Abstract
Objective
To identify the prognostic variables that predict disease-specific survival and second local recurrence-free survival in patients with recurrent retroperitoneal liposarcoma so as to guide clinical management.
Summary Background Data
Local recurrence following complete resection of primary retroperitoneal liposarcoma is a common clinical problem that frequently leads to morbidity and mortality. Factors that determine survival in patients with a local recurrence after complete resection of the primary and re-recurrence after resection of the first local recurrence have not been clearly defined.
Methods
From a prospective sarcoma database we selected 105 patients who had at least one local recurrence following complete resection of a primary retroperitoneal liposarcoma between July 1982 and December 2005. Of these patients, 61 underwent complete resection of their first local recurrence. Study end-points included second local recurrence-free survival for these 61 patients and disease-specific survival for all 105 patients. Univariate analysis was performed with the Kaplan-Meier method and log-rank test, and multivariate analysis with the Cox’s proportional hazards model and score test. Local recurrence growth rate was defined as the radiographic size of the local recurrence divided by the time to local recurrence from the primary resection.
Results
Median follow-up was 65 months. Local recurrence size, primary histologic variant and grade, and local recurrence growth rate were independent predictors of disease-specific survival. For those undergoing re-resection, local recurrence size and local recurrence growth rate independently influenced development of a second local recurrence. Only patients with local recurrence growth rates of less than 0.9 cm/month were associated with improved survival following aggressive resection of the local recurrence.
Conclusions
Local recurrence growth rate is strongly associated with disease-specific survival and local control for patients with completely resected locally recurrent retroperitoneal liposarcoma. Despite aggressive operative management patients with a local recurrence growth rate greater than 0.9 cm/month were associated with poor outcomes and should be considered for enrollment in clinical trials employing novel agents.
INTRODUCTION
Retroperitoneal liposarcomas constitute a challenging management problem for the surgeon due to their propensity for local recurrence following resection. Approximately 15% of soft tissue sarcomas reside in the retroperitoneum/intra-abdominal location, and liposarcoma, the single most common histology, accounts for approximately 50% of these malignancies. Current chemotherapy is ineffective for the majority of patients, and toxicity limits adequate dosing by radiation therapy. Complete surgical resection remains the most effective treatment modality for the majority of patients. Gross margin negative resection is attainable in over 80% of patients when the liberal use of en bloc adjacent organ resection is utilized. This aggressive approach yields a median survival of 83 months and a 5-year disease-specific survival of 60%.1-4
Local recurrence following complete resection of primary retroperitoneal liposarcoma is common with 50% of well-differentiated and 80% of dedifferentiated tumors recurring within 5 years.3, 5 This is not surprising given the large tumor size and its proximity to vital structures, which limit the surgeon’s ability to achieve negative surgical margins. In contrast to extremity sarcomas, most patients who die of retroperitoneal soft tissue sarcoma die from the effects of local recurrence. Seventy five percent of patients with primary retroperitoneal liposarcoma succumb to this disease in the absence of distant metastases.3, 6-11 Despite this unique tumor biology and the consequent importance of local control, objective criteria or consensus to guide the re-resection of local recurrence in this disease is lacking, and the decision for reoperation is often dictated by patient preference or demand and the impending anatomic restrictions that would prevent future resection due to the tumor’s proximity to adjacent vital structures.
The classification of liposarcoma into three biological types encompassing five subtypes: 1) well-differentiated/dedifferentiated, 2) myxoid/round cell, and 3) pleomorphic, based on morphological features and cytogenetic aberrations, is now widely accepted.12 Well-differentiated, dedifferentiated, myxoid and round cell liposarcoma subtypes account for 56%, 37%, 5% and 2% of primary retroperitoneal liposarcoma, respectively. Histologic subtype defines grade and represents the most important prognostic factor for disease-specific survival and local recurrence in primary retroperitoneal liposarcoma.13 However, the prognostic factors predictive of survival for patients presenting with locally recurrent retroperitoneal liposarcoma have not been delineated and there are no reliable guidelines that define the subset of patients most likely to benefit from surgical resection of their local recurrence.
The aim of this study was to analyze disease-specific survival and local recurrence-free survival in a large, well-characterized, and relatively homogeneous cohort of prospectively followed patients with retroperitoneal liposarcoma managed at a single institution, and to define prognostic factors to guide the operative management of patients with locally recurrent retroperitoneal liposarcoma following complete resection.
