Abstract
Early-life stress caused by the deprivation of maternal care has been shown to have long-lasting effects on the hypothalamic–pituitary–adrenal (HPA) axis in offspring of uniparental mammalian species. We asked if deprivation of maternal care in biparental species alters stress responsiveness of offspring, using a biparental avian species—the zebra finch, Taeniopygia guttata. In our experiment, one group of birds was raised by both male and female parents (control), and another was raised by males alone (maternally deprived). During adulthood, offspring of both groups were subjected to two stressors (restraint and isolation), and corticosterone concentrations were measured. Additionally, we measured baseline levels of the two corticosteroid receptors—glucocorticoid receptor (GR) and mineralocorticoid receptor (MR)—in the hippocampus, hypothalamus and cerebellum. Our results suggest that maternally deprived offspring are hyper-responsive to isolation in comparison with controls. Furthermore, mRNA levels of both GR and MR receptors are altered in maternally deprived offspring in comparison with controls. Thus, absence of maternal care has lasting consequences for HPA function in a biparental species where paternal care is available.
Keywords: zebra finch, stress, corticosterone, mineralocorticoid receptor, glucocorticoid receptor, maternal deprivation
1. Introduction
The hypothalamic–pituitary–adrenal (HPA) axis mediates the response to stressful events in vertebrates. In response to physical and psychological stressors, input from the limbic system and brain stem triggers release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the paraventricular nucleus (PVN) of the hypothalamus. These peptides stimulate the release of adrenocorticotropic hormone (ACTH) from the pituitary into the circulatory system. Once in circulation, ACTH is transported to the adrenal cortex, where it stimulates the release of glucocorticoids [1,2]. Glucocorticoids exert a multitude of acute effects, some of which include enhancing metabolism and channelling energy to muscles, stimulation of the immune system, inhibiting reproductive behaviours, enhancing cognitive abilities and cerebral glucose utilization, and decreasing appetite. These effects may aid in the survival of an organism [3]. Chronically elevated glucocorticoid levels in an organism, on the other hand, have deleterious effects in the long term [4,5]. The two receptors that mediate the effects of glucocorticoids are the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR). GR is bound only when levels are intermediate to high, such as during the peak of a circadian response or after the initiation of a stress response. In contrast, the affinity of MR for glucocorticoids is 5–10-fold higher than GR, as a result of which it is occupied even during periods of basal secretion [6]. In mammals, in addition to mediating the effects of glucocorticoids in the nervous system and the rest of the body, GR and MR play a key role in the regulation of the HPA axis via both negative and positive feedbacks. The balance of expression of both the GR and MR is thought to be critical to maintain homeostasis within an organism [7–9].
The HPA axis of uniparental mammalian species has been shown to be vulnerable to alterations of the social environment during development. Periods of deprivation of maternal care in species such as rodents and non-human primates have been shown to alter the HPA axis response of offspring to different stressors in adulthood [10,11]. In response to a stressor such as restraint, ACTH and corticosterone levels in maternally deprived rats remained elevated for a longer duration of time when compared with control animals [12]. In addition, maternal deprivation has anxiogenic effects. Maternally deprived rat offspring are highly fearful in adulthood, performing poorly in behavioural tests of novelty that involve feeding or exploring a novel environment such as an elevated plus-maze [13]. Additionally, levels of GR mRNA expression in the hippocampus of maternally deprived rat offspring in adulthood are lower than those of control offspring [14]. CRH levels in the PVN of maternally deprived offspring are higher when compared with control offspring [14,15]. These altered expression patterns of GR and CRF contribute to a hyper-responsive HPA axis in maternally deprived offspring [15–18].
Little is known about the effects of maternal or paternal deprivation in biparental species, where both parents contribute to raising offspring. In the present study, we sought to address the effect of maternal deprivation on the HPA axis of offspring of a monogamous and biparental avian species—the zebra finch, Taeniopygia guttata. In our experiment, zebra finch offspring were raised by their fathers (maternally deprived) in complete absence of their mothers from an early stage or by both parents (control). When these offspring reached adulthood, their plasma corticosterone concentrations were measured in response to restraint and isolation in separate experiments. In addition, levels of GR and MR mRNA were measured in specific brain regions to address the possibility that any differences in corticosterone concentrations between maternally deprived offspring and control offspring in response to stressors might be accompanied by changes in corticosteroid receptor expression.
