Abstract
Stressful conditions early in life can give rise to exaggerated stress responses, which, while beneficial in the short term, chronically increase lifetime exposure to stress hormones and elevate disease risk later in life. Using zebra finches Taeniopygia guttata, we show here that individuals whose glucocorticoid stress hormones were experimentally increased for only a brief period in early post-natal life, inducing increased stress sensitivity, had reduced adult lifespans. Remarkably, the breeding partners of such exposed individuals also died at a younger age. This negative effect on partner longevity was the same for both sexes; it occurred irrespective of the partner's own early stress exposure and was in addition to any longevity reduction arising from this. Furthermore, this partner effect continued even after the breeding partnership was terminated. Only 5 per cent of control birds with control partners had died after 3 years, compared with over 40 per cent in early stress–early stress pairs. In contrast, reproductive capability appeared unaffected by the early stress treatment, even when breeding in stressful environmental circumstances. Our results clearly show that increased exposure to glucocorticoids early in life can markedly reduce adult life expectancy, and that pairing with such exposed partners carries an additional and substantial lifespan penalty.
Keywords: corticosterone, lifespan, early environment, mate choice, Taeniopygia guttata
1. Introduction
The vertebrate stress response, which is highly conserved across taxonomic groups, facilitates escape from life-threatening situations by suspending non-essential activities and processes in favour of those that promote short-term survival [1]. This response involves activation of the hypothalamic–pituitary–adrenal (HPA) axis and leads to the release of glucocorticoid stress hormones [1]. Prolonged exposure to high levels of stress hormones is damaging, since key body maintenance activities are disrupted and brain physiology can be altered [2–4]. Exposure to high levels of stress hormones in early life can increase glucocorticoid exposure throughout life by altering an individual's HPA phenotype (i.e. the way in which stress hormones change on encountering stressors). Such individuals can develop a more stress-reactive phenotype (i.e. glucocorticoid concentrations on encountering stressors are higher and/or there is a slower return to baseline levels, which can also be elevated). While this phenotypic change might help affected individuals cope with stressful environments in the short term, the chronically increased stress hormone exposure can have negative effects over the longer term [5–8].
Exposure to stressful conditions in early life has been found to reduce subsequent sexual attractiveness, thought to be because affected individuals are poorer breeders [9]. However, the effects of the phenotypic changes induced by early stress exposure on breeding capabilities are unclear, and may depend on the environmental circumstances pertaining at the time of breeding [10,11]. An alternative reason why discrimination against individuals that experienced early-life stress as potential mates occurs could be because they have adverse effects on the health of their breeding partners. Such effects on partner longevity have not hitherto been investigated.
We examined the effect of increased exposure to stress hormones by a direct manipulation of corticosterone (CORT) levels in nestling zebra finches Taeniopygia guttata for a 16-day period during the nestling phase. We followed the survival of the control and experimental birds for the next 3 years. Birds were allocated a breeding partner of the same or opposite treatment and allowed to breed under stressful and non-stressful environmental conditions. This enabled us to examine the effects of early-life stress exposure on adult breeding performance in different environmental circumstances, and to examine the effect of partner exposure to stress hormones on life expectancy.
2. Material and methods
(a). Early-life corticosterone treatment and effect on adult stress responsiveness
The subjects were bred and held in the University of Glasgow, and the experiment ran from November 2007 to January 2010. Five days after they hatched, 68 chicks were allocated to the early CORT and 90 to the control treatment. Families were split between treatments where possible, and family was taken into account in the statistical analyses. We experimentally elevated levels of circulating CORT (the primary glucocorticoid hormone in birds) in the experimental birds by oral dosage with CORT in a peanut oil carrier between days 12 and 28 after hatching; control birds were given carrier only. In order to determine the natural variation in CORT levels secreted by zebra finch nestlings in response to a standardized environmental stressor, and to allow us to manipulate birds within biologically relevant levels, pilot work was undertaken prior to the start of the experiment to establish appropriate dose levels. A separate sample of unrelated zebra finch nestlings aged 12 and 16 days were chosen at random from our breeding colony (12 days old: n = 8; 16 days old: n = 10) and subjected to the standard capture–restraint protocol for measuring acute responses to environmental stressors in birds [12,13]. Blood samples (less than 50 µl) were collected upon initial capture (within 2 min) and at 10 and 30 min during restraint in an opaque box, and analysed for CORT as described below. Based on the effect of exogenous CORT administration on plasma levels in previous studies [14], we scaled the CORT levels administered in the current study to produce an elevation in peak plasma CORT in the region of one standard deviation above the mean for each age class (i.e. to a 10 min peak in 12–15-day-old birds of 25 ng ml−1 and in 16–28-day-old birds of 32 ng ml−1). These levels are similar to those of nestling zebra finches reported in other studies [15]. Chicks received the appropriate experimental or control dose twice per day with an interval of 5 h. The total daily amount administered was 6.2 µg of CORT per day (Sigma Aldrich; 0.124 mg ml−1 in peanut oil) between days 12 and 15, increasing to 8.15 µg of CORT per day between days 16 and 28.