METHODS
Patients
Review of the prospective database of adult patients (age ≥ 16 years) with STS treated at Memorial Sloan-Kettering Cancer Center identified a cohort of 207 patients who had their primary retroperitoneal liposarcoma treated between July 1, 1982 and October 31, 2005. Of these, 180 patients (87%) had complete resection of their primary and 105 patients (58%) subsequently had a local recurrence. Of these, 61 patients (58%) had complete resection of their local recurrence. These 105 and 61 patients with locally recurrent retroperitoneal liposarcoma form the basis of this study, as shown in Table 1.
Table 1.
Selection of Patients for Inclusion (1982-2005)
| Patient Group | N |
|---|---|
| All soft tissue sarcomas treated | 6682 |
| All retroperitoneal sarcomas treated | 607 |
| All retroperitoneal liposarcomas treated | 355 |
| Primary retroperitoneal liposarcomas treated | 207 |
| Completely resected | 180/207 (87%) |
| Local recurrence after complete resection | 105/80 (58%) |
| Local recurrence completely resected | 61/105 (58%) |
Patient, tumor, and treatment variables were correlated to recurrence and survival endpoints. Continuous variables analyzed included age at presentation and tumor size (measured as the sum of largest dimensions). Categorical patient/tumor variables analyzed included sex, histologic grade, histologic subtype (well-differentiated, dedifferentiated, myxoid, or round cell). These variables were studied for both the primary and recurrent tumors where the data was available. Treatment variables analyzed included micro margin status following primary resection, and gross margin status (i.e. complete versus incomplete resection) following first LR resection.
Pathology
The histologic features were reviewed by one of the authors (C.A.) and a minimum of one 4 μm thick hematoxylin and eosin stained histologic section was examined per centimeter of tumor diameter. Histologic subtype and grade was assigned by following the published criteria of the World Health Organization classification of tumors of soft tissue and bone. Histologic subtype was classified as well-differentiated, dedifferentiated, myxoid/round cell or pleomorphic. Retroperitoneal fatty tumors containing mature adipocytes with occasional atypical cells with irregular hyperchromatic nuclei and rare or absent lipoblasts or those lesions with lipoblasts and minimal fibrosis (<25% of the sampled tumor) were labeled lipoma-like well-differentiated liposarcoma. Tumors with atypical stromal cells associated with significant fibrosis (>25%) were designated as sclerosing well-differentiated liposarcoma. Lesions with regions of non-lipogenic spindle cell sarcoma arising within a fatty tumor or in the bed of a previously resected low grade lipomatous tumor were identified as dedifferentiated liposarcoma.
The majority of retroperitoneal tumors initially identified as malignant fibrous histiocytoma in the prospective database, on careful review for evidence of adjacent areas of well-differentiated liposarcoma, could be reclassified as dedifferentiated liposarcoma. Tumors with uniform round to oval shaped primitive non-lipogenic mesenchymal cells and a variable number of small or signet-ring lipoblasts in a prominent myxoid stroma with or without delicate arborizing vasculature were classified as myxoid liposarcoma. A subset of myxoid liposarcoma shows histological progression to round cell morphology that is characterized by solid sheets of back-to-back primitive round cells with a high nuclear/cytoplasmic ratio and conspicuous nucleoli with no intervening myxoid stroma. Pure myxoid liposarcoma is considered low grade. For the present study high histologic grade was defined as greater than 5% round cell areas.
Pleomorphic liposarcoma is characterized by pleomorphic spindle and giant cells as well as sheets of pleomorphic lipoblasts which contain enlarged and hyperchromatic nuclei scalloped by cytoplasmic vacuoles.
Statistical Analysis
Second local recurrence and disease-specific survival were the endpoints of this study. Second local recurrence-free survival was defined as time from the first local recurrence resection to time of the second local recurrence or time of death or last follow-up. Disease-specific survival after local recurrence was defined as time from the first local recurrence (time of diagnosis for the 105-patient analysis and time of resection for the 61-patient analysis) to time of death or last follow-up. Deaths confirmed to be caused by the disease (59 among the 105 patients) were treated as an endpoint for disease-specific survival; other deaths (six among the 105 patients) were considered censored observations.