2. Material and methods
(a). Breeding colonies, female deprivation manipulation and subsequent housing
Male and female zebra finches that were raised in the laboratory in the same colony and had prior breeding experience were allowed to pair in aviaries. Birds were housed on a 14 L : 10 D cycle. Humidity ranged from 30 to 70 per cent and room temperatures were maintained at 22°C. Each breeding aviary (0.94 × 0.76 × 0.94 m) was divided into two halves. In one half, both male and female birds raised their young (control offspring), whereas in the other half, male birds raised the young alone (maternally deprived offspring). Adult females were removed from this half of the aviary when nestlings were between 2–12 days old as nestlings in our experiments belonging to the different nests hatched within 10 days of each other. Chicks did not need to be provided with supplemental crop feeding (they gained weight normally) and the adult males raised them to independence. Although, the maternally deprived offspring did not have any tactile contact with adult females after adult females were removed from the aviary, they had visual and auditory contact with adult females in the adjacent half of the aviary once they had fledged. At 45–55 days of age, offspring from both groups were moved into unisex aviaries. At this age, zebra finches are no longer dependent on their parents for food and can be sexed based on their plumage coloration [19]. The unisex male and female aviaries comprising adult control and maternally deprived birds were in the same room, and they could therefore see and hear individuals of the opposite sex.
(b). Corticosterone concentrations in control and maternally deprived birds measured in response to restraint and isolation
Adult control and maternally deprived offspring of both sexes were sampled between 100 and 150 days of age. All blood sampling was performed between 09.00 and 12.00 h. Birds were isolated from their peers by placing them in single cages (45 × 25 × 23 cm) in a room that had no other birds in it for either 10 min (isolation 10) or 30 min (isolation 30). Birds were subjected to restraint by placing them in brown paper bags in a lit room for either 10 min (restraint 10) or 30 min (restraint 30). On one day, one control and one maternally deprived bird were captured from their aviaries and bled within 3 min to measure baseline concentrations (‘baseline’) of corticosterone. Following that, a different control and maternally deprived bird were subjected to 10 min of isolation or restraint. Another pair of control and maternally deprived birds was exposed to 30 min of isolation or restraint. Immediately after capture for baseline concentrations or after isolation or restraint, a blood sample of approximately 100 µl was obtained within 3 min, by piercing the alar vein of the left wing using a 26-gauge sterile needle (used once for each bird) and gathering the welling blood using heparinized capillary tubes. Samples were centrifuged at 12 000 r.p.m. and plasma was stored at −80°C.
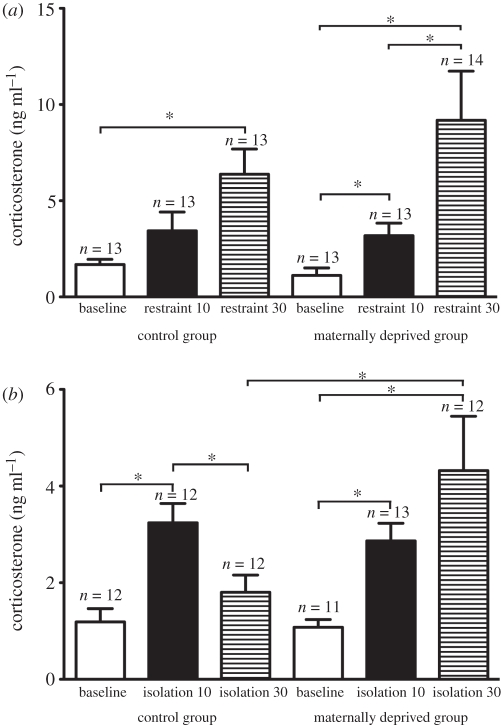
These procedures were carried out over several days to achieve the following numbers of subjects for the restraint experiment: n = 13 for control baseline group, n = 13 for control restraint 10 min group, n = 13 for control restraint 30 min group, n = 13 for maternally deprived baseline group, n = 13 for maternally deprived restraint 10 min group, n = 14 for maternally deprived restraint 30 min group (figure 1a). Numbers were as follows for the isolation experiment: n = 12 for control baseline group, n = 12 for control isolation 10 min group, n = 12 for control isolation 30 min group, n = 11 for maternally deprived baseline group, n = 13 for maternally deprived isolation 10 min group, n = 12 for maternally deprived isolation 30 min group (figure 1b). This was not a repeated-measures design experiment. Birds were randomly assigned to baseline or stressor treatment groups as follows. Within the isolation experiment, a single bird could be assigned to any one of the baseline, isolation 10 or isolation 30 groups. Within the restraint experiment, a single bird could be assigned to any one of the baseline, restraint 10 or restraint 30 groups. The same pool of birds was subjected to isolation and restraint, with a month between the two stress paradigms.
Figure 1.
Corticosterone concentrations (mean ± s.e.m.) in control and maternally deprived birds of both sexes in (a) baseline, restraint 10 and restraint 30 groups, and (b) baseline, isolation 10 and isolation 30 groups. *p < 0.05.
