyne and polygyne laboratory colonies illuminates dynamics of population growth in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 97, 466–475 10.1603/0013-8746(2004)097[0466:COMAPL]2.0.CO;2 (doi:10.1603/0013-8746(2004)097[0466:COMAPL]2.0.CO;2) [DOI] [Google Scholar]
- 22.Hayashi Y., Kitade O., Kojima J.-I. 2003. Parthenogenetic reproduction in neotenics of the subterranean termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Entomol. Sci. 6, 253–257 10.1046/j.1343-8786.2003.00030.x (doi:10.1046/j.1343-8786.2003.00030.x) [DOI] [Google Scholar]
- 23.Fournier D., Estoup A., Orivel J., Foucaud J., Jourdan H., Le Breton J., Keller L. 2005. Clonal reproduction by males and females in the little fire ant. Nature 435, 1230–1234 10.1038/nature03705 (doi:10.1038/nature03705) [DOI] [PubMed] [Google Scholar]
- 24.Ohkawara K., Nakayama M., Satoh A., Trindl A., Heinze J. 2006. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biol. Lett. 2, 359–363 10.1098/rsbl.2006.0491 (doi:10.1098/rsbl.2006.0491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearcy M., Aron S., Doums C., Keller L. 2004. Conditional use of sex and parthenogenesis for worker and queen production in ants. Science 306, 1780–1783 10.1126/science.1105453 (doi:10.1126/science.1105453) [DOI] [PubMed] [Google Scholar]
- 26.Templeton A. 1982. The prophecies of parthenogenesis. In Evolution and genetics of life histories (eds Dingle H., Hegmann J. P.), pp. 75–102 Berlin, Germany: Springer [Google Scholar]
- 27.Yamamoto Y., Matsuura K. In press Genetic influence on caste determination underlying the asexual queen succession system in a termite. Behav. Ecol. Sociobiol. [Google Scholar]
- 28.Austin J. W., Szalanski A. L., Cabrera B. J. 2004. Phylogenetic analysis of the subterranean termite family Rhinotermitidae (Isoptera) by using the mitochondrial cytochrome oxidase II gene. Ann. Entomol. Soc. Am. 97, 548–555 10.1603/0013-8746(2004)097[0548:PAOTST]2.0.CO;2 (doi:10.1603/0013-8746(2004)097[0548:PAOTST]2.0.CO;2) [DOI] [Google Scholar]
- 29.Light S. F. 1944. Parthenogenesis in termites of the genus Zootermopsis. Univ. Calif. Pub. Zool. 43, 405–412 [Google Scholar]
- 30.Grassé P.-P. 1949. Ordre des Isoptères ou termites. In Traité de Zoologie (ed. Grassé P.-P.), pp. 408–544 Paris: Masson [Google Scholar]
- 31.Afzal M., Salihah Z. 1985. Sex-ratio, occurrence of parthenogenesis, ovarian development and oviposition behavior of the primary reproductives of Bifiditermes beesoni (Gardner) (Isoptera, Kalotermitidae). Z. Angew. Entomol.–J. App. Entomol. 100, 132–146 [Google Scholar]
- 32.Chhotani O. B. 1962. Further observations on biology and parthenogenesis in the termite Kalotermes beesoni (Kalotermitidae). In Termites in the Humid Tropics: Proc. of the New Delhi Symp., pp. 73–75 Paris: UNESCO [Google Scholar]
- 33.Stansly P. A., Korman A. K. 1993. Parthenogenic development in Velocitermes spp. (Isoptera, Nasutitermitinae). Sociobiology 23, 13–24 [Google Scholar]