We examined the effect of this treatment on circulating CORT levels in a sub-sample of the birds (34 experimental and 34 controls) involved in this experiment when they were 14 days old, and on their stress reactivity in later life. These data have been reported in detail elsewhere [16] and are summarized here. The average levels of plasma CORT 10 min after dosing averaged 8.7 ± 1.3 ng ml−1 in the control birds and 28.5 ± 2.1 ng ml−1 in the CORT-treated birds, confirming that our dosing of the experimental birds had the desired effect, and mimicked the kind of CORT increase that occurs in response to an environmental stressor. Previous studies with nestling zebra finches show that such oral CORT administration causes a rise in circulating CORT that peaks around 10 min after dosing; levels return to baseline within 2 h [14]. Thus, our treatment elevated CORT levels in the treated nestlings for up to 4 h per day, such as would occur in response to natural stressors such as episodes of food shortage [17].
Examination of the HPA phenotype when the birds were young adults confirmed that, as in mammalian studies, the exposure to elevated stress hormones in early life increases stress reactivity [16]. When the birds were tested using the standard capture–handling–restraint protocol, the CORT-treated birds showed an exaggerated and prolonged response to acute stress; both increased CORT secretion and a slower return to baseline levels were recorded. We found no difference in baseline CORT levels between the treatment groups [16].
(b). Survival
Following the early-life treatment, the birds were maintained in family groups until 60 days of age, then in sex- and treatment-specific groups (n = 8–10 birds per 120 × 50 × 50 cm cage), other than when breeding. The body mass of the birds was measured before and near the end of each breeding event. Photoperiod was 14 L : 10 D, and temperature was between 20°C and 24°C. Survival of birds was tracked over the following 3 years.
(c). Breeding performance in different environmental circumstances
To examine the breeding capabilities of the treated (CORT) birds and their effects on the longevity of their breeding partners, we established breeding pairs among early CORT and control birds during their second year of life. Birds were allowed to breed with the same unrelated partner in 60 × 50 × 50 cm cages three times during their second year of life, survival permitting. Pairs comprised either both members being early-CORT or control birds (n = 16 and 26 pairs, respectively), or early-CORT males or females were paired with a control partner (n = 18 and 17 pairs, respectively). During one or two of the breeding events, the environmental conditions during breeding were made more stressful by manipulation of food predictability [18]. The breeding environment was made stressful by restricting access to food for 25 per cent of the daylight hours on a random schedule from 10 days prior to pairing until nests either failed or chicks fledged. Such unpredictable food availability has been shown to increase circulating stress hormone concentrations [18]. Clutch size, clutch mass, the number fledging and fledging mass of the brood were recorded. Pairs were separated for 30 days between each breeding event. The first two breeding events were in either stressful or non-stressful conditions, and conditions were switched for the final breeding event; this was counterbalanced across pair types.
(d). Data analyses
Cox mixed-effects models were fitted by maximum likelihood using the statistical program in R (v. 2.9.0, R package ‘coxme’ mixed-effects Cox models). We included male and female families as random factors to take into account any family effects since there were some siblings in the same treatment groups; the number of times the birds bred in a stressful environment and their sex were also included. A censor variable was included to allow inclusion of adults still alive at the completion of the study. We used a backward elimination process to exclude independent variables with p > 0.05. We analysed reproductive performance and body mass in each breeding event using general linear mixed models (GLMMs) in SASS 9.2, with pair type, breeding environment and pair type × breeding environment as fixed factors; female and male families were included as random factors in all analyses except for clutch mass in breeding round 2, where the model was not resolvable with paternal family included. Clutch size and the number of chicks fledged were rank-transformed to improve normality, and all other variables met the assumptions of parametric testing. The analyses of clutch mass and brood fledging mass were restricted to pairs that produced a clutch and hatched chicks, respectively. We used backward elimination to exclude independent variables with p > 0.05.