(c). Enzyme-immunoassay to measure corticosterone
We used corticosterone EIA kits (Cayman Chemical, cat. no. 500651). The cross-reactivity is 100 per cent for corticosterone, 19.6 per cent for 11-deoxy corticosterone and 1.01 per cent for progesterone. We ran samples in duplicate at a dilution of 1 : 10 in buffer and took the average of both of readings to obtain the final value of each sample. As females were in unisex aviaries and therefore not depositing egg yolk, samples did not require hormone extraction prior to assay. The intra- and interassay variances were 8.13 per cent and 9.6 per cent, respectively.
(d). Tissue collection and quantitative real-time PCR to measure glucocorticoid receptor and mineralocorticoid receptor mRNA levels
Birds were sacrificed in a CO2 chamber. The time interval between the stressor exposure and sacrifice was two to four months. Immediately after sacrifice, brains were dissected out and placed on dry ice until frozen, then stored at −80°C.
Brains were then sectioned in a cryostat at 200 µm thickness. A Palkovitz punch set was used to punch out three brain areas of interest (hippocampus, hypothalamus and cerebellum), and these were stored at −80°C. RNA was isolated from these brain regions using Trizol (Invitrogen, Carslbad, CA, USA). Genomic DNA contamination was removed by DNase I treatment (Invitrogen, Carlsbad, CA, USA) followed by reverse transcription using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time PCR (QPCR) was carried out on cDNA from different brain regions using gene-specific primer pairs for the GR, MR and the house-keeping gene β-actin (BA). All primer pairs amplified single products with no dimer pairs and had a standard efficiency of no less than 99 per cent. The following primer pairs were designed based on published zebra finch nucleotide gene sequences. GR (GeneID: 100008583) forward primer: 5′ TGA AGA GCC AGT CCC TGT TCG AG. GR reverse primer: 5′CAA CCA CAT CAT GCA TAG AGT CCA GCA. MR (GeneID: 751776) forward primer: 5′AAG AGT CGG CCA AAC ATC CTT GTT CT. MR reverse primer: 5′AAG AAA CGG GTG GTC CTA AAA TCC CAG. BA (GeneID: 751978) forward primer: 5′TCA TCA CCA TTG GCA ATG AGA GGT TCA G. BA reverse primer: 5′GCA TAC AGG TCC TTA CGG ATG TCC A. PCR products that were amplified by the above primer pairs were sequenced and homology verified using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
All real-time PCR reactions were run in triplicate along with no-template controls and contained the following: 10 µl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems), 2 µl of forward and reverse primer at a final concentration of 100 nM, 4 µl of H2O and 2 µl of the appropriate cDNA. Reactions were run on an Applied Biosystems 7900 HT Sequence Detection System at the Cornell University Life Sciences Core Laboratory Centre under the manufacturer's default conditions (SDS v. 2.1 software) using 60°C as a melting temperature. Gene copy number was determined for each tissue sampled from each individual using standard curve analysis for all gene primer sets, including housekeeping genes. BA gene expression levels did not differ between groups. Briefly, the raw cycle threshold (Ct) values were converted to copy number with the standard curve. Each target gene copy number was normalized using the BA copy number from the same tissue sample. Normalized data are reported as a ratio of copy numbers of GR or MR to BA.
(e). Statistics
SPSS v. 17.0 was used for statistical analyses. Corticosterone values were analysed using univariate ANOVA. GR and MR values were analysed using mixed linear model analysis. Receptor data were ln transformed to meet assumptions of normality and equality of variances. Pairwise contrasts using the Bonferroni method of adjustment for multiple comparisons were performed. p-values described as significant in (§3) are lower than the Bonferroni-corrected alpha.
3. Results
(a). Corticosterone concentrations in control and maternally deprived offspring subjected to 10 or 30 min of restraint
Overall, there was no significant interaction between treatment and restraint duration (F2,72 = 2.310, p = 0.107) or main effect of treatment (F1,72 = 0.776, p = 0.381) on plasma corticosterone concentration (figure 1a). There was a significant main effect of restraint duration (F2,72 = 38.767, p < 0.001). For control offspring, corticosterone concentrations in the baseline group were significantly lower than those in the birds exposed to 30 min of restraint (p < 0.001) but not 10 min of restraint (p = 0.09). Corticosterone concentrations of birds exposed to 10 min of restraint were significantly lower than in the birds exposed to 30 min of restraint (p = 0.016). For maternally deprived offspring, baseline corticosterone concentrations were significantly lower than those in the birds exposed to 10 min of restraint (p < 0.001) and 30 min of restraint (p < 0.001). Corticosterone concentrations of the birds exposed to 10 min of restraint were significantly lower than in the birds exposed to 30 min of restraint (p < 0.001). There were no significant differences between control and maternally deprived groups for baseline concentrations or for 10 min or 30 min of restraint.
