Abstract
Apoptosis is a tightly regulated physiologic process of programmed cell death that occurs in both normal and pathologic tissues. Numerous in vitro or in vivo studies have indicated that cardiomyocyte death through apoptosis and necrosis is a primary contributor to the progression of anthracycline-induced cardiomyopathy. There are now several pieces of evidence to suggest that activation of intrinsic and extrinsic apoptotic pathways contribute to anthracycline-induced apoptosis in the heart. Novel strategies were developed to address a wide variety of cardiotoxic mechanisms and apoptotic pathways by which anthracycline influences cardiac structure and function. Anthracycline-induced apoptosis provides a very valid representation of cardiotoxicity in the heart, an argument which has implications for the most appropriate animal models of damaged heart plus diverse pharmacological effects. In this review we describe various aspects of the current understanding of apoptotic cell death triggered by anthracycline. Differences in the sensitivity to anthracycline-induced apoptosis between young and adult hearts are also discussed.
Keywords: pediatric, cardiomyopathy, anthracycline, apoptosis
Introduction
Heart failure in childhood causes significant morbidity and mortality. The etiologies are diverse [1-3]. One of the most common causes is chemotherapy, such as anthracycline-induced cardiotoxicity. The anthracyclines, primarily doxorubicin, also including daunomycin, epirubicin and idarubicin, are among the most widely used and successful chemotherapeutics for childhood cancers, but their cumulative and dose-dependent cardiac toxicity has been the major concern of oncologists for decades [4, 5]. With the increasing population of cancer survivors, there is a growing need to develop preventive strategies and effective therapies against anthracycline-induced cardiotoxicity, in particular, the late onset cardiomyopathy.
Apoptosis, a Greek word that means falling of leaves from trees in autumn in response to the impending threat of freezing and damage in winter [6], is a genetically programmed cell death which proceeds through distinct morphological changes such as nuclear condensation, DNA fragmentation, shrinkage of the cell body, membrane blebbing and cellular fragmentation into apoptotic bodies. These apoptotic bodies are then engulfed by neighboring healthy cells or macrophages [7]. Apoptosis deletes cells with little tissue disruption and no inflammatory response. Two major apoptotic signaling cascades have been described and are generally referred to as the extrinsic (or receptor-mediated) and intrinsic (or mitochondrial) pathways. Apoptotic cell death is an essential process in normal and diseased pediatric heart. Recent in vitro and in vivo studies provided compelling evidence that terminally differentiated cardiomyocytes, can and do undergo programmed cell death. Apoptosis has been shown to be involved in numerous pathophysiological consequences, contributing to many diseases including cancer, immunity disorders, and cardiovascular disorders. Cardiomyocyte death has been found in major heart diseases, including cardiomyopathies, myocardial infarction (MI), end-stage heart failure, arrhythmogenic right ventricular dysplasia, etc [8-10]. Besides adult cardiac problems, numerous human and animal studies have shown distinct roles of apoptosis in normal and abnormal aspects of the pediatric heart. These studies have been instrumental in demonstrating the importance of cardiomyocyte apoptosis and in the characterization of the distinct apoptotic pathways.
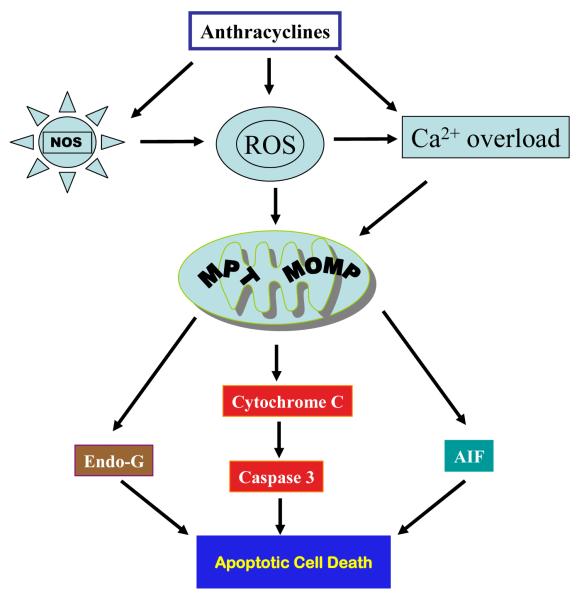
Although intensive investigations on anthracycline-induced cardiotoxicity have continued for decades, the underlying mechanisms responsible for anthracycline-induced cardiotoxicity remain incompletely understood. The mechanism for anthracycline-induced cadiotoxicity has been suggested to be attributable, at least in part, to the generation of free reactive oxygen species (ROS), which then activate mitochondrial-mediated apoptotic signaling pathway leading to caspase 3 activation and cardiomyocyte apoptosis [11-16] (Fig. 1). In this review, we will focus on the current understanding of molecular mechanisms underlying anthracycline-induced apoptosis and on the differences in the sensitivity to anthracycline-induced apoptotic signals between adult and young cardiomyocytes.
