Abstract
Plant toxins are sequestered by many animals and the toxicity is frequently advertised by aposematic displays to deter potential predators. Such ‘unpalatability by appropriation’ is common in many invertebrate groups and also found in a few vertebrate groups. However, potentially lethal toxicity by acquisition has so far never been reported for a placental mammal. Here, we describe complex morphological structures and behaviours whereby the African crested rat, Lophiomys imhausi, acquires, dispenses and advertises deterrent toxin. Roots and bark of Acokanthera schimperi (Apocynaceae) trees are gnawed, masticated and slavered onto highly specialized hairs that wick up the compound, to be delivered whenever the animal is bitten or mouthed by a predator. The poison is a cardenolide, closely resembling ouabain, one of the active components in a traditional African arrow poison long celebrated for its power to kill elephants.
Keywords: Lophiomys, Acokanthera, ouabain, toxicity, aposematic, mammal
1. Introduction
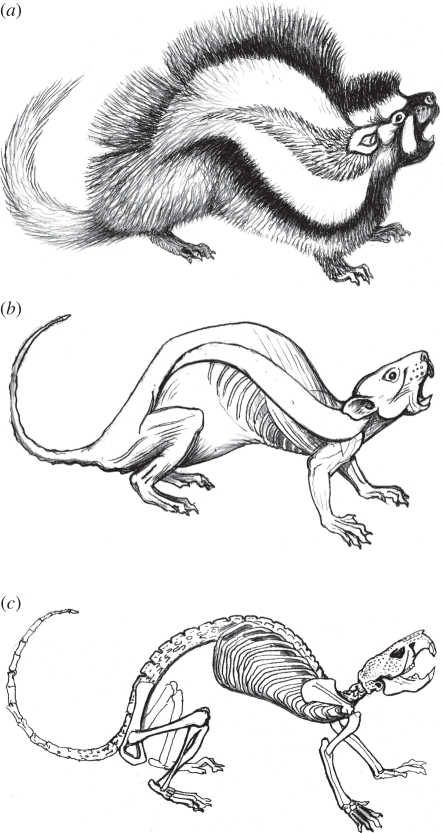
Since the discovery of the African crested rat, Lophiomys imhausi [1], the adaptive significance of the rodent's specializations, notably its peculiar coloration and generally sluggish behaviour, has escaped satisfactory explanation [2]. The ability to expose a bold black and white fur pattern has invited speculation about mimicry of spiny porcupines Hystricidae [3] or skunk-like zorillas Ictonyx striatus (Mustelidae). Indeed, Lophiomys has been held to exemplify a rare example of Batesian mimicry among mammals with the zorilla as a model [4]. While it is possible that a piebald face and flank stripes might have sufficient resemblance to the zorilla's pattern to deter some predators, Lophiomys is probably too poor a copy of the zorilla to qualify as its mimic. Furthermore, it is commonly overlooked that the crested rat is normally inconspicuous and that its long grey-tipped fur envelops its body completely (figure 1). Its black and white flanks are only exposed when the animal is disturbed or excited.
Figure 1.
(a) Lophiomys imhausi warning display; note lateral hair tract at visual centre of signal design. (b) Outline of skinned specimen in same position with lateral-line tract outlined; note how long hair transforms visual appearance. (c) Skeleton; note robust and elongated vertebral column, and possible role of scapulae as protective shields above vital centres. For a view of the animal's routine state, see the figure of electronic supplementary material, video S1.
The belief that Lophiomys is poisonous has long been current in Kenya [2,5,6]. Of particular interest is a tract of hairs that are of unique structure. We have confirmed the suggestion that this tract of hairs can acquire toxic properties and we assert here that they, together with other specialized behavioural, morphological and anatomical adaptations, have led to a powerful defence, highly unusual for a mammal. Flaring of the fur is triggered by external interference or attack on the animal, whereupon white and black banding of the longer hairs on either side of the lateral line effects outlines of the tract in a bold white and black ‘target’ design. An aggravated rat pulls its head back into its shoulders and turns its flared tract towards its adversary as if actively soliciting an attack. This display may or may not be accompanied by vocalizations.