(b). Corticosterone concentrations in control and maternally deprived offspring subjected to 10 or 30 min of isolation
Overall, there was a significant interaction between treatment (control or maternally deprived) and duration of isolation on plasma corticosterone concentration (F2,67 = 4.795, p = 0.011; figure 1b). There was a strong trend for main effect of treatment (F1,67 = 3.535, p = 0.064) and a significant effect of duration on corticosterone concentration (F2,67 = 18.59, p < 0.001). For the control offspring, corticosterone concentrations of the group subjected to 10 min of isolation were significantly higher than the baseline group (p < 0.001) and the group subjected to 30 min of isolation (p = 0.003). Baseline corticosterone concentrations did not differ significantly from the group subjected to 30 min of isolation (p = 0.118). For maternally deprived offspring, baseline corticosterone concentrations were significantly lower than those in the groups exposed to 10 min (p < 0.001) or 30 min of isolation (p < 0.001). The corticosterone concentrations of the groups exposed to 10 or 30 min of isolation were not significantly different from one another (p = 0.284). Corticosterone concentrations in control offspring exposed to 30 min of isolation were significantly lower than those in maternally deprived offspring exposed to 30 min of isolation (p = 0.001).
(c). Glucocorticoid receptor mRNA levels in the hippocampus, hypothalamus and cerebellum of control and maternally deprived birds
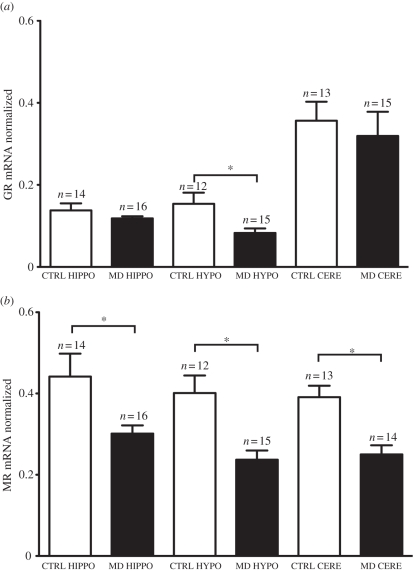
Overall, there was no significant interaction between treatment group (control or maternally deprived) and brain region (hippocampus, hypothalamus and cerebellum; F2,53.49 = 1.495, p = 0.233; figure 2a). There was a significant main effect of treatment (F1,28.18 = 6.78, p = 0.014) and region (F2,53.49 = 46.59, p < 0.001). Within the hypothalamus, GR mRNA levels were significantly lower in the maternally deprived group when compared with the control group (p = 0.012). GR mRNA levels did not differ between control and maternally deprived groups within the hippocampus (p = 0.681) and cerebellum (p = 0.165).
Figure 2.
(a) GR mRNA and (b) MR mRNA levels (mean ± s.e.m.) in control (CTRL) and maternally deprived (MD) adult zebra finch offspring in the hippocampus (HIPPO), hypothalamus (HYPO) and cerebellum (CERE). *p < 0.05.
(d). Mineralocorticoid receptor mRNA levels in the hippocampus, hypothalamus and cerebellum of control and maternally deprived birds
Overall, there was no significant interaction between treatment group (control or maternally deprived) and brain region (hippocampus, hypothalamus and cerebellum; F2,52.79 = 0.926, p = 0.402; figure 2b). There was a significant main effect of treatment (F1,27.97 = 24.38, p < 0.001), but not of region (F2,52.79 = 2.33, p = 0.107). MR mRNA levels of the maternally deprived group were significantly lower than control group within the hippocampus (p = 0.014), hypothalamus (p < 0.001) and cerebellum (p < 0.001).
4. Discussion
Our results show that maternal deprivation in zebra finches leads to an altered HPA axis response to social isolation in adulthood. Our most interesting finding from the corticosterone measurements was that the adult maternally deprived offspring had higher circulating corticosterone concentrations after 30 min of social isolation than control offspring that had been raised by both parents. That is, concentrations had returned to baseline (the response had terminated) in the controls but not the maternally deprived birds. These data suggest that the absence of mothers and adult females led to HPA axis hyper-responsiveness in adulthood. Both mothers and unrelated adult females may have influenced HPA axis development through adult–offspring interactions, as zebra finches are a highly social species, and in aviaries it is difficult to rule out the role of one over the other. Although the effects of perturbations of the early social environment and long-term effects on the HPA axis of birds have not been explored thus far, a recent study addressed the impact of nest attendance on proximal stress physiology of Florida scrub-jay nestlings [20]. The authors found that corticosterone levels of nestlings were positively correlated with the proportion of time female adults spent away from their nests. Overall, both Rensel et al. [20] and our present study suggest that, as in mammals, early-life changes in the levels of maternal care in biparental avian species can alter stress physiology of offspring both acutely and into adulthood.