Local recurrence growth rate for the first local recurrence was defined as the tumor size (i.e. the maximum dimension on cross-sectional imaging for a solitary mass, and the sum of all maximum dimensions for more than one mass) divided by the time from primary resection to local recurrence. The survival probabilities were estimated using the Kaplan-Meier method. The associations of the examined clinical, pathologic, and treatment variables with the survival outcome were examined using the log-rank test for categorical variables and the score test for continuous variables. To examine the association of the examined clinical, pathologic, and treatment variables with the survival outcome while adjusting for important prognostic factors, variables significant on univariate analysis at the 0.05 level were entered into a Cox proportional hazards model. When there are more than three such variables, only the top three variables with the smallest univariate p-values (the top two for the 61-patient analysis) were used as the sample size is relatively small. The optimum cut-off point for local recurrence growth rate was found using the Minimum P value method in the univariate setting.14
RESULTS
Patient, Primary Tumor, and Primary Treatment Variables
During the time period under study (1982-2005), 105 patients who had undergone complete resection for primary retroperitoneal liposarcoma at Memorial Sloan-Kettering Cancer Center had recurred locally. The distribution of clinical and pathologic characteristics in these patients is illustrated in Table 2. The median age of these patients was 60 years (range 24 to 84 years). Thirty-six (34%) patients were women. One hundred one (96%) presented with primary disease only, and four (4%) with synchronous metastatic disease. Forty-eight (46%) were well-differentiated, forty-nine (47%) were dedifferentiated, four (4%) were myxoid, and three (3%) were round cell histology. Fifty-two (49%) were high grade and the remaining fifty-three (51%) low grade. The median size of the primary tumor was 27 cm (range 5 to 70 cm). Fifty-three (51%) underwent a microscopic margin negative resection, and sixty-five (62%) required a contiguous organ resection to achieve complete resection. Seventeen (16%) received adjuvant radiation (9 external beam, 8 brachytherapy) and ten (10%) received adjuvant chemotherapy.
Table 2.
Characteristics of 105 patients with Locally Recurrent Retroperitoneal Liposarcoma
| Variable | Median (Range) | N (%) |
|---|---|---|
| Age (years) | 60 (24-84) | |
|
| ||
| Primary Tumor Size (cm) | 27 (5-70) | |
|
| ||
| Time to Local Recurrence (mo) | 21 (2-160) | |
|
| ||
| Sex | ||
| Female | 36 (34.3) | |
|
| ||
| Histologic Grade/Subtype | ||
| Low/Well-differentiated | 48 (46.2) | |
| High/Dedifferentiated | 49 (47.1) | |
| Low/Myxoid | 4 (3.8) | |
| High/Round Cell | 3 (2.9) | |
|
| ||
| Microscopic Margin Negative | 53 (51) | |
|
| ||
| Contiguous Organ Resection | 65 (62) | |
|
| ||
| Adjuvant Radiation | ||
| EBRT | 9 (8) | |
| Brachy | 8 (7) | |
|
| ||
| Adjuvant Chemotherapy | 10 (9) | |
Factors Predicting Disease-Specific Survival for the First Local Recurrence
The median follow-up was 65 months. The median time to the first local recurrence following complete resection in all 105 patients was 21 months (range 2 to 160 months). Factors influencing disease-specific survival measured from the time of the first local recurrence are illustrated in Table 3. Patient-specific variables such as age and sex did not influence disease-specific survival. Tumor-specific variables that predicted survival were primary histologic grade and histologic subtype, local recurrence size, and local recurrence growth rate. The only treatment variable that was associated with improved survival was complete resection of the local recurrence. Microscopic margin-free resection did not show significant association with disease-specific survival. Three variables were entered to a multivariate model and remain significant: primary histologic grade (p=0.010, HR=2.49), local recurrence growth rate (p=0.015, HR=1.20), and complete resection of the local recurrence (p=0.010, HR=2.70).
Table 3.
Analysis of Disease-Specific Survival in 105 Completely Resected Retroperitoneal Liposarcoma Patients with a Local Recurrence
| Variable | P Value (Univariate) |
P Value (Multivariate) |
Hazard Ratio [95% CI] (Multivariate) |
|---|---|---|---|
| Sex | 0.860 | ||
| Age (year) | 0.186 | ||
| Primary Grade High vs. Low * |
<0.001 | 0.010 | 2.49 [1.25, 4.98] |
| Primary Subtype * | <0.001 | ||
| Primary Size (cm) | 0.117 | ||
| Primary Micro Margin | 0.358 | ||
| LR Resection Incomplete/None vs. Complete |
<0.001 | 0.010 | 2.70 [1.27, 5.77] |
| LR Size (cm) ^ | <0.001 | ||
| Time to LR (month) | 0.010 | ||
| LR Growth Rate (cm/month) ^ |
<0.001 | 0.015 | 1.20 [1.04, 1.40] |
Primary grade and primary subtype are highly correlated. Only the former was entered to the multivariate model as it is more significant in the univariate setting.