A complex of defensive traits in the crested rat would have evolved against natural predators; however, most reliable observations about their efficiency and effectiveness have come from numerous reports of encounters with domestic dogs. One dog, on seeing a crested rat after the former had survived a putative near-death encounter, displayed every sign of fearful aversion (P. Bengough 2010, unpublished observation). Several descriptions of symptoms exhibited by dogs that have bitten Lophiomys range from mild lack of coordination, mouth-frothing and signs of general distress to collapse and rapid death, apparently from heart failure (M. Coverdale 2008, unpublished observations). One autopsy of a poisoned dog described pale mucous membranes and white blood cells with toxic granules, defective blood-clotting and associated generalized bleeding (S. Ghalay 2009, unpublished observation). In two cases, dogs that were seen to have attacked a crested rat took weeks to recover from severe and disabling symptoms (M. Coverdale & T. Hobbs 2008, unpublished observations).
These reports and anecdotes all demonstrate that although there is little or no evidence of the rat physically piercing any tissue, its defence is surprisingly effective against larger mammalian predators. The adaptations that enforce deterrence and details of the mechanisms involved are equally surprising.
The closest equivalent to this phenomenon reported to date concerns hedgehogs (Erinaceus europeus) chewing the parotid glands of toads Bufo and slathering the resultant mixture of saliva and toad venom onto its spines [7]. While this self-annointing is known among hedgehogs to make pricks from hedgehog spines more painful and irritating, there is, as yet, no evidence for lethal/terminal effects on the predator. The primary effect of such behaviour and use of substances seems to be to augment the pain and discomfort inflicted by the spines. In the case of Lophiomys, there is no evidence that a response to the toxins is dependent on any kind of wound. Mere contact between the mucous membranes of the predator's mouth and toxin in the rat's hairs would seem sufficient to precipitate symptoms of acute poisoning. Further research may reveal a pathology whereby mucous membrane cells are permeated directly without the need to actually wound an aggressor. For example, the possibility that Lophiomys saliva might introduce facilitating agents such as non-polar cardenolides deserves investigation. Unlike the hedgehog, which relies on its spines for both deterrent and punitive effects, the exposure of Lophiomys to damaging bites would seem to have selected for a suite of mitigating structures. These include an armoured skull, enlarged vertebrae, and dense and thick skin, in addition to fearless behaviour, all of which imply reliance upon a form of deterrence that must be extremely fast-acting in order not to be consistently more costly and dangerous to the prey than to the predator.
Although the gross structure of flank hairs has been described under low-power microscopy, and its possible function discussed [2], some more detailed features of the microstructure of these hairs under scanning electron microscope (SEM) were first described by Stoddart [8], who, like Kingdon [2], suggested that the hairs might be adapted to absorb secretions from an apparent ‘belt of glandular tissue fringed both above and below by short stiff hairs’ that lie ‘at the base of the crest and extend the whole of its length’ (p. 552 in [8]). General characterization of the dorsal hair above the flank hair tracts as a ‘crest’ is misleading inasmuch as dense, long hair grows over most of the body. An excited Lophiomys is more aptly described as ‘parting’ the fur along its flanks, rather than ‘raising its crest’. Furthermore, the specialized flank hairs do not ‘fringe’ a supposed glandular area but are, instead, densely but loosely rooted in a leaf-shaped tract of apparently differentiated skin that tapers away from behind the ears to end over the pelvic–femoral angle. Noting the uniquely complex structure of Lophiomys flank hairs, Stoddart suggested that pungent odours might serve to protect the animal from predators. To the human nose, Lophiomys does not smell strongly and dogs have been reported to show no sign of being deterred until actual oral contact with the rat has been made. This implies that an absence of olfactory cues and the deterrence must be learned by the predator from direct contact with the rat or from individuals observing the reactions of other predators.