3. Results
(a). Effect of early-life exposure to increased stress hormones on lifespan
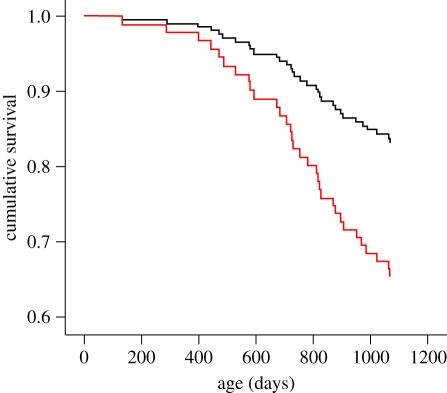
We found that experimental elevation of the stress hormone CORT, within the natural range, and for only a brief period in early life, substantially reduced later life expectancy (figures 1 and 2); CORT treatment did not affect survival of the birds as juveniles or young adults (see figure 1 for statistics), but the negative consequences became progressively more marked as the birds grew older (figure 1). There were no sex differences in survival patterns, with males and females being affected in the same way (statistics in figure 1). We also found no difference in the adult body mass of males or females in relation to early-stress treatment (mean ± s.e. adult body mass measured just prior to the onset of breeding: males, control 16.78 ± 0.32, early CORT, 17.36 ± 0.34; females, controls, 17.56 ± 0.24, early cort, 17.60 ± 0.27; GLMMs with family as a random factor: females, F1,50.35 = 0.02, p = 0.90, males, F1,51.61 = 2.01, p = 0.16).
Figure 1.
Survival up to 3 years of age of birds exposed to elevated corticosterone (CORT) levels in early life (red) and control birds (black). The period of CORT treatment was between days 12 and 28 post-hatching. Survival was analysed using a Cox mixed-effects model. As can be seen from the figure, there was no difference in survival of the birds as juveniles or young adults (to 400 days: z = 0.48, p = 0.63), and there was no difference between the sexes (z = 0.00, p = 1.00). Thereafter, survival was also influenced by the treatment of the breeding partner (figure 2).
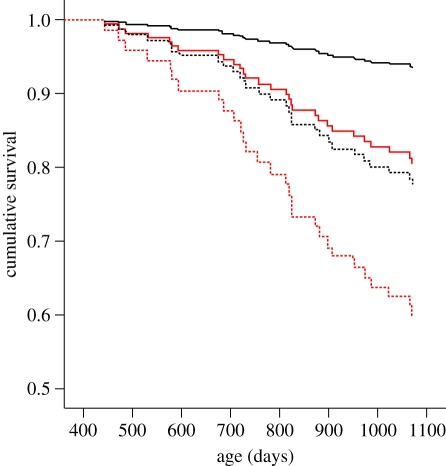
Figure 2.
The survival of birds exposed to stress in early life in relation to their own early-life treatment and that of their breeding partners. The survival of control birds is shown in black, and birds exposed to elevated stress hormones in early life (CORT treatment) is shown in red. Solid lines are for birds whose mate was a control bird, and dashed lines for birds whose mate was a CORT bird. Breeding took place three times during the second year of life, between 380 and 600 days (Cox mixed-effects model: treatment effect, z = 2.37, p = 0.02; partner effect on survival, z = 2.22, p = 0.03; breeding environment and sex had no effect, z = −1.07, p = 0.29 and z = −1.55, p = 0.12, respectively).
(b). Effect of early-life stress exposure on the longevity of breeding partners
Adult life expectancy was also strongly influenced by the early-life experience of the birds' breeding partners (figure 2). Control birds had their highest long-term survival when their mate was also a control bird. However, the longevity of control birds that had an early CORT bird as their mate fell to that of the CORT-exposed individuals. The lifespan reduction in the CORT birds was not mitigated by being paired with a non-exposed partner (figure 2). The negative effects of being paired to an early CORT bird were additive; survival of early CORT birds fell even further when their partner had also experienced stress during early life (figure 2). There were again no differences between males and females in these survival patterns, nor did the conditions under which the birds bred (stressful or non-stressful) have any effect on survival probability (see figure 2 legend for statistics).