Figure 1.
Simplified mitochondrial pathway of anthracycline-induced cardiotoxicity via activation of distinct apoptotic mechanisms following mitochondrial outer membrane permeabilization (MOMP) and/or mitochondrial permeability transition (MPT).
Pediatric cardiomyopathy
The etiologies of heart failure in childhood are strikingly different from adults and can result from 1) congenital structural defects; 2) inherited cardiomyopathies (i.e. abnormalities of sarcomeric or cytoskeletal proteins); 3) acquired disease (i.e. infection such as viral myocarditis [17] or exposure to cardiotoxic agents such as anthracycline chemotherapy for cancer [18, 19]); 4) ischemia-reperfusion injury during open-heart surgery to repair structural defects [20, 21]. Among the diverse causes, congenital heart defects are the leading cause of heart failure in children and represent approximately 1% of all live birth, making this the most common birth defect in humans [22]. As a result of abnormal heart morphogenesis, they present most commonly during infancy between birth and one year of age. Dilated or hypertrophic cardiomyopathies are most common in children over one year of age and remain the principal indication for cardiac transplantation in children throughout childhood [23]. The prognosis of dilated cardiomyopathy in children is poor, with a 5-year survival rate of only 60% [24].
Despite the importance of heart failure in infants and children, this disease is still under-studied during these ages. On the contrary, there are rich literature and a relatively better understanding of the cellular molecular aspects of heart failure in adults. As a result, most new concepts for management of heart failure in children today are based on translation of adult treatment strategies with little preclinical evidence supporting their use in children [1, 3].
Anthracycline cardiotoxicity
The anthracyclines are among the most widely used and successful anticancer drugs ever developed. Despite extensive and long-standing clinical use (more than 40 years), they still play a major role in the treatment of a wide spectrum of hematologic malignancies and solid tumors. Anthracycline chemotherapy, together with other improvements to treatment, has significantly improved cancer survival, particularly among children, with an increase in the 5-year survival rates from less than 50% in the 1970s to about 80% currently [25-27]. Unfortunately, the therapeutic potential of anthracycline is limited by their cumulative and dose-dependent cardiac toxicity [4, 5]. Three types of anthracycline-induced cardiotoxicity have been described: acute (within the first week of treatment), early-onset (within a year) and late-onset (more than one year after completion of treatment). Most patients who develop significant cardiotoxicity have a late-onset dilated cardiomyopathy.
Children and adolescents are particularly susceptible to the cardiotoxic effects of anthracycline chemotherapy [19, 28, 29]. About half of the young adult survivors of childhood cancer have received anthracyclines at some time points in their treatment. The frequency of cardiotoxic effects has been reported to be more than 50% among the survivors of childhood cancer and there is no safe dose in this population [18, 28-33]. At 30 years after diagnosis, cardiac complications are the leading noncancerous cause of chronic health condition in childhood cancer survivors. The standardized mortality rate for cardiac death in long-term survivors of childhood cancer is 8 times higher than expected [34]. There are currently more than 300,000 long-term survivors of childhood cancer in the United States, and this number is increasing [35]. Hence, the development of novel therapeutic strategies to improve the survivor outcome is of high clinical importance.
