Abstract
Local spatio-temporal resource variations can strongly influence the population dynamics of small mammals. This is particularly true on islands which are bottom-up driven systems, lacking higher order predators and with high variability in resource subsidies. The influence of resource fluctuations on animal survival may be mediated by individual movement among habitat patches, but simultaneously analysing survival, resource availability and habitat selection requires sophisticated analytical methods. We use a Bayesian multi-state capture–recapture model to estimate survival and movement probabilities of non-native black rats (Rattus rattus) across three habitats seasonally varying in resource availability. We find that survival varies most strongly with temporal rainfall patterns, overwhelming minor spatial variation among habitats. Surprisingly for a generalist forager, movement between habitats was rare, suggesting individuals do not opportunistically respond to spatial resource subsidy variations. Climate is probably the main driver of rodent population dynamics on islands, and even substantial habitat and seasonal spatial subsidies are overwhelmed in magnitude by predictable annual patterns in resource pulses. Marked variation in survival and capture has important implications for the timing of rat control.
Keywords: bottom-up, capture–recapture, Mediterranean, rainfall, Rattus rattus, survival
1. Introduction
At all scales, biological communities are regulated by spatio-temporal resource availability, and these effects cascade through food webs [1–4]. Ecosystems on islands tend to be strongly bottom-upregulated environments, with marked seasonal and inter-annual variations in resource availability. This variation can be driven by climatic patterns [5] and marine to terrestrial interfaces in resource exchange, including seabird-driven nutrient subsidy [6]. The impact of these inputs on population dynamics can differ among habitats according to resource quality and availability [7,8]. The population dynamics of animal populations on such islands are therefore likely to be strongly driven by spatial and temporal variation in these resource inputs [9], although the relative contribution of each to community regulation is less well known [10]. In this study, we are interested in determining the role of spatial (habitat) and temporal (climatic) resource variation on the survival, movement and capture probabilities of non-native black rats (Rattus rattus). We selected a small Mediterranean island, Bagaud Island, where different types of subsidies were found in close spatial proximity, to conduct a long-term capture–recapture survey on the resident introduced black rat population.
The population dynamics of small mammals are commonly used for studying population regulation processes and have been well studied in continental systems. In high-latitude continental systems, where small mammals have coevolved with high-order predators, predator-mediated cycles generally dominate population processes [11], while in tropical or arid continental regions rainfall-mediated cycles appear to dominate [12–14]. However, on island systems lacking high-order predators, the processes governing small mammal population fluctuations are poorly known.
Small mammals such as rodents have been widely introduced to most island groups throughout the world [15]. At the population level, introduced rodents are able to exploit a wide range of resources and establish populations in habitats of variable quality [16–19]. As generalist consumers and released from natural predators and competitors, they are expected to show high diet flexibility and respond to seasonal increases in resource availability [18,20]. Individual movements among habitats can have a particularly important role in subsidizing rodent population dynamic parameters, such as population size or survival [10,21,22], accompanied by dietary shifts [18] and changes to ecological processes, such as seed dispersal [23]. The impacts of introduced rodents on native wildlife can be devastating (e.g. [24]) and understanding the processes affecting the dynamics of their populations on islands is crucial for implementing effective management actions.
In the Mediterranean, black rats have an ongoing negative impact on island ecosystems [25], including predation on land- and seabirds [26] and dispersing introduced plants by invasional facilitation [23]. Over the last century, Mediterranean islands have shown a marked variation in spatial and temporal resource availability, driven by changes in inter-annual rainfall patterns and by an increase in anthropogenically driven resource input. Human land-use and its intensity have increased on many islands, increasing introductions of alien plants, and providing anthropogenic subsidies to gull populations. The expansion of succulent plants (e.g. Carpobrotus spp., Opuntia spp., Agave spp. [27]) along continental and island coastal sites of the Mediterranean has provided new resources to island consumers, especially during summer, when fleshy fruits ripen [23]. In addition, roosting and nesting by gulls on offshore islands lead to substantial changes in native plant [28] and arthropod [29] communities in guano-fertilized areas. Food scraps, cracked eggs, dead chicks or adults also fuel scavengers. These changes can interact with one another, possibly leading to facilitated ecological meltdown on some islands [23]. The relative influences of spatial subsidies (gulls and succulent plants) and temporal pulses (rainfall) in regulating introduced rat populations have never been investigated on Mediterranean islands.
Previous studies have shown that iceplant (Caprobrotus spp.) fruits and gull-derived resources are significant resources for introduced black rats on Mediterranean islands and that these enriched resources alters individual growth rates, reproductive output and rat population densities at a local scale [19,23]. We expect that the effect of resource variation on rat survival will be mediated by movement among habitat patches. The results of our investigation provide insight on rodent population dynamics and regulation, as well as valuable information to managers seeking to remove introduced rats from islands. We also demonstrate the power of a novel Bayesian approach for elucidating complex relationships from ecological datasets. In this study, we make use of recent advances in modelling of population dynamics [30–33] to develop an individual-based approach where survival depends upon biological (sex and age), temporal (month) and spatial (habitat) states.