Previous studies have shown that maternal deprivation during the stress hypo-responsive period (postnatal day 2 to day 14) in uniparental mammals can lead to lifelong changes in the responsiveness of the HPA axis [21]. If rat pups were subjected to more than 3 h of maternal separation or deprivation per day during this period, their serum ACTH and corticosterone levels measured after stressors were higher than control animals in adulthood [15]. In addition, mRNA levels of CRH have been shown to be higher in the PVN of the hypothalamus in maternally deprived rats in adulthood, along with lower levels of GR in the hippocampus, when compared with control rats [12,15,17,18]. Studies carried out in several biparental avian species, such as Florida scrub-jays (Aphelocoma coerulescens), mockingbirds (Mimus polyglottos), white-crowned sparrows (Zonotrichia leucophrys) and zebra finches, suggest that young birds also have a stress-hypo-responsive period [22–25]. Based on our results, it is likely that, as in mammals, the loss of maternal care during the putative stress-hypo-responsive period in birds led to a disruption (or reprogramming) in the development of the HPA axis that manifests itself in altered stress responsiveness in adulthood.
Recent studies have shown that both GR and MR receptors are expressed in the zebra finch brain. GR mRNA transcripts are concentrated in areas such as the hippocampus, hypothalamic nuclei and cerebellum. MR mRNA was detected in fewer areas, with highest concentration in the hippocampus [26,27]. We observed that mRNA levels of GR were lower in the hypothalamus of maternally deprived males in comparison with control birds, and mRNA levels of MR of maternally deprived birds were lower than mRNA levels of control birds specifically in the hypothalamus, hippocampus and the cerebellum. Although the effect of developmental manipulations on GR and MR expression has not previously been explored, studies have found interesting effects of adult manipulations on levels of GR and MR mRNA expression in the avian brain. Hodgson et al. [27] have shown that in a line of zebra finches selected for a high corticosterone response to an acute stressor (restraint for 20 min), MR but not GR mRNA levels were decreased in the hippocampus. In another study, the authors showed that when wild-caught adult starlings were exposed to chronic unpredictable stress over a period of 16 days, levels of MR mRNA were lower in the hippocampus and levels of GR mRNA were lower in the hypothalamus when compared with control birds [26]. Similar to starlings exposed to chronic unpredictable stress, our experiments showed that maternally deprived zebra finches that had elevated levels of corticosterone in response to isolation also had lower levels of GR mRNA in the hypothalamus and lower levels of MR mRNA in the hippocampus when compared with control birds. Decreased levels of GR in the hypothalamus of mammals are associated with a hyperactive HPA axis [28]. Our data suggest that this phenomenon may also be true in birds, as maternally deprived birds that were hyper-responsive to isolation had lower levels of hypothalamic GR mRNA in comparison with controls. In mammals, the hippocampus has been shown to be an important site of negative feedback of the HPA axis [29,30]. Prolonged stress in mammals is associated with a decrease in binding of both GR and MR in the hippocampus [31,32]. Hence, lower levels of MR mRNA expression in the hippocampal region of maternally deprived birds in comparison with controls might be indicative of a hyper-responsive HPA axis resulting in a prolonged stress response. With regard to the cerebellum, a recent study has demonstrated that the binding capacity of MR decreased with age in the cerebellum of white-crowned sparrow nestlings [33]. This change was correlated with HPA axis hypo-responsiveness to handling stress. In our study, decreased levels of cerebellar MR mRNA correlated with hyper-responsiveness of the HPA axis. To our knowledge, the role of the cerebellum in the regulation of the HPA axis is as yet unexplored, and therefore it is unclear what consequences lower MR mRNA in the cerebellum may have in maternally deprived birds. In mammals, it has been proposed that an imbalance of MR and GR results in dysregulation of the HPA axis and behaviour [34,35]. Overall, it is possible that an alteration in the ratio of expression of corticosteroid receptors in the brain contributed to the hyper-responsive HPA axis of maternally deprived birds.
In our study, we did not address the mechanism by which the loss of maternal care impacted the HPA axis. Studies in uniparental rats have shown that pups of mothers that demonstrate low levels of maternal care have decreased levels of GR in the hippocampus, an effect mediated via epigenetic mechanisms [36–39]. Perhaps the absence of female zebra finches and alteration of the early social environment leads to changes in HPA responsiveness to stressors via epigenetic changes. Female zebra finches clump and allopreen their offspring, and it is possible that a reduction in these tactile behaviours led to altered receptor gene expression that eventually impacted the functioning of the HPA axis of the offspring. It is unclear from our experiments, however, if the differences that we observed in mRNA levels of corticosteroid receptors contributed to or were a result of a hyper-responsive HPA axis.