Apoptosis in anthracycline-induced cardiotoxicity
Because anthracyclines are such effective anticancer drugs, their mechanisms of action have been under intense investigation for many years. Cardiomyocyte death, which occurs within hours after anthracycline exposure and during the late process of ventricular remodeling, is one of the most studied mechanisms for anthracycline-induced cardiomyopathy [11-15]. Cell death is classified by the morphology of the affected cells: apoptosis, necrosis and autophagy. Most experimental studies and histopathology of endomyocardial biopsies from human patients have provided evidence that anthrocycline-induced cardiac toxicity is associated with cardiomyocyte apoptosis and necrosis [11, 12]. Recent evidence indicates that autophagy and senescence can also be related to anthracycline-induced cardiomyopathy [36-39].
Oxidative stress generated by anthracyclines has been the most studied cause of cardiotoxicity and is believed acting as a major trigger for cardiomyocyte death [40-42]. ROS such as superoxide and hydroxyl radical are formed when the quinine moiety of anthracyclines is reduced to semiquinone [40-42]. These drugs are also able to combine with iron, generating toxic and highly charged ROS. Also, mitochondrial damage induced by ROS or directly by anthracyclines can further lead to respiratory chain failure and ROS liberation [43, 44]. Endothelial nitric oxide synthase (eNOS) reductase domain converts anthracycline to an unstable semiquinone intermediate that favors ROS generation [45]. Oxidative stress can also occur via induction of NOS, leading to nitric oxide and peroxynitrite formation [46]. Oxidative stress leads to many deleterious effects on cell membrane (lipid peroxidation) [47] and subcellular apparatuses, specifically cardiac mitochondria [48]. Ultimately these changes can lead to cell death by apoptosis and necrosis, and organ damage.
Cardiac mitochondria are the key mediators of anthracycline-induced cardiomyocyte death [48] (Fig.1). Mitochondrial damage induced by ROS or directly by anthracyclines include, but are not limited to, mitochondrial membrane damage due to lipid peroxidation, impaired mitochondrial oxidative phosphorylation and adenosine triphosphate synthesis, impaired mitochondrial calcium homeostasis resulting in loss of membrane stability [48], increased mitochondrial DNA mutations [49], impaired mitochondrial creatine kinase activity and function [50], disruption of cardiac mitochondrial biogenesis [51], and mitochondrial fragmentation [52]. All these events can trigger cardiomyocyte death by activating mitochondrial intrinsic apoptotic pathway or necrosis. For example, mitochondrial calcium overload triggers mitochondrial permeability transition (MPT), resulting in a loss of mitochondrial membrane potential, mitochondrial swelling, and outer membrane rupture, consequently release of cytochrome c, apoptosis inducing factor (AIF), and endonuclease G (EndoG) from mitochondria. Following mitochondrial release, cytochrome c forms a complex with the adaptor protein Apaf-1, dATP, and caspase 9, resulting in the formation of apoptosome. Apoptosome formation leads to the proteolytic cleavage and concomitant activation of caspase 9. Active caspase 9 directly cleaves and activates caspase 3. When a critical amount of activated caspase 3 is present within a cell, apoptosis is elicited. In addition, MPT can also trigger necrosis through inducing inner membrane rupture. Multiple studies have shown that anthracyclines induce cardiomyocyte apoptosis via the mitochondrial intrinsic pathway [16, 53-57].
In addition to mitochondrial damage, numerous signaling pathways are activated by ROS or by anthracyclines leading to the activation of the intrinsic apoptotic pathway. Cytochrome c release from mitochondria is regulated by the members of the Bcl-2 family, which includes three groups: anti-apoptotic members Bcl-2, Bcl-XL, and Mcl-1, pro-apoptotic members Bax and Bak, and BH3 only proteins such as Bad, Bid, Nix and BNip3 that enhance apoptosis via inhibition of anti-apoptotic Bcl-2 proteins or activation of pro-apoptotic Bax and Bak. Activation of BH3-only proteins by stress stimuli promotes Bax/Bak translocation from the cytosol to the outer membrane of mitochondria, resulting in increased mitochondrial outer membrane permeabilization (MOMP), leading to protein release from the intermembrane space to the cytoplasm, particularly the apoptogenic molecule cytochrome c. Anthracycline-induced cardiomyocyte apoptosis is associated with increased Bax/Bcl-2 ratio and down-regulations of anti-apoptotic factors, which can result from multiple mechanisms which include, but are not limited to, 1) activation of p53 tumor suppressor protein leading to increased Bax expression [58, 59]; 2) down-regulation of transcriptional factor GATA-4 leading to decreased Bcl-XL expression [57, 60]; 3) activation of the stress-activated protein kinase c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) [56, 61, 62]; 4) down-regulation of apoptosis repressor with a caspase recruitment domain (ARC) (mediated by ubiquitin-proteasome system mediated degradation) leading to increased Bax translocation to mitochondria [63, 64]; 5) inactivation of PI-3K/Akt survival pathway which has multiple impacts on apoptotic signaling [51, 65-68]; 6) dysregulation of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop resulting in decreased Bcl-2 expression [69].