2. Data
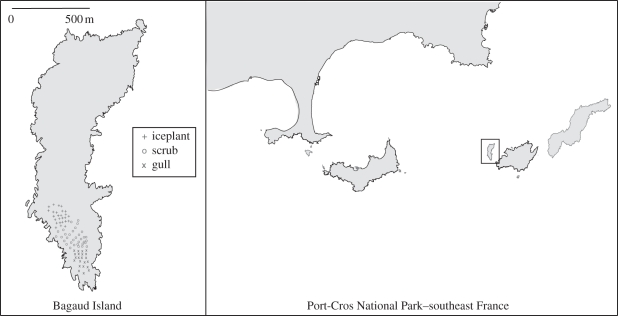
Bagaud Island (43°01″ N, 6°22″ E, 58 ha; 57 m.a.s.l.; figure 1) is a protected nature reserve in Port-Cros National Park (southeast France). The climate is temperate Mediterranean, with average monthly temperatures from 9.5°C to 24.7°C and monthly precipitation from 1.0 to 151.6 mm (Levant Island Meteorological Office 1998–2008). Significant between year variation in climate also occurs, with much drier than average summers (e.g. less than16 mm of rainfall from June to September in 2007, table 1). Black rats probably arrived on Bagaud Island hundreds of years ago when the nearby Port-Cros Island was occupied by humans during the Roman period [25], and are the highest order resident predator on the island.
Figure 1.
Bagaud Island in Port-Cros National Park.
Table 1.
Session dates, rainfall (courtesy of Levant Island Meteorological Office), time ti since start of last session, number of individuals captured ni (total and by per habitat), and the total number of marked individuals Mi+1.
| year | month | rainfall | session | season | ti |
ni |
Mi+1 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| total | gull | scrub | iceplant | |||||||
| 2007 | Apr | 12.6 | 1 | spring | 0 | 53 | 21 | 16 | 16 | 53 |
| May | 128.0 | 2 | spring | 26 | 13 | 3 | 3 | 7 | 61 | |
| Jun | 2.2 | 3 | summer | 36 | 60 | 30 | 17 | 13 | 114 | |
| Jul | 1.0 | 4 | summer | 34 | 54 | 30 | 12 | 12 | 149 | |
| Aug | 10.2 | 5 | summer | 39 | 34 | 17 | 6 | 11 | 159 | |
| Sep | 2.4 | |||||||||
| Oct | 24.4 | 6 | autumn | 55 | 38 | 14 | 12 | 12 | 169 | |
| Nov | 78.6 | |||||||||
| Dec | 28.4 | 7 | winter | 54 | 40 | 15 | 12 | 13 | 183 | |
| 2008 | Jan | 91.2 | 8 | winter | 48 | 43 | 19 | 8 | 16 | 196 |
| Feb | 8.0 | |||||||||
| Mar | 52.4 | |||||||||
| Apr | 56.2 | 9 | spring | 86 | 35 | 14 | 13 | 8 | 208 | |
| May | 46.0 | |||||||||
| Jun | 45.6 | 10 | summer | 42 | 42 | 20 | 12 | 10 | 238 | |
| Jul | 1.2 | 11 | summer | 43 | 106 | 57 | 25 | 24 | 321 | |
| Aug | 1.0 | 12 | summer | 42 | 49 | 33 | 9 | 7 | 334 | |
| Sep | 35.4 | |||||||||
| Oct | 57.8 | 13 | autumn | 40 | 35 | 16 | 6 | 13 | 340 | |
| Nov | 188.4 | |||||||||
| Dec | 220.4 | |||||||||
| 2009 | Jan | 110.8 | 14 | winter | 112 | 91 | 35 | 19 | 37 | 395 |
Black rats were captured over 14 sessions spanning 22 months from April 2007 to January 2009, in 81 permanent trap stations (BTS-Mécanique, Manufrance, Saint Etienne, France) over three distinct habitats (total area 4.25 ha; total length 500 m). Trapping sessions were separated by 26–122 days, and within each trapping session lasted from three to eight nights. All rats captured were marked with a unique sub-cuticle pit tag (FDX-B, IER Paris, France), weighed, sexed, assigned age class juvenile or adult, and released. We identified three habitats in close proximity and without geographical barriers (figure 1), but with resources that vary in quality and availability by season. The gull colony (‘GU’; Larus michahellis; 1.00 ha) is a ruderal open grassland mainly composed of a nitrogen-enriched plant community (e.g. Fabaceae, Poaceae, Juncaceae). The gull habitat has significant levels of guano burn, especially during the gull breeding season in spring (March–May), when vegetation cover expands and an increase in the amount of N-enriched plants, arthropods, food scraps and gull-derived items (egg shells and gull feathers) is observed in rat stomachs [19]. The iceplant habitat (‘IC’; Carpobrotus sp.; 1.25 ha) is also an open area dominated by a monoculture which produces large fleshy fruits that mature during the dry Mediterranean summer (June–August) and are largely eaten by rats during that season of overall poor in situ productivity [19]. Although resource peaks in both habitats are seasonal, their impacts can persist throughout the year (e.g. guano burn and monoculture dominance). The intermediate scrub habitat (‘SC’; 2.00 ha), mainly composed of Pinus halepensis, Erica arborea, Myrtus communis, Arbutus unedo and Pistacia lentiscus, provides substantially more canopy coverage, but with reduced resource availability and a fruit production period more spread out over the year, thus serving as a comparative baseline. Reproductive output and densities fluctuate seasonally across habitats, and the growth rate of young rats is significantly higher in gull and iceplant habitat compared with the scrub, especially during years of low fresh water input [34]. These observations suggest that both resource availability within each habitat and rainfall pulses may be significant drivers of black rat population dynamics. We expect rats would have higher survival rates in subsidized habitats (gull and iceplant) and that the seasonal change of resource quality within habitats would encourage inter-habitat migration. Owing to the linear configuration of the three habitats, rats must pass through the scrubland when moving between gull and iceplant habitats. Radio-tracking confirms rats could move across the entire study area should they choose [19].