LR growth rate and LR size are highly correlated. Only the former was entered to the multivariate model as it is more significant in the univariate setting.
Factors Predicting Disease-Specific Survival Following Resection of First Local Recurrence
Sixty-five (62%) patients underwent resection of the first local recurrence. Sixty-one (58%) had a documented complete gross resection. The four incomplete resections were performed with the intention of symptom (e.g. abdominal fullness and discomfort, ureteral obstruction) relief. Of the forty (38%) that were not re-resected, the reasons for observation or non-surgical management could be categorized based on 5 main components: 1. presence of synchronous metastatic disease, 2. extensive (e.g. involving major vascular or bony structures) or multifocal recurrence, 3. a short disease-free interval, 4. asymptomatic small-burden disease, especially in patients with a well-differentiated, low-grade tumor, and 5. prohibitive patient age or co-morbidity. Table 4 demonstrates that the factors influencing the disease-specific survival of patients undergoing re-resection of the first local recurrence include local recurrence histologic grade and subtype, local recurrence size, and local recurrence growth rate. Primary histologic grade also influences the disease-specific survival of these patients. Two variables most significant in the univariate setting were entered to a multivariate model and both remain significant: local recurrence histologic grade (p=0.005, HR=4.10) and local recurrence growth rate (p=0.001, HR=2.19).
Table 4.
Analysis of Disease-Specific Survival in 61 Patients with a Locally Recurrent Retroperitoneal Liposarcoma Completely Resected
| Variable | P Value (Univariate) |
P Value (Multivariate) |
Hazard Ratio [95% CI] (Multivariate) |
|---|---|---|---|
| Sex | 0.444 | ||
| Age (year) | 0.446 | ||
| Primary Grade | 0.024 | ||
| Primary Subtype | 0.074 | ||
| Primary Size (cm) | 0.401 | ||
| Primary Micro Margin |
0.283 | ||
| LR Grade High vs. Low |
<0.001 | 0.005 | 4.10 [1.54, 10.94] |
| LR Subtype | 0.001 | ||
| LR Size (cm) | 0.009 | ||
| Time to LR (month) | 0.153 | ||
| LR Growth Rate (cm/month) |
<0.001 | 0.001 | 2.19 [1.36, 3.53] |
Factors Predicting Second Local Recurrence-free Survival Following Resection of First Local Recurrence
Sixty-one patients underwent complete gross resection of their local recurrence. Forty-two (69%) of these patients recurred locally. Two of these patients had concurrent metastatic disease. Factors predicting local recurrence-free survival include local recurrence size and local recurrence growth rate as shown in Table 5. In particular, local recurrence growth rate (p<0.001, HR=2.70) remain as a significant prognostic factor when adjusting for local recurrence histologic grade.
Table 5.
Analysis of Second Local Recurrence-Free Survival in 61 Patients with a Locally Recurrent Retroperitoneal Liposarcoma Completely Resected
| Variable | P Value (Univariate) |
P Value (Multivariate) |
Hazard Ratio [95% CI] (Multivariate) |
|---|---|---|---|
| Sex | 0.180 | ||
| Age (year) | 0.095 | ||
| Primary Grade | 0.848 | ||
| Primary Subtype | 0.710 | ||
| Primary Size (cm) | 0.836 | ||
| Primary Micro Margin | 0.198 | ||
| LR Grade High vs. Low | 0.063 | 0.390 | 1.36 [0.67, 2.74] |
| LR Subtype | 0.066 | ||
| LR Size (cm) ^ | 0.002 | ||
| Time to LR (month) | 0.117 | ||
| LR Growth Rate (cm/month) ^ |
<0.001 | <0.001 | 2.70 [1.71, 4.27] |
LR growth rate and LR size are highly correlated. Only the former was entered to the multivariate model as it is more significant in the univariate setting.