On a final note, it is important to consider the consequences of a hyper-responsive HPA axis in maternally deprived offspring. Corticosterone is critical for the survival of an organism during both times of emergency and increased energetic demands, although chronically elevated levels of corticosterone are known to have harmful effects on physiology, neuron morphology and cognition in mammals [5]. A study in Galapagos marine iguanas has shown that individuals that had reduced efficacy of negative feedback and suppression of corticosterone secretion formed a large percentage of the population of individuals that died during El Niño conditions. Therefore individuals that were better in suppressing a corticosterone response after a stressor (capture and handling) could cope with starvation better than others [40]. Hence it is possible that control zebra finches that had baseline levels of corticosterone after 30 minutes of isolation would survive stressful conditions better than maternally deprived birds. On the other hand, it is possible that maternally deprived offspring are better prepared to cope with a challenging environment. A study has shown that under stressful conditions adult rat offspring of low-maternal-care mothers performed better in hippocampus-dependent tasks of learning in comparison with offspring of high-maternal-care mothers [41]. This suggests that in uniparental rats, through maternal care, the endocrine and cognitive systems of offspring can be ‘programmed’ to be increasingly sensitive and receptive to stressful or adverse situations, potentially ensuring the survival of offspring in environments where dangers are imminent, although potentially rendering an organism more susceptible to stress-related pathology in the long term [42–45]. Therefore perhaps maternally deprived zebra finch offspring, as compared with controls, would have a better chance of survival in stressful environments.
In conclusion, our study is one of the first to shed light on the long-term consequences of altering the early social environment of a socially monogamous and biparental species. We have shown that removal of all females, including mothers, from the early social environment of zebra finch offspring, altered HPA axis responsiveness and the expression of corticosteroid receptors in these offspring.
Acknowledgements
All animal procedures conformed to Federal and State regulations and were approved by the Cornell University IACUC.
We would like to thank Prof. Andrew H. Bass, Department of Neurobiology and Behaviour, Cornell University, for his immense generosity in allowing us to perform all real-time PCR experiments in his laboratory. We would also like to thank animal facilities manager Tim Van Deusen for his help in setting up aviaries. This work was supported by a National Science Foundation grant (IBN 0130986) to E.A.-R., NIH grant (NIDCD R01DC00092) to Andrew H. Bass and an American Psychological Association Dissertation Research Award to S.B.B.
References
- 1.Lightman S. L. 2008. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 20, 880–884 10.1111/j.1365-2826.2008.01711.x (doi:10.1111/j.1365-2826.2008.01711.x) [DOI] [PubMed] [Google Scholar]
- 2.McEwen B. S., et al. 1995. Stress and the brain: a paradoxical role for adrenal steroids. Vitam. Horm. 51, 371–402 10.1016/S0083-6729(08)61045-6 (doi:10.1016/S0083-6729(08)61045-6) [DOI] [PubMed] [Google Scholar]
- 3.McEwen B. S. 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185 10.1016/j.ejphar.2007.11.071 (doi:10.1016/j.ejphar.2007.11.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotsky P. M., Owens M. J., Nemeroff C. B. 1998. Psychoneuroendocrinology of depression—hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North Am. 21, 293–307 10.1016/S0193-953X(05)70006-X (doi:10.1016/S0193-953X(05)70006-X) [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 6.De Kloet E. R. 1991. Brain corticosteroid receptor balance and homeostatic control. Front. Neuroendocrinol. 12, 95–164 10.1016/S0079-6123(08)62132-9 (doi:10.1016/S0079-6123(08)62132-9) [DOI] [PubMed] [Google Scholar]
- 7.De Kloet E. R., Karst H., Joels M. 2008. Corticosteroid hormones in the central stress response: quick-and-slow. Front. Neuroendocrinol. 29, 268–272 10.1016/j.yfrne.2007.10.002 (doi:10.1016/j.yfrne.2007.10.002) [DOI] [PubMed] [Google Scholar]