3. Model
We constructed an individual effects multi-state model of capture probability and survival over the 22 months in our study. Trapping sessions occurred in 14 months from which we estimate constant nightly capture probability, pj, over kj nights and monthly survival, 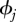 . For months where no trapping occurred pj is constrained to zero and
. For months where no trapping occurred pj is constrained to zero and 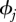 specified as the previous month. The model incorporates survival rates and capture probabilities at each month, and seasonal movement probabilities between each of the three habitats. Spatial resource variation is incorporated in the model as fixed effects for each habitat. Temporal resource pulses are incorporated in the model as a fixed linear effect for rainfall. We use only rainfall as a proxy for all climate pulses in order to avoid over-fitting [35]. Spatio-temporal resource pulses are modelled by the fixed effect interaction term of habitat and season, i.e. a seasonal subsidy in any particular habitat. In addition to spatial and temporal fixed effects, survival and capture probabilities may vary with sex, age and individual random effects.
specified as the previous month. The model incorporates survival rates and capture probabilities at each month, and seasonal movement probabilities between each of the three habitats. Spatial resource variation is incorporated in the model as fixed effects for each habitat. Temporal resource pulses are incorporated in the model as a fixed linear effect for rainfall. We use only rainfall as a proxy for all climate pulses in order to avoid over-fitting [35]. Spatio-temporal resource pulses are modelled by the fixed effect interaction term of habitat and season, i.e. a seasonal subsidy in any particular habitat. In addition to spatial and temporal fixed effects, survival and capture probabilities may vary with sex, age and individual random effects.
We use a modified version of Royle [31] to account for the number of captures within a session. For individual i in month j, we treat xij as the number of times individual i was trapped out of kj nights in month j. X = (xij) is the matrix of trapping records, and Z = (zij) where zij = 1 if individual i is alive in month j, else 0. Thus for our survival process model, we have the conditional relationship
and for our capture observation model, we have the relationship conditional on the state process
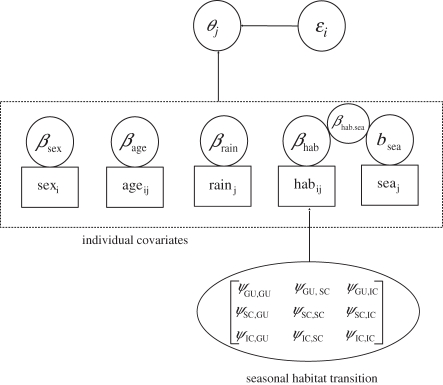
We wish to make inference on 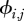 , survival of individual i from month j − 1 to j. We note similarities with Gimenez et al. [30] and Schofield et al. [33]. The value of zij is only partially observed as an individual's state is only known if it is captured. Similarly, we treat age class, A = (aij) (adult or juvenile), and habitat, H = (hij), as partially observed states dependent on capture. For age classes, we can impute missing (unobserved) values based on the well-known rapid maturation of wild rats [36] relative to our time between trapping sessions. We treat habitat as a state variable with missing (unobserved) values, upon which we impose a seasonal transition probability matrix with probabilities ψt,f,g for moving in season t from habitat f to g between months, where t = spring, summer, autumn or winter, and where f and g = IC, SC or GU accordingly [33,37]. Sex, si, is assigned upon first capture. To account for potential additional individual heterogeneity in survival and capture probability, we included random effects in our model. Random effects were assumed to be Normal(0,σ2), but owing to poor mixing were re-parametrized to the mathematically equivalent σ.Normal(0,1), which speeds mixing by constraining to the standard normal distribution.
, survival of individual i from month j − 1 to j. We note similarities with Gimenez et al. [30] and Schofield et al. [33]. The value of zij is only partially observed as an individual's state is only known if it is captured. Similarly, we treat age class, A = (aij) (adult or juvenile), and habitat, H = (hij), as partially observed states dependent on capture. For age classes, we can impute missing (unobserved) values based on the well-known rapid maturation of wild rats [36] relative to our time between trapping sessions. We treat habitat as a state variable with missing (unobserved) values, upon which we impose a seasonal transition probability matrix with probabilities ψt,f,g for moving in season t from habitat f to g between months, where t = spring, summer, autumn or winter, and where f and g = IC, SC or GU accordingly [33,37]. Sex, si, is assigned upon first capture. To account for potential additional individual heterogeneity in survival and capture probability, we included random effects in our model. Random effects were assumed to be Normal(0,σ2), but owing to poor mixing were re-parametrized to the mathematically equivalent σ.Normal(0,1), which speeds mixing by constraining to the standard normal distribution.
We model the fixed effects, β, of sex, age class, habitat, season, habitat–season interaction, log10(rain) and individual random effects, ε, as covariates on capture and survival. This is achieved using a logistic generalized linear-mixed model:
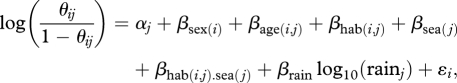 |
where θ = p or 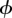 and the intercept
and the intercept 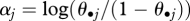 (i.e. the average log-odds ratio when all covariates are zero).
(i.e. the average log-odds ratio when all covariates are zero).
Where the 95% credible interval for the fixed effect excludes zero, we assume the effect is statistically significant. We estimate the average nightly capture probability and survival probability for each month incorporating all covariates across all alive individuals, and then estimate a geometric mean monthly survival across our entire study. The mean survival estimate is used to estimate demographic parameters such as mean lifespan [−1/ln(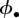 )] and maximum age (less than 10% survival) of rats on Bagaud Island [38]. The entire model can be conceptually represented by a directed acyclical graph (figure 2).
)] and maximum age (less than 10% survival) of rats on Bagaud Island [38]. The entire model can be conceptually represented by a directed acyclical graph (figure 2).
Figure 2.
Directed acyclical graph (DAG) for the Bagaud Island black rat model where θ = p or 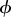 .