Disease-Specific Survival by Local Recurrence Growth Rate
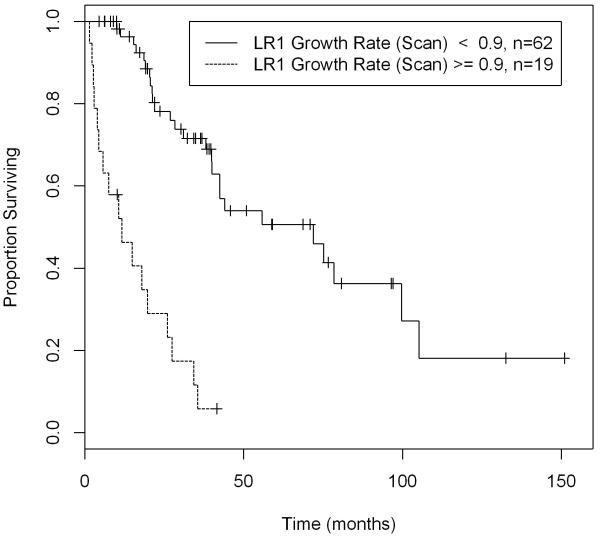
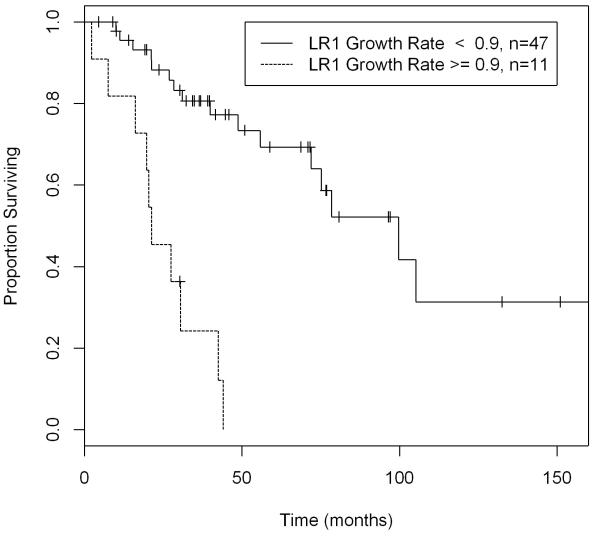
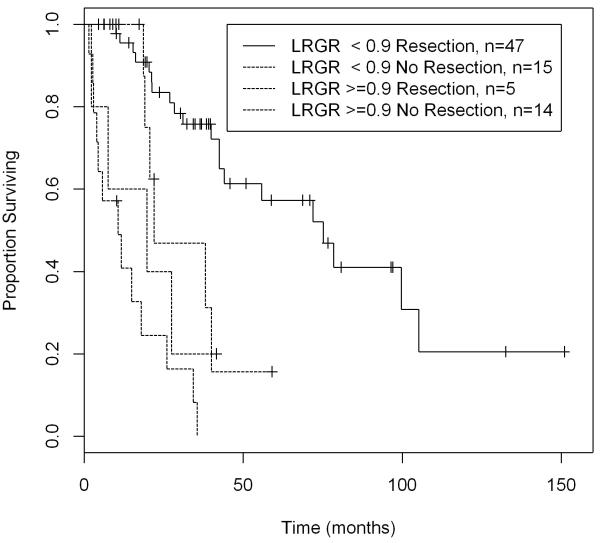
The cut-off point for local recurrence growth rate was found using the Minimum P-value method in the univariate setting. The median disease-specific survival was 65 months for patients with tumors with local recurrence growth rate of less than 0.9 cm/month, and only 13 months for those with local recurrence growth rate equal to or greater than 0.9 cm/month when all 105 patients are considered. (Figure 1) For the 61 patients undergoing complete resection of their local recurrence, the median disease-specific survival was 100 months for patients with tumors with local recurrence growth rate of less than 0.9 cm/month, and only 21 months for those with local recurrence growth rate equal to or greater than 0.9 cm/month. (Figure 2) The 105 patients were further categorized based on whether or not they underwent resection: patients undergoing resection had a significantly longer disease-specific survival compared to no resection only when the local recurrence growth rate is less than 0.9 cm/month (p-value=0.014). Despite aggressive resection, patients with tumors exhibiting a high local recurrence growth rate were associated with a poor disease-specific survival. (Figure 3)
Figure 1.