- 8.De Kloet E. R., Joels M., Holsboer F. 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 10.1038/nrn1683 (doi:10.1038/nrn1683) [DOI] [PubMed] [Google Scholar]
- 9.De Kloet E. R. 2000. Stress in the brain. Eur. J. Pharmacol. 405, 187–198 10.1016/S0014-2999(00)00552-5 (doi:10.1016/S0014-2999(00)00552-5) [DOI] [PubMed] [Google Scholar]
- 10.Veenema A. H. 2009. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front. Neuroendocrinol. 30, 497–518 10.1016/j.yfrne.2009.03.003 (doi:10.1016/j.yfrne.2009.03.003) [DOI] [PubMed] [Google Scholar]
- 11.Levine S. 2000. Influence of psychological variables on the activity of the hypothalamic–pituitary–adrenal axis. Eur. J. Pharmacol. 405, 149–160 10.1016/S0014-2999(00)00548-3 (doi:10.1016/S0014-2999(00)00548-3) [DOI] [PubMed] [Google Scholar]
- 12.Lippmann M., Bress A., Nemeroff C. B., Plotsky P. M., Monteggia L. M. 2007. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur. J. Neurosci. 25, 3091–3098 10.1111/j.1460-9568.2007.05522.x (doi:10.1111/j.1460-9568.2007.05522.x) [DOI] [PubMed] [Google Scholar]
- 13.Daniels W. M. U., Pietersen C. Y., Carstens M. E., Stein D. J. 2004. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab. Brain Dis. 19, 3–14 10.1023/B:MEBR.0000027412.19664.b3 (doi:10.1023/B:MEBR.0000027412.19664.b3) [DOI] [PubMed] [Google Scholar]
- 14.Meaney M. J., Diorio J., Francis D., Widdowson J., LaPlante P., Caldji C., Sharma S., Seckl J. R., Plotsky P. M. 1996. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 18, 49–72 10.1159/000111395 (doi:10.1159/000111395) [DOI] [PubMed] [Google Scholar]
- 15.Plotsky P. M., Meaney M. J. 1993. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median-eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 18, 195–200 10.1016/0169-328X(93)90189-V (doi:10.1016/0169-328X(93)90189-V) [DOI] [PubMed] [Google Scholar]
- 16.Meaney M. J. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 10.1146/annurev.neuro.24.1.1161 (doi:10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]
- 17.Francis D. D., Meaney M. J. 1999. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 9, 128–134 10.1016/S0959-4388(99)80016-6 (doi:10.1016/S0959-4388(99)80016-6) [DOI] [PubMed] [Google Scholar]
- 18.Anisman H., Zaharia M. D., Meaney M. J., Merali Z. 1998. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16, 149–164 10.1016/S0736-5748(98)00025-2 (doi:10.1016/S0736-5748(98)00025-2) [DOI] [PubMed] [Google Scholar]
- 19.Zann R. A. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press [Google Scholar]
- 20.Rensel M. A., Wilcoxen T. E., Schoech S. J. 2010. The influence of nest attendance and provisioning on nestling stress physiology in the Florida scrub-jay. Horm. Behav. 57, 162–168 10.1016/j.yhbeh.2009.10.009 (doi:10.1016/j.yhbeh.2009.10.009) [DOI] [PubMed] [Google Scholar]
- 21.Sapolsky R. M., Meaney M. J. 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 396, 64–76 10.1016/0165-0173(86)90010-X (doi:10.1016/0165-0173(86)90010-X) [DOI] [PubMed] [Google Scholar]
- 22.Rensel M. A., Boughton R. K., Schoech S. J. 2010. Development of the adrenal stress response in the Florida scrub-jay (Aphelocoma coerulescens). Gen. Comp. Endocrinol. 165, 255–261 10.1016/j.ygcen.2009.07.002 (doi:10.1016/j.ygcen.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 23.S Sims C. G., Holberton R. L. 2000. Development of the corticosterone stress response in young Northern Mockingbirds (Mimus polyglottos). Gen. Comp. Endocrinol. 119, 193–201 10.1006/gcen.2000.7506 (doi:10.1006/gcen.2000.7506) [DOI] [PubMed] [Google Scholar]
- 24.Wada H., Hahn T. P., Breuner C. W. 2007. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. Gen. Comp. Endocrinol. 150, 405–413 10.1016/j.ygcen.2006.10.002 (doi:10.1016/j.ygcen.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 25.Wada H., Salvante K. G., Wagner E., Williams T. D., Breuner C. W. 2009. Ontogeny and individual variation in the adrenocortical response of zebra finch (Taeniopygia guttata) nestlings. Physiol. Biochem. Zool. 82, 325–331 10.1086/599320 (doi:10.1086/599320) [DOI] [PubMed] [Google Scholar]
- 26.Dickens M., Romero L. M., Cyr N. E., Dunn I. C., Meddle S. L. 2009. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mRNA expression in the European starling (Sturnus vulgaris) brain. J. Neuroendocrinol. 21, 832–840 10.1111/j.1365-2826.2009.01908.x (doi:10.1111/j.1365-2826.2009.01908.x) [DOI] [PubMed] [Google Scholar]