.
Model selection in a Bayesian framework is difficult when missing data or random effects, both of which we have, are present in a model specification [33,39]. We focused on building a biologically realistic model incorporating covariates that are reasonably expected to have an influence on rodent population dynamics (and where data were available).
In our population, migration into or out of our study area during the two year study is confounded with capture probability and survival, but we assume such movements on the boundaries are random and rare, only affecting our precision without bias [40]. We assumed a closed state population within months, i.e. that an individual did not move between states (i.e. age or habitats) during any trapping session. This was not true for some individuals (n = 18, 2.6% of observations) who did move between habitats within months. For these individuals, we took the first location of capture as the animal's habitat for that month. Our estimates of capture probability are only applicable to those n individuals captured within our study and alive in any given month. Temporary emigration will negatively bias capture probability [41], but we treat capture probability only as a nuisance parameter. Confounding in the last session prevents estimation of the final survival and capture probabilities [31].
Our model formulation gives a total of 79 independent parameters to estimate, after considering constraints within our habitat transition matrix (rows sum to one). Following others [30,31,37], we use uniform priors for baseline monthly survival rate and capture probability. Perceivably uninformative priors on coefficients of logistic models can substantially alter the distribution of the modelled response (bi-modally weighting it towards extremes [42]). We do not expect survival or capture to change drastically (greater than ±1 on logit scale) with covariates, and so we use conservative prior distributions appropriate to our binary state variables in order to retain relatively uninformative prior distributions on survival rate and capture probability when incorporating covariates (electronic supplementary material, appendix S1); Normal(0,1) priors for fixed effects parameters and Uniform(0,5) for random effect standard deviations. Finally, we use a Dirichlet [1] prior for seasonal habitat transition probabilities (rows sum to one). We ran the model as two chains from randomly drawn initial values for 55 000 iterations discarding the first 5000 iterations of each chain as burn-in after which convergence was achieved. We performed model analysis entirely in WinBUGS v. 1.4, followed by a suite of standard Markov chain Monte Carlo (MCMC) diagnostics [43], and testing sensitivity to covariate priors. We considered fixed effects significant if their 95% credible interval did not include zero [44].
4. Results
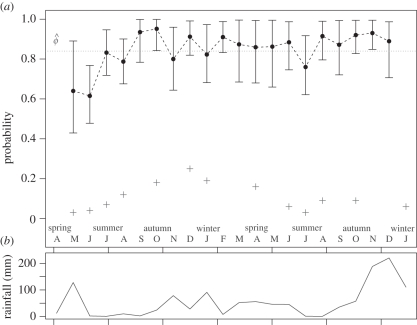
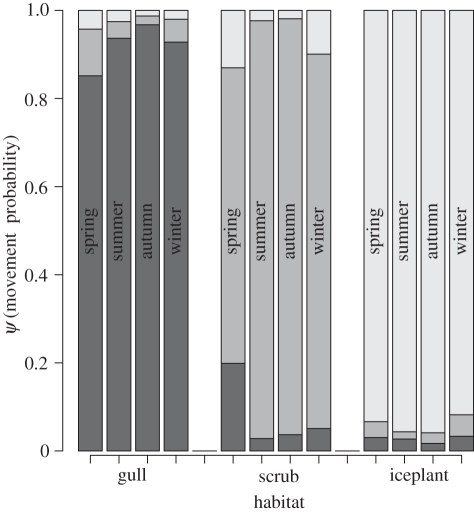
A total of 395 individuals were captured 685 times. Average monthly survival estimates ranged from 0.62 to 0.95, with clear seasonal trends (figure 3; electronic supplementary material, appendix S2). Mean nightly capture probabilities ranged from 0.03 to 0.32 (figure 3; electronic supplementary material, appendix S2), and were significantly lower in summer or during high rainfall (table 2). Juvenile rats had a lower probability of capture (table 2). Survival was only significantly affected by log(rainfall) (table 2), although this variable also had high auto-correlation within the MCMC chains. Neither habitat nor sex had a significant effect on rat survival or capture probability, and no evidence of a seasonal–habitat interaction was detected (table 2). The seasonal habitat transition matrix showed that rats were most likely to stay within the same habitats between season (ψ > 0.84), except in spring when there was increased movement out of scrub predominantly into gull habitat (figure 4). Estimates of survival rates, capture probabilities, fixed and random effects were robust to more uninformative prior distribution specifications (Normal(0,10) for fixed effects, Uniform(0,10) for random effects). This was despite the fact that for logistic models more uninformative prior distributions on covariates heavily inform the prior distribution of the modelled response (electronic supplementary material, appendix S1). Based on our mean monthly survival estimate, the mean lifespan of rats on Bagaud Island is 5.74 months (95% credible interval 4.79–7.01), and only 10 per cent of rats would survive beyond 13 months.
Figure 3.
(a) Average monthly survival across all habitats and nightly capture probability estimates. Bars indicate 95% credible intervals (filled circles, monthly survival; plus symbols, monthly capture). (b) Monthly rainfall. April 2007–January 2009 (months indicated).
Table 2.