Disease-Specific Survival of 105 Completely Resected Retroperitoneal Liposarcoma Patients with a Local Recurrence by LR Growth Rate
Figure 2.
Disease-Specific Survival of 61 Patients with Completely Resected Locally Recurrent Retroperitoneal Liposarcoma by LR Growth Rate
Figure 3.
Despite aggressive resection, patients with tumors exhibiting a local recurrence growth rate ≥ 0.9 cm/mo were associated with a poor disease-specific survival (N=105)
DISCUSSION
The surgical management of primary retroperitoneal liposarcoma and the prognostic variables influencing its resultant outcome are well-established. Complete surgical resection is feasible in approximately 80% of cases treated aggressively at specialized centers, and is the treatment of choice which results in a median disease-specific survival of 65 months. The quality of surgical resection has been demonstrated to be an independent prognostic factor predicting survival in this disease.5, 15 It has previously been shown that histologic subtype defines histologic grade (i.e. well-differentiated and myxoid subtypes are low grade, and dedifferentiated and round cell subtypes are high grade), and that this variable predicts local recurrence and disease-specific survival.13, 16 Therefore, the ability to achieve a complete resection and the histologic grade or subtype of the tumor are the most important respective therapeutic and biologic factors prognostic for survival in patients with primary retroperitoneal liposarcoma.
There is limited data to guide the surgical management of locally recurrent retroperitoneal liposarcoma following complete resection of the primary. When the patient presents with a symptomatic recurrence, the role of surgical intervention is somewhat clearer. There is evidence to support the use of incomplete, palliative surgical resection in patients with symptomatic recurrences to alleviate debilitating symptoms, with suggestions of potentially improving survival.17 Several reports have demonstrated that no survival benefit is derived from incomplete resections, and assert that this approach be reserved for treatment of incapacitating symptoms.1, 2, 8 The majority of patients who present with locally recurrent disease will not have symptoms that require surgical palliation. These patients present with resectable and oftentimes asymptomatic recurrences detected either on surveillance cross-sectional imaging or found in clinical follow-up. How then does the surgeon counsel the patient with an asymptomatic, resectable local recurrence? Should all of these patients be operated on, or is there a subset of patients that will not benefit from this aggressive approach? What factors should be considered in the timing of the reoperation?
In previously published work from our institution examining 500 patients with retroperitoneal soft tissue sarcomas, there was a suggestion that although complete resection at the initial operation for the primary may positively influence outcome, once the tumor recurs, the outcome of these patients is dictated by tumor biology itself or its influence on our ability to deliver effective treatment.2 This implies that resection of local recurrences should be undertaken only for symptomatic patients, since it does not change outcome significantly. However, given that approximately 60% of the first local recurrences are technically resectable with an aggressive approach, and since there is a lack of effective alternative therapeutic modalities, surgical resection is usually recommended in patients with good performance status and a reasonable but admittedly arbitrary disease-free interval “cut-off”. There appears to be an apparent discordance between the observed tumor biology of locally recurrent disease and the generally accepted clinical practice to address local recurrences. This discrepancy is most evident in patients presenting with a local recurrence following a short disease-free interval. The only justification for re-operation is the implication that there is a biological difference between those tumors that have an extremely short recurrence-free interval and those that are incompletely resected. There is certainly no definition of what constitutes a reasonable disease-free interval.
These controversial questions were evaluated in a relatively homogeneous group of patients with locally recurrent retroperitoneal liposarcoma following complete resection of the primary performed at Memorial Sloan-Kettering Cancer Center. Liposarcoma was specifically chosen for study given its unique status as the single most common histology of soft tissue sarcoma found in the retroperitoneum, its high rate of local recurrence often in the absence of distant disease, and as a disease entity where the local recurrence is intimately associated with patient survival.
The findings in this study challenge the general acceptance that most patients with local recurrences following complete resection of their retroperitoneal soft tissue sarcoma amenable to surgical resection should undergo reoperation. Although complete resection of the local recurrence clearly influences the disease-specific survival of these patients, the data also demonstrate that for the specific subgroup of patients with locally recurrent retroperitoneal liposarcoma, those with local recurrence growth rates greater than 0.9 cm/month, (ergo “the 1 cm/month rule”) do not benefit from this aggressive surgical approach. This subset of patients will have a disease-specific survival similar to those patients who do not undergo surgical re-resection. This data enable the treating physician to select patients that will have a very poor prognosis even with aggressive surgery and may be best treated with an alternative modality of treatment or with novel systemic agents. Although some may argue that the best local control afforded to these patients is surgical resection, an aggressive surgical approach has a 3 to 6% mortality and is not without potential major complications, and hence is not warranted with evidence demonstrating lack of benefit to the patient’s overall outcome. In the absence of symptoms, it would be difficult to justify an aggressive surgical approach in this subset of patients with local recurrence growth rates greater than 0.9 cm/month.