The observed differences in longevity were substantial and continued through the third year of life, when the birds were no longer with their breeding partners, but housed in single-sex groups. By the time the birds were 3 years old, over 40 per cent of those that had bred in pairs where both members received the early-CORT treatment had died, compared with only 5 per cent of those in pairs where neither partner had received the early-CORT treatment (figure 2).
(c). Effect of early-life exposure to stress hormones on breeding performance
In contrast to the effects on survival, breeding capacity was unaffected by the early-CORT treatment. Reproductive success per breeding event (clutch size or mass, number of chicks fledged and brood fledging mass; table 1) in either stressful or non-stressful breeding environments was not significantly affected by early-CORT exposure, breeding environment nor an interaction between the two (table 2). We found no significant differences among the different pair types in the degrees of mass change during breeding in either males or females (GLMM with family as a random factor: effect of pair-type breeding round 1, p = 0.34; round 2, p = 0.81; round 3, p = 0.41; no significant interaction between sex and pair type).
Table 1.
Breeding performance of the four different pair types during the three breeding rounds.
| pair type | breeding round 1 |
breeding round 2 |
breeding round 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | mean | s.e. | n | mean | s.e. | n | mean | s.e. | |
| clutch size | |||||||||
| control birds | 26 | 5.08 | 0.28 | 26 | 4.69 | 0.23 | 25 | 4.20 | 0.37 |
| control F early-CORT M | 18 | 4.33 | 0.34 | 16 | 4.69 | 0.30 | 18 | 4.33 | 0.44 |
| control M early-CORT F | 17 | 5.00 | 0.35 | 16 | 4.75 | 0.30 | 16 | 4.94 | 0.46 |
| early-CORT birds | 16 | 5.25 | 0.36 | 16 | 5.00 | 0.30 | 11 | 4.55 | 0.56 |
| clutch mass | |||||||||
| control birds | 26 | 6.05 | 0.29 | 26 | 5.43 | 0.30 | 23 | 5.54 | 0.36 |
| control F early-CORT M | 15 | 6.06 | 0.38 | 16 | 5.44 | 0.39 | 15 | 6.30 | 0.45 |
| control M early-CORT F | 17 | 5.94 | 0.35 | 16 | 5.57 | 0.39 | 15 | 6.34 | 0.45 |
| early-CORT birds | 16 | 6.18 | 0.36 | 16 | 5.74 | 0.39 | 11 | 5.44 | 0.52 |
| number of chicks fledged | |||||||||
| control birds | 26 | 2.85 | 0.38 | 26 | 2.15 | 0.03 | 24 | 2.00 | 0.35 |
| control F early-CORT M | 17 | 1.77 | 0.47 | 18 | 1.28 | 0.40 | 18 | 2.00 | 0.40 |
| control M early-CORT F | 17 | 3.00 | 0.47 | 16 | 1.94 | 0.43 | 16 | 3.06 | 0.43 |
| early-CORT birds | 15 | 2.20 | 0.50 | 14 | 2.21 | 0.46 | 11 | 1.64 | 0.51 |
| brood fledging mass | |||||||||
| control birds | 20 | 49.34 | 4.58 | 19 | 39.61 | 4.07 | 15 | 44.13 | 4.36 |
| control F early-CORT M | 10 | 41.99 | 6.48 | 9 | 35.07 | 5.91 | 12 | 41.59 | 4.87 |
| control M early-CORT F | 14 | 47.32 | 5.48 | 11 | 39.95 | 5.35 | 15 | 46.49 | 4.36 |
| early-CORT birds | 12 | 39.15 | 5.92 | 11 | 38.15 | 5.35 | 7 | 35.90 | 6.38 |
Table 2.