In addition to the activation of the intrinsic mitochondrial apoptotic pathway, activation of extrinsic apoptotic pathway also contributes to anthracycline-induced cardiomyocyte apoptosis [70-73]. In the extrinsic pathway, death ligands, such as FasL and TNFα, bind their receptors and stimulate recruitment of the adaptor proteins Fas-associated via death domain (FADD) and TNFR associated death domain (TRADD). FADD and TRADD recruit caspase 8 into a complex named death-inducing signaling complex (DISC), where it undergoes dimerization and concomitant activation. Activated caspase 8 then activates caspase 3 directly or indirectly through the mitochondria via activation of Bid which translocates to mitochondria and activates Bax and Bak to trigger the release of cytochrome c. Anthracyclines activate the extrinsic apoptotic pathway by several mechanisms which include 1) activation of nuclear factor-activated T cell-4 (NFAT4) by increased mitochondrial ROS production and activation of the calcium/calcineurin signaling pathway, leading to up-regulation of Fas/FasL [74]; 2) activation of transcription factor NF-κB by ROS leading to increased Fas/FasL and p53 [75-77]; 3) down-regulated expression of FLIP, a FLICE/caspase-8 inhibitory protein, by ROS thereby sensitizing Fas-mediated apoptosis [72]; 4) down-regulation of ARC, an endogenous inhibitor of extrinsic pathway through interaction with Fas, FADD, and caspase 8 to prevent the formation of DISC [63, 64].
Additional mechanisms for anthracycline-induced apoptosis include endoplasmic/sarcoplasmic reticulum (ER/SR)-mediated apoptotic pathway leading to the activation of caspase 12 [78], and caspase-independent but AIF- and/or EndoG-dependent apoptosis [79-82]. Although a majority of the studies documented the involvement of apoptosis in anthracycline-induced cardiotoxocity, other reports have challenged the induction of apoptosis in these pathologies [83-85]. This controversy may be explained by the low prevalence of cardiomyocyte apoptosis in these hearts (typically <1%) and a wide variety of experimental conditions used in the studies including differences in dosage and frequency of anthracycline administration, in timing of assays, and in animal species and so on [86].
Potential therapeutic targets in anthracycline-induced apoptosis
Several approaches have been used to reduce the incidence of anthracycline-induced cardiotoxicity [11, 12, 87]. These strategies includes 1) dose limitation; 2) close cardiac monitoring; 3) alteration of dosage schedule such as using low-dose prolonged continuous infusion; 4) development of new anthracycline analogs that retain chemotherapeutic potential but with reduced cardiotoxicity; 5) liposome-encapsulation which limits the drugs to escape the tight capillary junctions of the heart, but not the discontinuous capillary system in tumors; 6) the administration of protective agents such as antioxidants, iron chelators and free radical scavengers. Although many efforts have been focused on reducing anthracycline-associated cardiotoxicity, it continues to have a high incidence.
Therapeutic blockage of cardiomyocyte programmed death is obviously a challenge that might require identification of numerous players in the apoptotic cascade and the right time-point to begin treatment without compromising anthracycline toxicity to tumor cells. Inhibition of the propagation and execution stages of anthracycline-induced apoptosis, e.g. inhibition of caspases may delay or block cell death and could be used to recover cardiac function. Therefore, a combination of an antiapoptotic therapy together with other cardioprotective therapies may be more effective. In addition, models of anthracycline-induced cardiotoxicity will probably help clarify the significance of combined therapies. Future novel cardioprotective therapeutic strategies might be tested in both the intrinsic and extrinsic apoptotic pathways using genetic and biochemical approaches. In keeping with this view, unraveling the sequence of key apoptotic factors recruited during anthracycline-induced apoptosis should enable us to identify regulatory molecules to be targeted for producing an optimal effect.