2. Hair morphology
While glandular structures underlying the flank tract have yet to be demonstrated, a more exact and detailed description of the hairs is needed, especially in the light of their now-known toxin-holding function. We have investigated the structure and function of the crested rat's highly specialized hairs in this context. Rather than growing at the ill-defined base of a ‘crest’, the hairs grow along two lateral lines that run across the flanks (figure 1a). The animal exposes these tracts of somewhat shorter hair (mean 28 mm, range 18–32 mm) by means of specially modified dermal muscles (figure 1b). These muscles erect the animal's long (mean 46 mm, range 38–52 mm and externally grey) fur upwards above the lateral line, while simultaneously deflecting fur downwards below the lateral line.
Hair is parted not only in response to disturbance, but also to allow the animal to apply poison to the specialized flank hairs. When presented with pieces of Acokanthera branch and root, we observed a living wild-caught crested rat to gnaw and masticate the bark and selectively ‘slaver’ the lateral-line fur between repeated bouts of gnawing and chewing. Such mastication appears to liberate the ouabain from the bark and mix it with saliva to form a coarse colloid, which is then specifically applied only to the lateral-line hairs. Fragments of bark that drop to the ground while applying the colloid are carefully picked up and chewed again. Over a period of 5 days, three separate short bouts of bark-chewing, followed by application of the colloid, were observed. This individual ignored leaves and raw green fruit of Acokanthera both as food and as material for mastication. Other individuals have been seen to carefully ‘groom’ their flank hair tracts, but in the absence of any plant material and without any obvious stimulus.
When relaxed, long grey fur covers the lateral-line tracts, and, to a large degree, screens the tracts from the light and repels moderate rain. If toxins in the lateral-line hairs are at all light-sensitive or soluble in water, as seems likely, then insulation from both elements may be just as important a role for the long hair as keeping the rodent warm or making it less conspicuous.
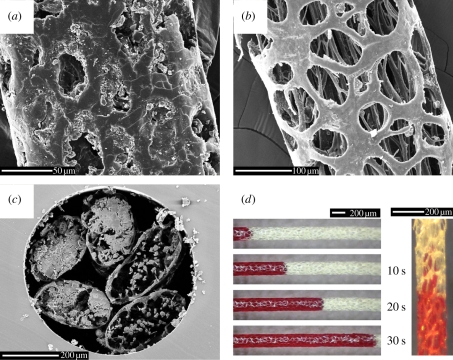
We examined the structure of the lateral-line hairs under both light electron microscope and SEM, and have discovered that the hair exemplifies unique, exceptional optimization and economy of structure for sequestration and delivery of toxins [9,10]. The central portion of each main shaft develops a thin but strong outer cylinder perforated by abundant vacuoles (figure 2). This perforated cylinder encloses fibrillar strands that remain mostly separate, but can adhere to each other or to the outer cylinder along short stretches. These long fibrils are numerous and cumulatively act as a ‘wick’, as we have demonstrated (figure 2d) and observed with our captive specimen. Indeed, as the colloid is applied to the hair, one can observe an almost instantaneous flip from reflective gloss to opaque invisibility, which suggests rapid absorption. In the electronic supplementary material, a short film demonstrates such absorption through capillary action along the interior of each hair cylinder (electronic supplementary material, video S1). Once a hair has become saturated, the colloid appears to dry as a semi-viscous secretion (figure 2a). The hairs' open lattice ensures that processed or ‘groomed’ hairs cannot be touched without contacting the poisonous colloid/secretion.
Figure 2.
Microscopy images of the uniquely adapted poison-delivery hairs growing in a tract along the lateral lines of the African crested rat Lophiomys imhausi. (a–c) Scanning electron microscopy images of the hair indicating (a) a section near the tip fully loaded with poison, (b) detail of the microfibres running up the centre of a washed hair from a section near the root, and (c) cross-sections of five hairs to show internal microfibres and how the saliva is stored. (d) Light microscopy of the ‘wicking’ effect using red ink over 30 s. See also the electronic supplementary material, video S1.