There are biases and limitations to this single institution, retrospective analysis consisting of a limited number of patients. Although the number of patients evaluable is small due to the rarity of both the disease and the subgroup that we are studying, with 105 patients it is the largest series to date of a homogeneous histology. There has been much debate as to what is the most accurate method of measuring tumor burden. The current literature supports the use of a single dimension measurement on cross-sectional imaging as a reliable method, and numerous studies comparing volume versus single dimension measurements have demonstrated that the unidimensional method is universally applicable, more reliable, and overall superior.18-21 There is no literature to support serial follow-up imaging for retroperitoneal soft tissue sarcomas. However, CT or MR imaging at 3 to 6 month intervals is performed as a routine at our institution. Although disease-free interval (i.e. the time from complete resection to when the local recurrence was first evident on imaging) is only as accurate as the interval at which the scans are obtained, this measurement is normalized to the tumor dimension by use of the parameter growth rate, accepting the limitation that growth rate is not necessarily a linear parameter. Growth rate in this study has been demonstrated as an independent prognostic factor that determines both disease-specific survival and local recurrence-free survival. These findings are in accordance to observations made in extremity sarcomas, where large, high-grade, short disease-free interval tumors demonstrate a 10% 5 year survival, whereas small, low-grade, long disease-free interval tumors result in 80% 5 year survival.22
The histologic subtype and hence tumor grade are well-established factors determining the outcome of patients with primary retroperitoneal liposarcoma. However, this study of patients with locally recurrent disease demonstrates that these factors appear to play less of a role in determining disease-specific survival and local re-recurrence in this subgroup. The subtype and grade of the primary tumor does not maintain its importance as an independent prognostic factor for survival. This is in part due to the dedifferentiation of previously well-differentiated tumors which is a known occurrence in 17 % of cases upon first local recurrence and in 44% of cases of second local recurrence.13 Furthermore. it is well accepted that following the complete gross resection of a dedifferentiated liposarcoma, the microscopic disease left behind not infrequently manifests as a local recurrence that contains just the well-differentiated component.23 The resection may change the outcome in these cases. This argues that the technical aspect of the re-resection does play a major role in determining the ultimate outcome of these patients. It also argues that the histologic subtype of the primary and even that of the LR may not be reliable markers for predicting outcome of locally recurrent RP liposarcoma.
From a patient counseling standpoint, the histologic subtype and tumor grade of the local recurrence, although a demonstrated independent prognostic factor, is not information that is available to aid in the decision making process for resection of the local recurrence in most patients, and therefore is more limiting compared to the growth rate, which is available for all patients in the pre-operative setting. We do not advocate preoperative biopsy of the recurrence to define the histologic subtype, given that there will always be an inherent sampling error in detecting regions that may harbor dedifferentiation. The concept that soft-tissue sarcoma tumor size and time to recurrence, and hence growth rate, influences outcome is not new. This study is an effort to try to qualitatively apply this to clinical practice and guide patient management.
Based on these results for patients presenting with asymptomatic local recurrence and growth rates exceeding or equal to 1 cm per month we are now recommending treatment with systemic chemotherapy or novel targeted therapeutic trials. Surgery is only considered in this sub group if they develop symptoms unresponsive to medical management such as obstruction and bleeding. For patients with local recurrence growth rates less than 1 cm/month immediate surgery is recommended for all symptomatic patients and for asymptomatic patients whose local recurrence is impinging on critical structures (particularly if further growth may result in the need to sacrifice critical organs) or has a solid appearance on CT scan (suspicious for a dedifferentiation). Many asymptomatic patients with a well-differentiated appearing local recurrence that is well away from critical structures may be safely followed off any therapy and monitored to determine if they develop other sites of disease before recommending complete surgical resection. Such an approach can extend the interval between surgical resections and enables the surgeon to be more confident that they are encompassing all sites of known disease with their planned procedure.