Caspase inhibitors has been shown to be effective in reducing myocardial reperfusion injury, which could at least be partially attributed to the attenuation of cardiomyocyte apoptosis [88, 89]. Inhibition of apoptotic DNA fragmentation and nuclear cleavage may block programmed cell death, however, blockage of earlier signaling steps required for anthracycline-induced apoptotic nuclear fragmentation might work better to ensure cell survival. For instance, blocking MOMP is likely to maintain long-term survival by abolishing the killing functions of downstream molecules including caspases. In fact MOMP inhibition can also block caspase-independent cell death found in autophagic or necrotic death. Therefore, inhibition of MOMP may have a wider range of cardioprotective actions than inhibition of caspases. MOMP can be blocked by the antiapoptotic proteins of the Bcl-2 family and is proven to have very effective cytoprotective effects in various tissues including the heart [90-93]. However, inhibition of mitochondrial permeabilization may not block cell death in conditions in which caspase activation is activated via the external pathway or, for instance, by of IAP (Inhibitor of Apoptosis Protein) antagonists, where cell death can occur without of MOMP.
Differences in anthracycline-induced cardiotoxicity in neonatal, young and adult, and old hearts
As suggested by clinical studies, children and adolescents are particularly susceptible to the cardiotoxic effects of anthracycline chemotherapy [19, 28, 29]. Children treated before the age of 4 years are especially vulnerable [29]. Potential underlying mechanisms include, but are not limited to, 1) increased cardiomyocyte apoptosis as neonatal cardiomyocytes appear to be more susceptible to doxorubicin-induced apoptosis compared to adult cardiomyocytes [94]; 2) impaired cardiac growth resulting in inadequate left ventricular mass and cardiomyopathy in younger patients whose hearts are less developed [28]; 3) increased proportion of fat in younger children (also in female sex) resulting in more sustained exposure and resultant cardiotoxicity due to the lipophilic nature of anthracyclines [95]; 4) cardiomyocyte atrophy and myofiber disarray which is observed in anthracycline treated juvenile mice [96]; 5) increased anthracycline-sensitive cardiac transcription factors in younger hearts including cardiac ankyrin repeat protein (CARP) which is present at a higher level in neonatal hearts than in adult hearts [97, 98]; 6) increased number of cardiac progenitor cells in younger hearts which can be more sensitive to anthracycline-induced cytotoxicity resulting in impaired cardiac regenerative capacity [99, 100]. It is possible that anthracycline-induced loss of cardiomyocytes, together with early damage of cardiac stem cells in pediatric patients, can cause permanent cardiotoxicity among long-term cancer survivors. Another effect of age is increased sensitivity in the old age group (more than 65 years) [101], possibly due to the alteration of doxorubicin pharmacokinetics [102, 103].
Decreased apoptotic potential has been demonstrated in postmitotic cells such as cardiomyocytes [104], skeletal muscle cells [105], and neuronal cells [106]. Reduced expression levels of Apaf-1, caspases, and some pro-apoptotic members of the Bcl-2 family, may contribute to the reduced apoptotic potential in postmitotic cells [82, 104-107]. Rapid down-regulation of key apoptotic regulatory proteins including Bim, Apaf-1 and caspase 3 was observed in mouse heart, from neonate to adult [82, 107]. Recent in vitro studies also support increased anthracycline-induced apoptotic signaling in neonatal cardiomyocytes compared with adult cardiomyocytes, associated with significant down-regulation of pro-apoptotic molecules [94]. The research in our laboratory has indicated that neonatal mouse cardiomyocytes exhibit increased anthracycline-induced apoptosis compared to the frequency in adult cardiomyocyte in vivo (Shi et al, unpublished observations). Given the unique properties of cardiomyocytes during postnatal development, it is therefore important to understand the molecular events involved in cardiomyocyte apoptosis in this age group.