Over 120 years ago, Arnaud [11] extracted and isolated ouabain, a crystalline glycoside from the bark and roots of the Acokanthera tree. Used for poisoning arrows in Somaliland [11], it has become famous as the weapon of choice by East African elephant hunters [12]. For more than two centuries, the bark extract also found use as a clinical treatment against congestive heart failure [13]. Ouabain's activity, as for all cardiac glycosides, comes from its capacity to inhibit the Na+/K+-ATPase, thus greatly increasing the force of cardiac muscle contractions [14].
3. Hair contents
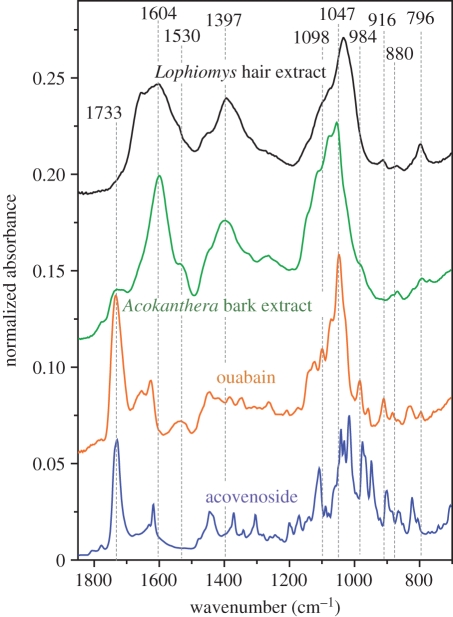
Our Fourier transform infrared (FT-IR) spectroscopy pilot study of the compounds found inside the hairs strongly suggests the presence of ouabain (figure 3). Specifically, ouabain seems to be the active ingredient transferred from the bark to the hair as our spectra had strong peaks at 1530, 916, 880 and 796 cm−1 and lacked peaks more typical for acovenoside, which is another toxic compound sometimes found in Acokanthera bark. In addition, our spectra showed signatures indicative of additional compounds such as lignins or tannins, suggesting that more detailed investigation is likely to elucidate a selective natural extraction system driven by the rats' masticatory behaviour and salivary chemistry. Importantly, the FT-IR spectra of the Acokanthera bark and of the compounds in the active part of the Lophiomys hair are very similar. This confirms our assertion that the compounds slathered onto the rat's lateral-line fur derived from the bark of Acokanthera.
Figure 3.
ATR FT-IR spectra of pure acovenoside, ouabain and extract from the Acokanthera bark and Lophiomys hairs.
While key to its unique toxicity, the highly modified lateral-line hairs of the crested rat are not its only interesting morphological modification. For example, a study of the animal's skull suggests that direct contact with predators has selected for extra shielding of the brain (figure 4). The skull has invited a comparison with the armoured head of a turtle [2] and earned one supposed subspecies the name L. i. testudo. Margins of the frontal, squamosal and parietal have extended around the orbit and over the cranium to create a ‘cranial helmet’, while dense bony ‘pimples’ (rugose osteoblasts) extend over the bridge of the nose and most of the external surfaces of the occiput. These cranial features are unique among rodents. Superficially similar elaboration occurs in the zygomatic region of the paca Agouti paca, where a position around the buccal cavity supports the hypothesis that this has more of an acoustic than protective function. By contrast, bony elaboration in Lophiomys skulls covers or surrounds solid tissues, the brain, nasal passages, eyes and temporal muscles.
Figure 4.
Skull of Lophiomys imhausi showing extensions of jugal, squamosal, frontal and parietal bones, and their granulated surfaces. Temporal fossae are completely roofed over, as are parts of the orbital fossae.