Posterior parameter summaries for fixed and random effects with 95% credible intervals. Fixed effects for habitat–season interaction are not shown (all not significant).
| capture | mean | s.d. | q0.025 | q0.975 | survival | mean | s.d. | q0.025 | q0.975 |
|---|---|---|---|---|---|---|---|---|---|
| sexF | 0.10 | 0.21 | −0.31 | 0.52 | sexF | 0.10 | 0.24 | −0.36 | 0.60 |
| ageJ | −0.58 | 0.21 | −1.00 | −0.18 | ageJ | 0.01 | 0.45 | −0.80 | 0.99 |
| habitatgull | 0.59 | 0.47 | −0.31 | 1.54 | habitatgull | −0.45 | 0.51 | −1.44 | 0.54 |
| habitaticeplant | 0.28 | 0.43 | −0.53 | 1.13 | habitaticeplant | −0.38 | 0.50 | −1.41 | 0.58 |
| seasonsummer | −1.61 | 0.67 | −2.86 | −0.22 | seasonsummer | 0.81 | 0.70 | −0.57 | 2.16 |
| seasonautumn | 0.20 | 0.74 | −1.23 | 1.62 | seasonautumn | 0.50 | 0.85 | −1.18 | 2.15 |
| seasonwinter | 0.27 | 0.81 | −1.32 | 1.87 | seasonwinter | 0.55 | 0.82 | −1.05 | 2.13 |
| log(rainfall) | −0.63 | 0.18 | −0.98 | −0.29 | log(rainfall) | 0.44 | 0.19 | 0.10 | 0.86 |
| σp | 1.03 | 0.14 | 0.78 | 1.34 | σΦ | 0.87 | 0.42 | 0.10 | 1.77 |
Figure 4.
Seasonal movement probabilities among habitats (all s.d. < 0.11). Black bars, to gull; dark grey bars, to scrub; light grey bars, to iceplant.
5. Discussion
Although both spatial subsidies and temporal resource pulses were regulated by season in our study, the temporal resource pulse, rainfall, had the strongest effect on survival. Rat survival increased by a factor of up to 1.6 with intense rainfall events, which themselves could vary by a factor of up to 200 from winter to summer. This relationship was logarithmic (i.e. nonlinear), suggesting that rainfall immediately enhances rat survival, but this effect rapidly becomes saturated. These results confirm previous studies conducted on tropical continental systems that showed a boost in rodent population dynamics, including individual survival [13], generated by an increase in primary productivity lagging behind rainfall pulses, as resource pulses move through trophic webs [45,46]. However, in the Galapagos correlations between rainfall pulses and rodent abundances were not systematic within the same island but depended mostly on habitat characteristics, such as vegetation cover or local climate [47].
In contrast, no consistent effect of spatial habitat subsidies on rat survival was found in our study. Rat survival tended to be lower in more open gull and iceplant habitats, but with high variation in the estimate of this effect. The absence of predictable spatial variation in survival may be caused by the seasonal island-wide rainfall pulses homogenizing micro-habitat differences across habitats following dry periods. Alternatively, although the habitats on Bagaud Island differentially subsidize individual growth rates, reproductive output and densities [34], they may not significantly alter survival. While seabird-derived resources can substantially enhance the population dynamics of high-order consumers on arid islands [9], the effects of seabirds vary among seabird-island systems, as a result of in situ productivity [48]. Studies of dry islands in the Gulf of California show that pulsed rainfall events and seabird colonies interact to influence the growth of plant populations [9], and in these environments trapping rates of rodent populations are spatially heterogeneous, and increase by a factor of 1.5–4 during years of intense rainfall, leading to invasion of less preferred habitats [7]. In contrast, we found rainfall decreased capture probabilities. Outside of rainfall, we also found no additional significant effect of season on rat survival. Following no habitat or season-specific effects on survival, we also found no evidence of season-specific habitat subsidies, although statistical power was poor for this high-level interaction. The impact of seasonal spatial subsidies could be diffused throughout the year, for example, where the chemical and physical disturbances caused by gulls favour the massive establishment of nitrogen-enriched flora [28] and alters arthropod assemblages over longer periods [29].
Investigating inter-habitat migration of individuals as a function of shifting intra-annual resource availability is a key issue for understanding how generalist consumers stabilize populations and adapt to spatio-temporal variation in availability of resources. This topic has been addressed for small mammal populations in continental systems [22,49,50] but has rarely been investigated for insular rodent populations (but see [7,18]). Overall, black rats moved very little among habitats tracking resources, contrary to other consumers that may increase mobility in response to pulsed resources [4]. The spring season did show a marked tendency for rats to move from scrubland to the adjacent gull habitat. This movement almost certainly represents adult rats seeking better quality resources, rather than juvenile dispersal, as juveniles were not caught prior to dispersal. Even this single instance of deliberate migration tracking resources remained moderate (ψ = 0.20). With no control on Bagaud Island, rats exist at high densities and it seems intra-specific territorial interactions limit the mobility of individuals, preventing access to high-quality resources by subordinate animals [19]. This occurs despite rats being physically capable of traversing our entire study area, as revealed by distances moved by a subsample of radio-tracked individuals [34].
Our modelling approach provided a powerful and sophisticated method with which to simultaneously analyse the effects of spatial and temporal resource variations on individual survival and movement. We were able to partition complex model components into simple conditional components to which we could specify relationships [32]. We estimated habitat movement rates independently of survival (sensu [37]). If survival differs between habitats, then state-transition and survival must be jointly considered (sensu [51]). However, we found no significant difference in survival among habitats, and given the strong posterior weighting towards remaining in the same habitat, we expect our state-transition matrix, ψ, to be a good indicator of movement rates. Without incorporating the influences of survival and different habitats from our model, it would have been impossible to determine movement rates from the raw trapping data alone (e.g. table 1). We were also able to estimate capture probability, accounting for different trapping session lengths. In particular, we found that capture probability was significantly lower in summer and for juveniles. The random effect for capture probability also compensated for unexplained heterogeneity owing to non-random trap placement, where individuals were exposed to different levels of trapping intensity.