General linear mixed models (GLMMs) were used to examine the potential effects of pair type (P), breeding environment (E) and pair type × breeding environment interactions (P × E) on measures of reproductive success (clutch size, clutch mass, the number of chicks fledged and brood fledging mass) during breeding rounds 1, 2 and 3. Male and female families were included as random factors to control for siblings within the analyses in all but the model for clutch mass in breeding round 2, where its inclusion rendered the model unresolvable. We used a Bonferroni correction to account for multiple comparisons and regarded variables as significant at α = 0.004.
| breeding round 1 |
breeding round 2 |
breeding round 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |||
| clutch size | |||||||||||
| P | 3, 46.8 | 1.84 | 0.15 | P | 3, 58.6 | 0.30 | 0.83 | P | 3, 50.5 | 0.77 | 0.51 |
| E | 1, 60.1 | 0.20 | 0.66 | E | 1, 64.0 | 0.06 | 0.80 | E | 1, 59.7 | 0.39 | 0.54 |
| P × E | 3, 60.0 | 1.45 | 0.24 | P × E | 3, 60.4 | 1.13 | 0.35 | P × E | 3, 47.6 | 0.59 | 0.62 |
| P | 3, 44.7 | 1.86 | 0.15 | P | 3, 60.1 | 0.35 | 0.79 | P | 3, 53.5 | 0.36 | 0.78 |
| E | 1, 54.6 | 0.43 | 0.52 | E | 1, 66.2 | 0.04 | 0.85 | E | 1, 61.9 | 0.57 | 0.45 |
| P | 3, 46.4 | 1.88 | 0.15 | P | 3, 61.0 | 0.36 | 0.79 | E | 1, 61.4 | 0.69 | 0.41 |
| clutch mass | |||||||||||
| P | 3, 49.8 | 0.28 | 0.84 | P | 3, 23.8 | 0.05 | 0.98 | P | 3, 26.1 | 0.63 | 0.60 |
| E | 1, 60.9 | 1.79 | 0.19 | E | 1, 14.9 | 0.8 | 0.39 | E | 1, 45.0 | 0.52 | 0.47 |
| P × E | 3, 49.5 | 1.16 | 0.33 | P × E | 3, 1.0 | 0.85 | 0.64 | P × E | 3, 40 | 0.26 | 0.85 |
| P | 3, 49.7 | 0.30 | 0.82 | P | 3, 27.1 | 0.09 | 0.97 | P | 3, 33.5 | 0.61 | 0.61 |
| E | 1, 58.7 | 2.56 | 0.11 | E | 1, 20.7 | 0.61 | 0.44 | E | 1, 45.7 | 0.44 | 0.51 |
| E | 1, 62.6 | 2.72 | 0.10 | E | 1, 39.3 | 0.59 | 0.45 | E | 1, 46.7 | 0.73 | 0.40 |
| number of chicks fledged | |||||||||||
| P | 3, 59.6 | 1.58 | 0.20 | P | 3, 49.0 | 0.87 | 0.46 | P | 3, 44.0 | 1.90 | 0.14 |
| E | 1, 64.1 | 0.26 | 0.61 | E | 1, 62.1 | 1.18 | 0.28 | E | 1, 57.9 | 0.21 | 0.65 |
| P × E | 3, 55.2 | 0.98 | 0.41 | P × E | 3, 51.9 | 0.98 | 0.41 | P × E | 3, 54.5 | 0.13 | 0.94 |
| P | 3, 61.2 | 1.72 | 0.17 | P | 3, 47.7 | 0.85 | 0.47 | P | 3, 50.0 | 2.06 | 0.12 |
| E | 1, 67.6 | 0.30 | 0.59 | E | 1, 57.0 | 0.55 | 0.46 | E | 1, 60.0 | 0.26 | 0.61 |
| P | 3, 61.3 | 1.79 | 0.16 | E | 1, 56.7 | 0.39 | 0.53 | P | 3, 50.0 | 2.11 | 0.11 |
| brood fledging mass | |||||||||||
| P | 3, 34.6 | 0.48 | 0.70 | P | 3, 32.6 | 0.5 | 0.68 | P | 3, 21.1 | 0.49 | 0.69 |
| E | 1, 45.2 | 0.23 | 0.63 | E | 1, 39.2 | 4.44 | 0.04 | E | 1, 32.0 | 0.42 | 0.52 |
| P × E | 3, 42.6 | 1.49 | 0.23 | P × E | 3, 40.2 | 1.34 | 0.28 | P × E | 3, 28.8 | 1.36 | 0.27 |
| P | 3, 35.6 | 0.55 | 0.65 | P | 3, 33.6 | 0.31 | 0.82 | P | 3, 36.9 | 0.66 | 0.58 |
| E | 1, 40.4 | 0.03 | 0.85 | E | 1, 40.0 | 4.61 | 0.04 | E | 1, 43.0 | 0.05 | 0.82 |
| E | 3, 35.8 | 0.58 | 0.63 | E | 1, 42.7 | 4.39 | 0.04 | E | 3, 38.1 | 0.74 | 0.54 |
4. Discussion
The exposure to elevated stress hormones early in post-natal life markedly reduced adult longevity. Since our experimental protocol involved direct manipulation of circulating CORT levels, and this was done only during a brief period in early growth, the observed differences in survival can be attributed to the phenotypic changes induced by the elevated glucocorticoid exposure that occurred during development.