4. Other adaptations
Moreover, there are other interesting macro-morphological modifications associated with the rat's toxicity. For example, the animal has an unusually dense, tough and close-textured dermis, which can be interpreted as predator-selected. The skin is resistant to all but the sharpest of teeth, claws or beaks. The fresh skin of one Lophiomys specimen showed bruising consistent with the rat having been previously savaged by dogs, but the dogs' teeth were either unable to penetrate the tough dermis or the dogs desisted before their teeth could tear through. Species-specific specializations include an elongated vertebral column with three extra thoracic vertebrae and one extra lumbar vertebra forming a backbone that is exceptionally robust and flexible. All vertebrae have enlarged the bodies and short spines (figure 1c). The clavicle is highly atrophied, allowing the broad scapulae exceptional mobility, possibly enhancing the protection of the neck and anterior thorax. An exceptionally large, weakly sacculated stomach is unique among rodents [15], implying a complex physiology for processing plant material [16], resembling that of a fibre-digesting forestomach fermenter. Yet such a conclusion would be vitiated by the fact that easily digested plant parts are the main source of nutrition [16]. The observation that non-fibrous foods are the norm has been confirmed from extensive analysis of faeces in the wild (J. Kingdon 2010, unpublished observation). This could imply other, as yet unknown, functions for the digestive tract of Lophiomys. Given that substantial quantities of very poisonous material frequently enter the rodent's mouth, albeit temporarily, it is plausible that detoxification might be among the specializations of the Lophiomys digestive tract.
Possibly, a key to future studies of the animal's ability to deal with the toxicity of Acokanthera bark to its own health is the salivary glands of crested rats, which are unusually large for their taxon. Because some mammals' saliva produces proteins that bind to plant polyphenols [17,18], it is possible that Lophiomys salivary secretions are adapted to augment or process ouabain (and possibly other plant toxins) in significant but currently unknown ways, among them enhancement of the toxin's capacity to permeate cells. Among some ouabain-insensitive invertebrates, the location of an ouabain-binding site on the DNA sequence has been narrowed to a single amino acid substitution (asparagine for histidine) at position 122 of Na+/K+-ATPase α-subunit gene [19,20]. Possibly, some similar adaptation prevents ouabain from damaging the rat's cellular functions. Ingestion of ouabain into the mouth presents no visible risk to the rat, but what role the salivary glands (or digestive tract) play in this immunity remains to be elucidated. Once the physiological and genetic foundations of Lophiomys immunity are fully understood, there could be implications for molecular or pharmacological applications in human medical therapies.
In terms of understanding the long-term evolution of specialized hair, a recent phylogeny [21] identified four major muroid subdivisions, and allocated Lophiomys its own lineage close to the divergence between Gerbillinae and Murinae. Among the former is a distinct radiation of proto-gerbillines with modified hair: the spiny mice (Acomys), link rats (Deomys) and brush-furred mice (Lophuromys). While no very close affinity between these genera and Lophiomys can be envisaged, it raises the possibility that incremental elaboration of dorsal or flank hair might have very ancient evolutionary origins [22], particularly if selective advantage in relation to predators can be shown, as we do here for Lophiomys.
5. Conclusions
Our confirmation of toxicity by acquisition in Lophiomys suggests that the superficially random suite of peculiarities that we have listed can all be explained as adaptations connected by the evolutionary thread of predator selection. Without regular augmentation of toxins to the flanks, a canopied skull, dense dermis and reinforced skeleton would all be insufficient to deter predators. For both rat and predator, the bold (and memorable) flank pattern represents a costly signal [23]. Significant costs to Lophiomys would suggest that the survival of the rat will continue to rely on readily available supplies of ouabain within the range of northeast African Apocynacae species.