Introduced rats play a major role in island ecosystems. Not only do they have direct effects on other species, both as predators (e.g. [24]) and prey [52], but also through these strong direct effects they have cascading top-down indirect effects on other ecosystem components, such as invertebrates [53] and plants [54]. The magnitude of these indirect effects can also vary spatially [8]. Given dominant bottom-up regulation of introduced rats on islands, resource pulses leading to rat irruptions may generate strong direct effects such as extinctions of vulnerable native species [55], and also indirect effects such as increased food abundance for higher level introduced predators, where present [52]. On Bagaud Island, the low probability of movements recorded among habitats dampens the potential for rats to disperse introduced iceplant seeds over long distances, reducing the potential for invasional facilitation [23]. Our results have direct implications for the management of black rat populations on Mediterranean islands. As climate is the main driver of rat survival, the optimal time for an eradication programme would be during dry summer months, when the rat population is most limited by fresh water and food resources, even though at this time rat capture probability is also lowest.
Acknowledgements
Thanks to the National Science Foundation (NSF) funded Seabird-Predator (SEAPRE) Research Coordination Network (RCN) for facilitating L.R.'s visit to UC Berkeley. Thanks to Perry de Valpine and Jonas Knape for discussions and assistance on implementing Bayesian state-space models, and comments on earlier versions of the manuscript. We especially thank the Port-Cros National Park for authorization and logistic support and the volunteers who helped trap rats on Bagaud Island. J.C.R. was funded by Post-doctoral Fellowship UCAL081 from the New Zealand Foundation for Research, Science and Technology and L.R. by a doctoral fellowship from the ‘Ecole Doctorale des Sciences de l'Environnement’, Paul Cézanne University, and by the ANR (French National Research Agency) with the ALIENS project. Thanks to Rachel Fewster, Eric Vidal, Per Lundberg and others for comments on the manuscript.
References
- 1.Orr M., Zimmer M., Jelinski D. E., Mews M. 2005. Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy. Ecology 86, 1496–1507 10.1890/04-1486 (doi:10.1890/04-1486) [DOI] [Google Scholar]
- 2.Bissonette J. A., Storch I. 2007. Temporal dimensions of landscape ecology: wildlife responses to variable resources. New York, NY: Springer [Google Scholar]
- 3.Marczak L. B., Thompson R. M., Richardson J. S. 2007. Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88, 140–148 10.1890/0012-9658(2007)88[140:MTLHAP]2.0.CO;2 (doi:10.1890/0012-9658(2007)88[140:MTLHAP]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 4.Yang L. H., Bastow J. L., Spence K. O., Wright A. N. 2008. What can we learn from resource pulses. Ecology 89, 621–634 10.1890/07-0175.1 (doi:10.1890/07-0175.1) [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Piñero F., Polis G. A. 2000. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81, 3117–3132 [Google Scholar]
- 6.Polis G. A., Hurd S. D., Jackson C. T., Sánchez-Piñero F. 1997. El Niño effects on the dynamics and control of an island ecosystem in the Gulf of California. Ecology 78, 1884–1897 [Google Scholar]
- 7.Stapp P., Polis G. A. 2003. Influence of pulsed resources and marine subsidies on insular rodent populations. Oikos 102, 111–123 10.1034/j.1600-0706.2003.12445.x (doi:10.1034/j.1600-0706.2003.12445.x) [DOI] [Google Scholar]
- 8.Rayner M. J., Hauber M. E., Imber M. J., Stamp R. K., Clout M. N. 2007. Spatial heterogeneity of mesopredator release within an oceanic island system. Proc. Natl Acad. Sci. USA 104, 20 862–20 865 10.1073/pnas.0707414105 (doi:10.1073/pnas.0707414105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson W. B., Wait D. A., Stapp P. 2008. Resources from another place and time: responses to pulses in a spatially subsidized system. Ecology 89, 660–670 10.1890/07-0234.1 (doi:10.1890/07-0234.1) [DOI] [PubMed] [Google Scholar]
- 10.Holt R. D. 2008. Theoretical perspectives on resource pulses. Ecology 89, 671–681 10.1890/07-0348.1 (doi:10.1890/07-0348.1) [DOI] [PubMed] [Google Scholar]
- 11.Hanski I., Hansson L., Henttonen H. 1991. Specialist predators, generalist predators, and the microtine rodent cycle. J. Anim. Ecol. 60, 353–367 10.2307/5465 (doi:10.2307/5465) [DOI] [Google Scholar]
- 12.O'Connell M. A. 1989. Population dynamics of neotropical small mammals in seasonal habitats. J. Mammal. 70, 532–548 10.2307/1381425 (doi:10.2307/1381425) [DOI] [Google Scholar]
- 13.Madsen T., Shine R. 1999. Rainfall and rats: climatically-driven dynamics of a tropical rodent population. Aust. J. Ecol. 24, 80–89 10.1046/j.1442-9993.1999.00948.x (doi:10.1046/j.1442-9993.1999.00948.x) [DOI] [Google Scholar]