CONCLUSION
In this study of patients with locally recurrent retroperitoneal liposarcoma following complete resection of the primary, local recurrence growth rate was found to predict DSS and local control. Although local recurrence resection was a predictor of DSS, when performed on patients with tumors having a local recurrence growth rate greater than 0.9 cm/month it was not associated with a survival benefit. Local recurrence growth rate can be used as a guide to direct management of patients with locally recurrent retroperitoneal liposarcoma.
Acknowledgments
Supported by the NIH Soft Tissue Sarcoma Program Project Grant P01CA47179 and the Kristen Ann Carr Fund
REFERENCES
- 1.Jaques DP, Coit DG, Hajdu SI, Brennan MF. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg. 1990;212(1):51–9. doi: 10.1097/00000658-199007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228(3):355–65. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221(2):185–95. doi: 10.1097/00000658-199502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storm FK, Mahvi DM. Diagnosis and management of retroperitoneal soft-tissue sarcoma. Ann Surg. 1991;214(1):2–10. doi: 10.1097/00000658-199107000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarenga JC, Ball AB, Fisher C, et al. Limitations of surgery in the treatment of retroperitoneal sarcoma. Br J Surg. 1991;78(8):912–6. doi: 10.1002/bjs.1800780806. [DOI] [PubMed] [Google Scholar]
- 6.Catton CN, O’Sullivan B, Kotwall C, et al. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29(5):1005–10. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 7.Gronchi A, Casali PG, Fiore M, et al. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100(11):2448–55. doi: 10.1002/cncr.20269. [DOI] [PubMed] [Google Scholar]
- 8.Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15(8):2832–9. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 9.Kilkenny JW, 3rd, Bland KI, Copeland EM., 3rd. Retroperitoneal sarcoma: the University of Florida experience. J Am Coll Surg. 1996;182(4):329–39. [PubMed] [Google Scholar]
- 10.Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18(8):1637–43. doi: 10.1200/JCO.2000.18.8.1637. [DOI] [PubMed] [Google Scholar]
- 11.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92(2):359–68. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CD, Akerman M, Dal Cin P, et al. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group Correlation between clinicopathological features and karyotype in lipomatous tumors. Am J Pathol. 1996;148(2):623–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238(3):358–70. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazumdar M, Smith A, Bacik J. Methods for categorizing a prognostic variable in a multivariable setting. Stat Med. 2003;22(4):559–71. doi: 10.1002/sim.1333. [DOI] [PubMed] [Google Scholar]
- 15.McGrath PC, Neifeld JP, Lawrence W, Jr., et al. Improved survival following complete excision of retroperitoneal sarcomas. Ann Surg. 1984;200(2):200–4. doi: 10.1097/00000658-198408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalal KM, Kattan MW, Antonescu CR, et al. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381–91. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata D, Lewis JJ, Leung DH, Brennan MF. Is there a role for incomplete resection in the management of retroperitoneal liposarcomas? J Am Coll Surg. 2001;193(4):373–9. doi: 10.1016/s1072-7515(01)01024-9. [DOI] [PubMed] [Google Scholar]
- 18.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91(6):523–8. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 19.Dachman AH, MacEneaney PM, Adedipe A, et al. Tumor size on computed tomography scans: is one measurement enough? Cancer. 2001;91(3):555–60. [PubMed] [Google Scholar]
- 20.Schuetze SM, Baker LH, Benjamin RS, Canetta R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist. 2008;13(Suppl 2):32–40. doi: 10.1634/theoncologist.13-S2-32. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin RS. SARC-CTOS imaging symposium: introduction to the problem from a clinical perspective. Oncologist. 2008;13(Suppl 2):1–3. doi: 10.1634/theoncologist.13-S2-1. [DOI] [PubMed] [Google Scholar]
- 22.Eilber FC, Kattan MW. Sarcoma nomogram: validation and a model to evaluate impact of therapy. J Am Coll Surg. 2007;205(4 Suppl):S90–5. doi: 10.1016/j.jamcollsurg.2007.06.335. [DOI] [PubMed] [Google Scholar]
- 23.van Dalen T, Plooij JM, van Coevorden F, et al. Long-term prognosis of primary retroperitoneal soft tissue sarcoma. Eur J Surg Oncol. 2007;33(2):234–8. doi: 10.1016/j.ejso.2006.09.020. [DOI] [PubMed] [Google Scholar]