While the details of this extraordinary relationship between mammal and plant require further field and laboratory work, our observations exemplify the power of predation to select for very unusual defences in prey species and, in this instance, astonishing integration of a large suite of behaviours with complex structures. The rat's behaviour in the presence of a predator directs the latter's attention and attack towards a well-advertised specific area, where a painful and poisonous surprise awaits. Micro-structural modification of the hairs growing within this specific area (presumably evolved by small increments) allows them to serve as exceptionally efficient receptacles for self-applied poison. Likewise, chemical adaptation of saliva and tissues probably maximizes deterrence to the predator, while avoiding or minimizing self-harm from the toxins. Finally, macro-structural modifications of separate elements of whole-body anatomy (skin and skeleton) have evolved to make survival more likely by attracting and then ‘managing’ the bites of a predator.
6. Methods summary
Microscopy was performed using an Olympus SZ40 dissection microscope with a Canon A640 digital camera. Samples for SEM studies were coated using an AuPd target for 180 s (resulting in a 6–10 nm layer) and imaged using a Jeol Neoscope (Nikon, UK) under high vacuum at either 10 or 15 keV.
For spectra acquisition, we used a Nicolet 6700 Fourier transform infrared spectrometer equipped with a liquid-nitrogen-cooled MCT-A detector together with a single bounce diamond attenuated total reflectance (ATR) accessory (Thermo Electron Corp., Madison, WI, USA). Spectra were acquired at 4 cm−1 resolution from 6000 to 500 cm−1 using a Happ-Genzel apodization, a Metz phase correction and no zero filling. Spectra were obtained from an average of 64 scans at 5.0632 cm s−1 mirror speed. All spectral operations were performed using Omnic 7.3 (Thermo Scientific, Madison, WI, USA). Spectra were neither deconvolved nor smoothed; the only applied correction was an offset at 4000 cm−1 and a normalization of the intensity. The ouabain and acovenoside spectra were recorded from a thin film cast from an aqueous solution on the ATR crystal. We obtained the Acokanthera bark extract spectrum by immersing the bark in 70°C demineralized water for 10 h before casting the solution on the ATR crystal. Lophiomys imhausi hair extract was prepared by washing several hairs with demineralized water. Both extracts were purified by using 0.2 µm pore size filters under vacuum. Unwashed and washed hair spectra were acquired by directly pressing different parts of specimen hairs with an anvil on the ATR crystal.
Acknowledgements
Hairs were taken from National Museums of Kenya skin Catalogue number NMK 180396 Coll Sept 2010 Field No D. 1.
J.K. initiated the study and wrote the first draft; B.A. provided hairs and background information; J.K., T.O. and M.K. documented poison-slathering behaviour; F.V. and C.H. performed scanning electron microscopy and structural analyses of the hairs; T.G. and M.B.-A. analysed the compounds within the hairs and are indebted to Cedric Dicko for pilot studies. All authors contributed throughout the analysis and writing process. Thanks to Philippa Bengough, Chris Thouless, Miles and Elizabeth Coverdale, Tennai, Susanna Rouse, Richard Mwenda, Surita Ghalay, Darcy Ogada, Tony Archer, Fumi Mizutani Wells, Ian Hardy, Henk Beentje, and John Michael Lock.