- 14.Brown J. H., Ernest S. K. M. 2002. Rain and rodents: complex dynamics of desert consumers. Bioscience 52, 979–987 10.1641/0006-3568(2002)052[0979:RARCDO]2.0.CO;2 (doi:10.1641/0006-3568(2002)052[0979:RARCDO]2.0.CO;2) [DOI] [Google Scholar]
- 15.Atkinson I. A. E. 1985. The spread of commensal species of Rattus to oceanic islands and their effects on island avifaunas. In Conservation of island birds (ed. Moors P. J.), pp. 35–81 Cambridge, UK: International Council for Bird Preservation Technical Publication No. 3 [Google Scholar]
- 16.Clark D. A. 1981. Foraging patterns of black rats across a desert-montane forest gradient in the Galapagos Islands. Biotropica 13, 182–184 10.2307/2388123 (doi:10.2307/2388123) [DOI] [Google Scholar]
- 17.Harper G. A., Dickinson K. J. M., Seddon P. J. 2005. Habitat use by three rat species (Rattus spp.) on Stewart Island/Rakiura, New Zealand. N. Z. J. Ecol. 29, 251–260 [Google Scholar]
- 18.Caut S., Angulo E., Courchamp F. 2008. Dietary shift of an invasive predator: rats, seabirds and sea turtles. J. Appl. Ecol. 45, 428–437 10.1111/j.1365-2664.2007.01438.x (doi:10.1111/j.1365-2664.2007.01438.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruffino L., Russell J. C., Pisanu B., Caut S., Vidal E. 2011. Low individual-level diet plasticity in an island-invasive generalist forager. Popul. Ecol. 10.1007/j.S10144-011-0265-6 (doi:10.1007/j.S10144-011-0265-6) [DOI] [Google Scholar]
- 20.Russell J. C., Ringler D., Trombini A., Le Corre M. 2011. The island syndrome and population dynamics of introduced rats. Oecologia. 10.1007/s00442-011-2031-z (doi:10.1007/s00442-011-2031-z) [DOI] [PubMed] [Google Scholar]
- 21.Polis G. A., Anderson W. B., Holt R. D. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. System. 28, 289–316 10.1146/annurev.ecolsys.28.1.289 (doi:10.1146/annurev.ecolsys.28.1.289) [DOI] [Google Scholar]
- 22.Lin Y. K., Batzli G. O. 2001. The influence of habitat quality on dispersal, demography and population dynamics of voles. Ecol. Monogr. 71, 245–275 10.1890/0012-9615(2001)071[0245:TIOHQO]2.0.CO;2 (doi:10.1890/0012-9615(2001)071[0245:TIOHQO]2.0.CO;2) [DOI] [Google Scholar]
- 23.Bourgeois K., Suehs C. M., Vidal E., Médail F. 2005. Invasional meltdown potential: facilitation between introduced plants and mammals on French Mediterranean islands. Ecoscience 12, 248–256 10.2980/i1195-6860-12-2-248.1 (doi:10.2980/i1195-6860-12-2-248.1) [DOI] [Google Scholar]
- 24.Towns D. R., Atkinson I. A. E., Daugherty C. H. 2006. Have the harmful effects of introduced rats on islands been exaggerated? Biol. Invasions 8, 863–891 10.1007/s10530-005-0421-z (doi:10.1007/s10530-005-0421-z) [DOI] [Google Scholar]
- 25.Ruffino L., et al. 2009. Invasive rats and seabirds after 2,000 years of an unwanted coexistence on Mediterranean islands. Biol. Invasions 11, 1631–1651 10.1007/s10530-008-9394-z (doi:10.1007/s10530-008-9394-z) [DOI] [Google Scholar]
- 26.Martin J.-L., Thibault J.-C., Bretagnolle V. 2000. Black rats, island characteristics, colonial nesting birds in the Mediterranean: consequences of an ancient introduction. Conserv. Biol. 14, 1452–1466 10.1046/j.1523-1739.2000.99190.x (doi:10.1046/j.1523-1739.2000.99190.x) [DOI] [Google Scholar]
- 27.Hulme P. E. 2004. Invasions, islands and impacts: a Mediterranean perspective. In Island ecology (ed. Fernandez Palacios J. M.), pp. 337–361 La Laguna, Spain: Asociación Española de Ecología Terrestre [Google Scholar]
- 28.Vidal E., Médail F., Tatoni T., Roche P., Vidal P. 1998. Impact of gull colonies on the flora of the Riou archipelago (Mediterranean islands of south-east France). Biol. Conserv. 84, 235–243 10.1016/S0006-3207(97)00130-4 (doi:10.1016/S0006-3207(97)00130-4) [DOI] [Google Scholar]
- 29.Orgeas J., Vidal E., Ponel P. 2003. Colonial seabirds change beetle assemblages on a Mediterranean island. Ecoscience 10, 38–44 [Google Scholar]
- 30.Gimenez O., Rossi V., Choquet R., Dehais C., Doris B., Varella H., Vila J.-P., Pradel R. 2007. State-space modelling of data on marked individuals. Ecol. Model. 206, 431–438 10.1016/j.ecolmodel.2007.03.040 (doi:10.1016/j.ecolmodel.2007.03.040) [DOI] [Google Scholar]
- 31.Royle J. A. 2008. Modeling individual effects in the Cormack–Jolly–Seber model: a state–space formulation. Biometrics 64, 364–370 10.1111/j.1541-0420.2007.00891.x (doi:10.1111/j.1541-0420.2007.00891.x) [DOI] [PubMed] [Google Scholar]
- 32.Calvert A. M., Bonner S. J., Jonsen I. D., Flemming J. M., Walde S. J., Taylor P. D. 2009. A hierarchical Bayesian approach to multi-state mark–recapture: simulations and applications. J. Appl. Ecol. 46, 610–620 10.1111/j.1365-2664.2009.01636.x (doi:10.1111/j.1365-2664.2009.01636.x) [DOI] [Google Scholar]
- 33.Schofield M. R., Barker R. J., MacKenzie D. I. 2009. Flexible hierarchical mark-recapture modeling for open populations using WinBUGS. Environ. Ecol. Stat. 16, 369–387 10.1007/s10651-007-0069-1 (doi:10.1007/s10651-007-0069-1) [DOI] [Google Scholar]