- 27.Hodgson Z. G., Meddle S. L., Roberts M. L., Buchanan K. L., Evans M. R., Metzdorf R., Gahr M., Healy S. D. 2007. Spatial ability is impaired and hippocampal mineralocorticoid receptor mRNA expression reduced in zebra finches (Taeniopygia guttata) selected for acute high corticosterone response to stress. Proc. R. Soc. B 274, 239–245 10.1098/rspb.2006.3704 (doi:10.1098/rspb.2006.3704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makino S., Hashimoto K., Gold P. W. 2002. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol. Biochem. Behav. 73, 147–158 10.1016/S0091-3057(02)00791-8 (doi:10.1016/S0091-3057(02)00791-8) [DOI] [PubMed] [Google Scholar]
- 29.Sapolsky R. M., Zolamorgan S., Squire L. R. 1991. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J. Neurosci. 11, 3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson L., Sapolsky R. 1991. The role of the hippocampus in feedback-regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118–134 10.1210/edrv-12-2-118 (doi:10.1210/edrv-12-2-118) [DOI] [PubMed] [Google Scholar]
- 31.Kitraki E., Karandrea D., Kittas C. 1999. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology 69, 331–338 10.1159/000054435 (doi:10.1159/000054435) [DOI] [PubMed] [Google Scholar]
- 32.Herman J. P., Ostrander M. M., Mueller N. K., Figueiredo H. 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychol. 29, 1201–1213 10.1016/j.pnpbp.2005.08.006 (doi:10.1016/j.pnpbp.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 33.Wada H., Breuner C. W. 2010. Developmental changes in neural corticosteroid receptor binding capacity in altricial nestlings. Dev. Neurobiol. 70, 853–861 10.1002/dneu.20819 (doi:10.1002/dneu.20819) [DOI] [PubMed] [Google Scholar]
- 34.Oitzl M. S., Champagne D. L., van der Veen R., De Kloet E. R. 2010. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 34, 853–866 10.1016/j.neubiorev.2009.07.006 (doi:10.1016/j.neubiorev.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 35.De Kloet E. R., Vreugdenhil E., Oitzl M. S., Joels M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 10.1210/er.19.3.269 (doi:10.1210/er.19.3.269) [DOI] [PubMed] [Google Scholar]
- 36.Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M., Meaney M. J. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 37.Champagne F. A., Francis D. D., Mar A., Meaney M. J. 2003. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359–371 10.1016/S0031-9384(03)00149-5 (doi:10.1016/S0031-9384(03)00149-5) [DOI] [PubMed] [Google Scholar]
- 38.Champagne F. A., Curley J. P. 2009. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 33, 593–600 10.1016/j.neubiorev.2007.10.009 (doi:10.1016/j.neubiorev.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 39.Szyf M., Weaver I. C. G., Champagne F. A., Diorio J., Meaney M. J. 2005. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front. Neuroendocrinol. 26, 139–162 10.1016/j.yfrne.2005.10.002 (doi:10.1016/j.yfrne.2005.10.002) [DOI] [PubMed] [Google Scholar]
- 40.Romero L. M., Wikelski M. 2010. Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc. R. Soc. B 277, 3157–3162 10.1098/rspb.2010.0678 (doi:10.1098/rspb.2010.0678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champagne D. L., Bagot R. C., van Hasselt F., Ramakers G., Meaney M. J., De Kloet E. R., Joëls M., Krugers H. 2008. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045 10.1523/JNEUROSCI.0526-08.2008 (doi:10.1523/JNEUROSCI.0526-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T. Y., et al. 2006. Maternal programming of defensive responses through sustained effects on gene expression. Biol. Psychol. 73, 72–89 10.1016/j.biopsycho.2006.01.009 (doi:10.1016/j.biopsycho.2006.01.009) [DOI] [PubMed] [Google Scholar]
- 43.Zhang T. Y., Meaney M. J. 2010. Epigenetics and the environmental regulation of the genome and its function. Annu. Rev. Psychol. 61, 439–466 10.1146/annurev.psych.60.110707.163625 (doi:10.1146/annurev.psych.60.110707.163625) [DOI] [PubMed] [Google Scholar]
- 44.Meaney M. J., Szyf M., Seckl J. R. 2007. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol. Med. 13, 269–277 10.1016/j.molmed.2007.05.003 (doi:10.1016/j.molmed.2007.05.003) [DOI] [PubMed] [Google Scholar]
- 45.Cameron N. M., Champagne F. A., Fish C., Ozaki-Kuroda K., Meaney M. J. 2005. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci. Biobehav. Rev. 29, 843–865 10.1016/j.neubiorev.2005.03.022 (doi:10.1016/j.neubiorev.2005.03.022) [DOI] [PubMed] [Google Scholar]