Adult stress responsiveness was significantly increased in the birds exposed to elevated CORT in early life, as evidenced by both increased CORT secretion in response to a standardized stressor and a slower return to baseline levels [16]. These patterns of CORT release are indicative of a reduced negative feedback within the HPA axis that is commonly induced by early-life stress [7]. The reduced adult lifespan in these early CORT birds is likely to be associated with the damaging effects of these observed changes to the HPA phenotype, which, by making the animal more stress-reactive, increase overall exposure to stress hormones, with the attendant negative health consequences [2,3,7,19,20]. We did not find any differences in body mass in relation to early-stress treatment, and the actual cause of death was unclear, the birds usually being found dead in their cage. There are a number of possible organ and system malfunctions that have been associated with chronic stress exposure [5], not all of which can be identified from post-mortem examination. Those corpses that were found soon after death have been frozen for future examination of body tissues for gross pathologies. There was no evidence of aggression among the birds, such as fighting or pecking, so attacks from other individuals were not a contributory factor; nor was there any evidence of infection. Increased exposure to stress hormones has been associated with a wide range of physiological effects [2], so a number of factors are likely to be involved.
We found no effect of the early-stress treatment on breeding performance in either stressful or non-stressful circumstances, nor did we find any evidence of differences in mass change during breeding. Our data, therefore, suggest that the phenotypic adjustments to the HPA system do not confer any significant breeding advantages or disadvantages, nor make the birds better able to cope with breeding under stressful conditions, at least over the age span when we allowed our birds to breed. Although it is possible that reproductive performance could be affected in old age, if this occurs it is likely to have a much smaller effect on fitness than the lifespan reduction.
A particularly surprising result of this study is the strong effect of exposure to elevated stress hormones in early life on the survival of breeding partners. Breeding with a bird that had been exposed to early-life stress substantially reduced adult lifespan in both males and females, and this effect was in addition to any survival reduction arising from an individual's own early-life CORT exposure. The causes of this lateral transfer are likely to be complex. Since birds did not choose their breeding partners in our study, but were randomly allocated mates from within the treatments, this effect is not confounded by any other factors, such as low-quality individuals being more likely to pair with each other. Behavioural studies of our birds during breeding showed that the early-CORT treatment was associated with a reduction in the incubation effort of exposed females, which was in part compensated for by their partner [21]. Similar effects could also have occurred during chick rearing, but we were not able to quantify this. Increased workload during breeding could, therefore, have contributed to the reduced adult life expectancy in birds mated to early-CORT birds. However, this seems unlikely since our birds only bred three times under the relatively benign conditions of captivity, where foraging and thermoregulatory costs were relatively low and breeding success was similar in all groups.
The most likely possibility is that social buffering of partners was reduced in the CORT-exposed birds, and/or that being mated to a CORT bird in itself increased exposure to stress hormones. Social buffering occurs when the presence of a familiar conspecific reduces stress responsiveness via the oxytocin-related hormone system; this has been reported in several vertebrate species and is known to have important consequences for health and longevity [22,23]. Zebra finches form strong pair bonds [24] and social buffering between mates has been shown to reduce glucocorticoid responsiveness to an environmental stressor [25]. However, the ability of a partner who is more stress-sensitive to provide social buffering can be reduced [26,27]. It has also been reported recently in female zebra finches that being mated to a partner of a non-preferred phenotype increases the levels of circulating CORT to between three and four times that of females mated to males of preferred phenotypes [28]. It is therefore possible that in our experiment, partner stress exposure was increased as a consequence of being mated to a CORT bird, and that the effects of this persisted even after the birds were no longer with their partners.