References
- 1.Milne-Edwards H. 1867. Memoires c, iii. Arch. Mus. Hist. Nat. Paris. [Google Scholar]
- 2.Kingdon J. East African mammals. An atlas of evolution in Africa, vol. 2, part B (hares and rodents). New York, NY:: Academic Press Inc; 1974. [Google Scholar]
- 3.Hollister N. 1919. East African mammals in the United States National Museum. II. Rodentia, Lagomorpha, and Tubulidentata. United States National Museum Bulletin 99. Washington, DC: Smithsonian Institution [Google Scholar]
- 4.Fogden M., Fogden P. Animals and their colours. London, UK:: Eurobook; 1974. [Google Scholar]
- 5.Euw J. V., Fishelson L., Parsons J. A., Reichstein T., Rothschild M. 1967. Cardenolides (heart poisons) in a grasshopper feeding on milkweeds. Nature 214, 35–39 10.1038/214035a0 (doi:10.1038/214035a0) [DOI] [PubMed] [Google Scholar]
- 6.Goldfinch G. H. 1923. Notes on the African Crested Rat (Lophiomys imhausi). Proc. Zool. Soc. Lond. 93, 1091 [Google Scholar]
- 7.Brodie E. D. 1977. Hedgehogs use toad venom in their own defence. Nature 268, 627–628 10.1038/268627a0 (doi:10.1038/268627a0) [DOI] [Google Scholar]
- 8.Stoddart D. M. 1979. Specialised scent releasing hair in the crested rat Lophiomys imhausi. J. Zool. 189, 551–553 10.1111/j.1469-7998.1979.tb03986.x (doi:10.1111/j.1469-7998.1979.tb03986.x) [DOI] [Google Scholar]
- 9.Wainwright S. A. 1988. Axis and circumference: the cylindrical shape of plants and animals. Cambridge, UK: Harvard University Press [Google Scholar]
- 10.Wainwright S. A., Biggs W. D., Currey J. D., Gosline J. M. 1982. Mechanical design in organisms. New York, NY: John Wiley and Sons [Google Scholar]
- 11.Arnaud M. 1888. Sur la mattiere cristallisee active de fleches empoisonnee des somalis extradite du bois d'Ovabio. C. R. Hebd. Seances l'Acad. Sci. Paris 106/107, 1011–1162 [Google Scholar]
- 12.Schmelzer G. H., Gurib-Fakim A. 2008. Medical plants 1. PROTA foundation. Wageningen, The Netherlands: Backhuys Publishers [Google Scholar]
- 13.Jacobs W. A., Bigelow N. M. 1932. Ouabain or g-Strophantin. J. Biol. Chem. 96, 647–658 [Google Scholar]
- 14.Ruegg U. T. 1992. Ouabain: a link in the genesis of high blood pressure. Experientia 48, 1102–1106 10.1007/BF01947997 (doi:10.1007/BF01947997) [DOI] [PubMed] [Google Scholar]
- 15.Vorontsov N. N. 1979. Evolution of the alimentary system in myomorph rodents. New Delhi, India: The Indian National Scientific Documentation Centre [Google Scholar]
- 16.Naumova E. I., Zharova G. K. 2003. Structure and functions of the digestive tract in the maned hamster Lophiomys imhausi. Zool. J. 82, 1368–1374 [Google Scholar]
- 17.Bennick A. 2002. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 13, 184–196 10.1177/154411130201300208 (doi:10.1177/154411130201300208) [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal G., Berenbaum M. 1992. Herbivores: their interactions with secondary plant metabolites. San Diego, CA: Academic Press [Google Scholar]
- 19.Labeyrie E., Dobler S. 2004. Molecular adaptation of Chrysochus leaf beetles to toxic compounds in their food plants. Mol. Biol. Evol. 21, 218–221 10.1093/molbev/msg240 (doi:10.1093/molbev/msg240) [DOI] [PubMed] [Google Scholar]
- 20.Torrie L. S., Radford J. C., Southall T. D., Kean L., Dinsmore A. J., Davies S. A., Dow J. A. T. 2004. Resolution of the insect ouabain paradox. Proc. Natl Acad. Sci. USA 101, 13 689–13 693 10.1073/pnas.0403087101 (doi:10.1073/pnas.0403087101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansa S. A., Weksler M. 2004. Phylogeny of muroid rodents: relationships within and among major lineages as determined by IRBP gene sequences. Mol. Phylogenet. Evol. 31, 256–276 10.1016/j.ympev.2003.07.002 (doi:10.1016/j.ympev.2003.07.002) [DOI] [PubMed] [Google Scholar]
- 22.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scr. 26, 331–348 10.1111/j.1463-6409.1997.tb00423.x (doi:10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 23.Payne R. J. H., Pagel M. 1997. Why do animals repeat displays? Anim. Behav. 54, 109–119 10.1006/anbe.1996.0391 (doi:10.1006/anbe.1996.0391) [DOI] [PubMed] [Google Scholar]