- 34.Ruffino L. 2010. Ecologie, dynamique de population, comportement et impact d'un rongeur introduit Rattus rattus sur les îles de Méditerranée. PhD dissertation (in French with English abstract), Université Paul Cézanne, Aix-en-Provence [Google Scholar]
- 35.Knape J., De Valpine P. 2011. Effects of weather and climate on the dynamics of animal population time series. Proc. R. Soc. B 278, 985–992 10.1098/rspb.2010.1333 (doi:10.1098/rspb.2010.1333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry J. S. 1945. The reproduction of the wild brown rat (Rattus norvegicus Erxleben). J. Zool. 115, 19–46 [Google Scholar]
- 37.Dupuis J. A. 1995. Bayesian estimation of movement and survival probabilities from capture–recapture data. Biometrika 82, 761–772 [Google Scholar]
- 38.Poole D. 2002. Bayesian estimation of survival from mark-recapture data. J. Agric. Biol. Environ. Stat. 7, 264–276 10.1198/10857110260141283 (doi:10.1198/10857110260141283) [DOI] [Google Scholar]
- 39.Celeux G., Forbes F., Robert C. P., Titterington D. M. 2006. Deviance information criteria for missing data models. Bayesian Anal. 1, 651–674 10.1214/06-BA122 (doi:10.1214/06-BA122) [DOI] [Google Scholar]
- 40.Kendall W. L. 1999. Robustness of closed capture–recapture methods to violations of the closure assumption. Ecology 80, 2517–2525 [Google Scholar]
- 41.Kendall W. L., Nichols J. D., Hines J. E. 1997. Estimating temporary emigration using capture–recapture data with Pollock's robust design. Ecology 78, 563–578 [Google Scholar]
- 42.Van Dongen S. 2006. Prior specification in Bayesian statistics: three cautionary tales. J. Theor. Biol. 242, 90–100 10.1016/j.jtbi.2006.02.002 (doi:10.1016/j.jtbi.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 43.Cowles M. K., Carlin B. P. 1995. Markov Chain Monte Carlo diagnostics: a comparative review. J. Am. Stat. Soc. 91, 883–904 [Google Scholar]
- 44.Nakagawa S., Cuthill I. C. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605 10.1111/j.1469-185X.2007.00027.x (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 45.Ernest S. K. M., Brown J. H., Parmenter R. R. 2000. Rodents, plants, and precipitation: spatial and temporal dynamics of consumers and resources. Oikos 88, 470–482 10.1034/j.1600-0706.2000.880302.x (doi:10.1034/j.1600-0706.2000.880302.x) [DOI] [Google Scholar]
- 46.Previtali M. A., Lima M., Meserve P. L., Kelt D. A., Gutiérrez J. R. 2009. Population dynamics of two sympatric rodents in a variable environment: rainfall, resource availability, and predation. Ecology 90, 1996–2006 10.1890/08-0405.1 (doi:10.1890/08-0405.1) [DOI] [PubMed] [Google Scholar]
- 47.Clark D. B. 1980. Population ecology of Rattus rattus across a desert-montane forest gradient in the Galápagos Islands. Ecology 61, 1422–1433 10.2307/1939051 (doi:10.2307/1939051) [DOI] [Google Scholar]
- 48.Mulder C. H. P., Anderson W. B., Towns D. R., Bellingham P. J. 2011. Seabird islands: ecology, invasion, and restoration. New York, NY: Oxford University Press [Google Scholar]
- 49.Morris D. W. 1987. Tests of density-dependent habitat selection in a patchy environment. Ecol. Monogr. 57, 269–281 10.2307/2937087 (doi:10.2307/2937087) [DOI] [Google Scholar]
- 50.Halama K. J., Dueser R. D. 1994. Of mice and habitats: tests for density-dependent habitat selection. Oikos 69, 107–114 10.2307/3545289 (doi:10.2307/3545289) [DOI] [Google Scholar]
- 51.Brownie C., Hines J. E., Nichols J. D., Pollock K. H., Hestbeck J. B. 1993. Capture–recapture studies for multiple strata including non-Markovian transitions. Biometrics 49, 1173–1187 10.2307/2532259 (doi:10.2307/2532259) [DOI] [Google Scholar]
- 52.Bonnaud E., Bourgeois K., Vidal E., Kayser Y., Tranchant Y., Legrand J. 2007. Feeding ecology of a feral cat population on a small Mediterranean island. J. Mammal. 88, 1074–1081 10.1644/06-MAMM-A-031R2.1 (doi:10.1644/06-MAMM-A-031R2.1) [DOI] [Google Scholar]
- 53.Towns D. R., Wardle D. A., Mulder C. P. H., Yeates G. W., Fitzgerald B. M., Parrish G. R., Bellingham P. J., Bonner K. I. 2009. Predation of seabirds by invasive rats: multiple indirect consequences for invertebrate communities. Oikos 118, 420–430 10.1111/j.1600-0706.2008.17186.x (doi:10.1111/j.1600-0706.2008.17186.x) [DOI] [Google Scholar]
- 54.Mulder C. P. H., Grant-Hoffman M. N., Towns D. R., Bellingham P. J., Wardle D. A., Durrett M. S., Fukami T., Bonner K. I. 2009. Direct and indirect effects of rats: does rat eradication restore ecosystem functioning of New Zealand seabird islands? Biol. Invasions 11, 1671–1688 10.1007/s10530-008-9396-x (doi:10.1007/s10530-008-9396-x) [DOI] [Google Scholar]
- 55.Harper G. A. 2005. Heavy rimu (Dacrydium cupressinum) mast seeding and rat (Rattus spp.) population eruptions on Stewart Island/Rakiura. N. Z. J. Zool. 35, 155–162 10.1080/03014223.2005.9518408 (doi:10.1080/03014223.2005.9518408) [DOI] [Google Scholar]