Variation in exposure to stress hormones in early life could occur for a number of reasons, such as variation in nutrition [29] or level of exposure to other stressors. It has been shown that such birds are discriminated against in mate choice tests [9]. Exposure to stress in early life has been found to affect the quality of male song [30], and is also likely to affect female attractiveness, though this has been less studied. The substantial lifespan penalty for the breeding partners of such birds suggests that birds exposed to stress in early life are discriminated against as breeding partners not simply because, as has generally been assumed, they make poor parents, but rather because they substantially reduce the lifespan of their breeding partners. That we found no difference between males and females in either the effect of early stress exposure on their own or partner longevity suggests that there will be selection pressure in both sexes in favour of avoiding these birds as mates.
Acknowledgements
We thank Graham Law and Alastair Kirk for assistance with bird husbandry, Ruedi Nager and Johan Nilsson for advice with statistical analyses, and Neil Metcalfe, Lynn Martin and two anonymous referees for constructive criticisms of an earlier draft of the manuscript. Funding was provided by a grant from the UK Biotechnology and Biological Sciences Research Council.
References
- 1.McEwen B. S., Wingfield J. C. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 10.1016/S0018-506X(02)00024-7 (doi:10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky R. M. 2000. Stress hormones: good and bad. Neurobiol. Dis. 7, 540–542 10.1006/nbdi.2000.0350 (doi:10.1006/nbdi.2000.0350) [DOI] [PubMed] [Google Scholar]
- 3.Weinstock M. 2008. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086 10.1016/j.neubiorev.2008.03.002 (doi:10.1016/j.neubiorev.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 4.Dickens M., Romero L. M., Cyr N. E., Dunn I. C., Meddle S. L. 2009. Chronic stress alters glucocorticoid receptor and mineralocorticoid receptor mrna expression in the European starling (Sturnus vulgaris) brain. J. Neuroendocrinol. 21, 832–840 10.1111/j.1365-2826.2009.01908.x (doi:10.1111/j.1365-2826.2009.01908.x) [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky R. M., Krey L. C., Mcewen B. S. 1986. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 7, 284–301 10.1210/edrv-7-3-284 (doi:10.1210/edrv-7-3-284) [DOI] [PubMed] [Google Scholar]
- 6.Romero L. M. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 10.1016/j.tree.2004.03.008 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 7.Cottrell E. C., Seckl J. R. 2009. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 3, 19. 10.3389/neuro.08.019.2009 (doi:10.3389/neuro.08.019.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews S. G., Phillips D. I. W. 2010. Minireview: transgenerational inheritance of the stress response: a new frontier in stress research. Endocrinology 151, 7–13 10.1210/en.2009-0916 (doi:10.1210/en.2009-0916) [DOI] [PubMed] [Google Scholar]
- 9.Husak J. F., Moore I. T. 2008. Stress hormones and mate choice. Trends Ecol. Evol. 23, 532–534 10.1016/j.tree.2008.06.007 (doi:10.1016/j.tree.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 10.Breuner C. W., Patterson S. H., Hahn T. P. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 10.1016/j.ygcen.2008.05.017 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 11.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645 10.1098/rstb.2007.0011 (doi:10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingfield J. C., Smith J. P., Farner D. S. 1982. Endocrine responses of white-crowned sparrows to environmental stress. Condor 84, 399–409 10.2307/1367443 (doi:10.2307/1367443) [DOI] [Google Scholar]