Conclusion and future directions
A large body of experimental evidence indicates that cardiomyocyte death through apoptosis and necrosis is a primary contributor to the progression of anthracycline-induced cardiomyopathy. Excessive oxidative stress, DNA damage, changes in calcium handling and cellular contractility, suppression of transcription factors that regulate cell survival and sarcomere protein synthesis, and disruption of sarcomere stability are identified as contributors to the mechanisms of cardiomyocyte death. These experimental results are supported by clinical data. Dexrazoxane, the only cardioprotective drug currently available clinically, is an intracellular iron chelator which has been proven to reduce cardiotoxicity including cardiomyocyte death induced by anthracyclines, via removing iron from its complex with anthracyclines, thereby reducing ROS formation [108-110]. Carvedilol, an adrenergic blocking agent with potent anti-oxidant activity, has been found to be protective against anthracycline-induced ROS generation and apoptosis in experimental studies [111, 112] and in clincial trials with adult patients undergoing anthracycline therapy [113]. Combined treatment of antharcyclines and trastuzumab, an antibody targeting the erbB2, shows synergistic cardiotoxic potential in metastatic breast cancer patients [114]; the increased cardiotoxicity is attributable to the inhibition of PI-3K/Akt-mediated survival signaling through inhibiting neuregulin/erbB2 interaction by trastuzumab resulting in increased apoptosis and necrosis in response to anthracycline treatment [65, 115].
Numerous studies evaluating anthracycline-induced cardiomyocyte death were performed in vitro or in vivo with a time window of hours or days after exposure to anthracyclines at high concentrations. Future studies using long-term animal models should be performed to evaluate the contribution of different types of cardiomyocyte death to the chronic and delayed anthracycline-induced cardiotoxicity associated with clinically relevant doses of the drugs. In addition to apoptosis and necrosis, future research should determine the contribution of other forms of cell death such as autophagy and senescence as well as the relative importance of each form of cell death in anthracycline-induced cardiotoxicity, especially the late-onset cardiotoxicity. As mentioned above, the mechanisms for the late-onset anthracycline cardiac toxicity in children remain under-explored. Future research should continually validate the essential mechanisms and develop therapeutic strategies to prevent premature cardiomyocyte death in pediatric patients who need anthracycline treatment.
Although considerable efforts to prevent cardiotoxicity have been made, a significant portion of patients, especially children, are still threatened by heart failure. At present, there is no general accepted method to provide selective protection of the heart from damage induced by anthracyclines. Knowledge derived from basic research has provided an increasing number of potential therapeutic targets for developing new strategies of cardioprotection against anthracycline-induced cardiotoxicity. Continuous efforts in elucidating the pathogenic mechanisms and identifying new therapeutic targets will certainly be helpful for the development of more effective therapies to minimize the most serious adverse effect of this broadly used anticancer agent in order to increase cancer cure rate and improve the life quality and expectancy of cancer survivors.
Acknowledgments
This work was supported by the Riley Children’s Foundation, the Indiana University Department of Pediatrics (Cardiology), and by National Institutes of Health (NIH P01 HL085098 to LW).