- 13.Soares M. C., Bshary R., Fusani L., Goymann W., Hau M., Hirschenhauser K., Oliveira R. F. 2010. Hormonal mechanisms of cooperative behaviour. Phil. Trans. R. Soc. B 365, 2737–2750 10.1098/rstb.2010.0151 (doi:10.1098/rstb.2010.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer K. A., Verhulst S. 2007. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata). Horm. Behav. 51, 273–280 10.1016/j.yhbeh.2006.11.001 (doi:10.1016/j.yhbeh.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 15.Wada H., Salvante K. G., Stables C., Wagner E., Williams T. D., Breuner C. W. 2008. Adrenocortical responses in zebra finches (Taeniopygia guttata): individual variation, repeatability, and relationship to phenotypic quality. Horm. Behav. 53, 472–480 10.1016/j.yhbeh.2007.11.018 (doi:10.1016/j.yhbeh.2007.11.018) [DOI] [PubMed] [Google Scholar]
- 16.Spencer K. A., Evans N. P., Monaghan P. 2009. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology 150, 1931–1934 10.1210/en.2008-1471 (doi:10.1210/en.2008-1471) [DOI] [PubMed] [Google Scholar]
- 17.Loiseau C., Sorci G., Dano S., Chastel O. 2008. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen. Comp. Endocrinol. 155, 101–108 10.1016/j.ygcen.2007.03.004 (doi:10.1016/j.ygcen.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 18.Pravosudov V. V., Kitaysky A. S., Wingfield J. C., Clayton N. S. 2001. Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli). Gen. Comp. Endocrinol. 123, 324–331 10.1006/gcen.2001.7684 (doi:10.1006/gcen.2001.7684) [DOI] [PubMed] [Google Scholar]
- 19.Seckl J. R. 2004. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 151, U49–U62 10.1530/eje.0.151U049 (doi:10.1530/eje.0.151U049) [DOI] [PubMed] [Google Scholar]
- 20.Glover V., O'Connor T. G., O'Donnell K. 2010. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 35, 17–22 10.1016/j.neubiorev.2009.11.008 (doi:10.1016/j.neubiorev.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 21.Spencer K. A., Heidinger B. J., D'Alba L., Evans N. P., Monaghan P. 2010. Then versus now: effect of developmental and current environmental conditions on incubation effort in birds. Behav. Ecol. 21, 999–1004 [Google Scholar]
- 22.Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398 10.1016/S0006-3223(03)00465-7 (doi:10.1016/S0006-3223(03)00465-7) [DOI] [PubMed] [Google Scholar]
- 23.Hennessy M. B., Kaiser S., Sachser N. 2009. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482 10.1016/j.yfrne.2009.06.001 (doi:10.1016/j.yfrne.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 24.Zann R. A. 1996. The zebra finch: a synthesis of field and laboratory studies, 1st edn. Oxford, UK: Oxford University Press [Google Scholar]
- 25.Remage-Healey L., Adkins-Regan E., Romero L. M. 2003. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm. Behav. 43, 108–114 10.1016/S0018-506X(02)00012-0 (doi:10.1016/S0018-506X(02)00012-0) [DOI] [PubMed] [Google Scholar]
- 26.Kiyokawa Y., Kikusui T., Takeuchi Y., Mori Y. 2004. Partner's stress status influences social buffering effects in rats. Behav. Neurosci. 118, 798–804 10.1037/0735-7044.118.4.798 (doi:10.1037/0735-7044.118.4.798) [DOI] [PubMed] [Google Scholar]
- 27.Kikusui T., Winslow J. T., Mori Y. 2006. Social buffering: relief from stress and anxiety. Phil. Trans. R. Soc. B 361, 2215–2228 10.1098/rstb.2006.1941 (doi:10.1098/rstb.2006.1941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith S. C., Pryke S. R., Buttemer W. A. 2011. Constrained mate choice in social monogamy and the stress of having an unattractive partner. Proc. R. Soc. B 278, 2798–2805 10.1098/rspb.2010.2672 (doi:10.1098/rspb.2010.2672). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poisbleau M., Demongin L., Chastel O., Eens M., Quillfeldt P. 2010. Reversed hatching order, body condition and corticosterone levels in chicks of southern rockhopper penguins (Eudyptes chrysocome chrysocome). Gen. Comp. Endocrinol. 169, 244–249 10.1016/j.ygcen.2010.09.007 (doi:10.1016/j.ygcen.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 30.Spencer K. A., Wimpenny J. H., Buchanan K. L., Lovell P. G., Goldsmith A. R., Catchpole C. K. 2005. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata). Behav. Ecol. Sociobiol. 58, 423–428 10.1007/s00265-005-0927-5 (doi:10.1007/s00265-005-0927-5) [DOI] [Google Scholar]