REFERENCES
- [1].Dadlani GH, Harmon WG, Simbre IV, Tisma-Dupanovic S, Lipshultz SE. Cardiomyocyte injury to transplant: pediatric management. Curr Opin Cardiol. 2003;18:91–7. doi: 10.1097/00001573-200303000-00003. [DOI] [PubMed] [Google Scholar]
- [2].Towbin JA, Bowles NE. Molecular genetics of left ventricular dysfunction. Curr Mol Med. 2001;1:81–90. doi: 10.2174/1566524013364077. [DOI] [PubMed] [Google Scholar]
- [3].Burch M. Heart failure in the young. Heart. 2002;88:198–202. doi: 10.1136/heart.88.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- [5].Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- [6].Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abdelwahid E, Pelliniemi LJ, Niinikoski H, Simell O, Tuominen J, Rahkonen O, et al. Apoptosis in the pattern formation of the ventricular wall during mouse heart organogenesis. Anat Rec. 1999;256:208–17. doi: 10.1002/(SICI)1097-0185(19991001)256:2<208::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [8].Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–48. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- [9].Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- [10].Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–73. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435–45. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–13. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ranek MJ, Wang X. Activation of the ubiquitin-proteasome system in doxorubicin cardiomyopathy. Curr Hypertens Rep. 2009;11:389–95. doi: 10.1007/s11906-009-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234-235:119–24. [PubMed] [Google Scholar]
- [15].Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- [16].Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–8. [PubMed] [Google Scholar]
- [17].Leonard EG. Viral myocarditis. Pediatr Infect Dis J. 2004;23:665–6. doi: 10.1097/01.inf.0000132280.36984.a9. [DOI] [PubMed] [Google Scholar]
- [18].Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- [19].Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–8. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- [20].Turina MI, Siebenmann R, von Segesser L, Schonbeck M, Senning A. Late functional deterioration after atrial correction for transposition of the great arteries. Circulation. 1989;80:162–7. [PubMed] [Google Scholar]
- [21].Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44:2065–72. doi: 10.1016/j.jacc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- [22].Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- [23].Boucek MM, Faro A, Novick RJ, Bennett LE, Keck BM, Hosenpud JD. The Registry of the International Society for Heart and Lung Transplantation: Fourth Official Pediatric Report--2000. J Heart Lung Transplant. 2001;20:39–52. doi: 10.1016/s1053-2498(00)00243-6. [DOI] [PubMed] [Google Scholar]
- [24].Burch M, Siddiqi SA, Celermajer DS, Scott C, Bull C, Deanfield JE. Dilated cardiomyopathy in children: determinants of outcome. Br Heart J. 1994;72:246–50. doi: 10.1136/hrt.72.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [26].Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:320–34. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–96. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- [28].Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–15. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- [29].Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- [30].Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- [31].Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. 2008;26:3777–84. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol. 2007;7:122–8. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- [33].Paulides M, Kremers A, Stohr W, Bielack S, Jurgens H, Treuner J, et al. Prospective longitudinal evaluation of doxorubicin-induced cardiomyopathy in sarcoma patients: a report of the late effects surveillance system (LESS) Pediatr Blood Cancer. 2006;46:489–95. doi: 10.1002/pbc.20492. [DOI] [PubMed] [Google Scholar]
- [34].Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr., Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–72. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- [35].Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–40. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- [36].Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol. 2009;134:82–90. doi: 10.1016/j.ijcard.2008.01.043. [DOI] [PubMed] [Google Scholar]
- [38].Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging cell. 2008;7:125–36. doi: 10.1111/j.1474-9726.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- [39].Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, et al. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol. 2009;297:H2169–81. doi: 10.1152/ajpheart.00068.2009. [DOI] [PubMed] [Google Scholar]
- [40].Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–74. [PubMed] [Google Scholar]
- [41].Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988;48:4766–9. [PubMed] [Google Scholar]
- [42].Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. Faseb J. 1990;4:3076–86. [PubMed] [Google Scholar]
- [43].Lebrecht D, Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–13. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- [44].Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–7. [PubMed] [Google Scholar]
- [45].Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation. 2007;116:506–14. doi: 10.1161/CIRCULATIONAHA.106.652339. [DOI] [PubMed] [Google Scholar]
- [46].Fogli S, Nieri P, Breschi MC. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. Faseb J. 2004;18:664–75. doi: 10.1096/fj.03-0724rev. [DOI] [PubMed] [Google Scholar]
- [47].Balanehru S, Nagarajan B. Intervention of adriamycin induced free radical damage. Biochem Int. 1992;28:735–44. [PubMed] [Google Scholar]
- [48].Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–7. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- [49].Nithipongvanitch R, Ittarat W, Velez JM, Zhao R, St Clair DK, Oberley TD. Evidence for p53 as guardian of the cardiomyocyte mitochondrial genome following acute adriamycin treatment. J Histochem Cytochem. 2007;55:629–39. doi: 10.1369/jhc.6A7146.2007. [DOI] [PubMed] [Google Scholar]
- [50].Tokarska-Schlattner M, Wallimann T, Schlattner U. Multiple interference of anthracyclines with mitochondrial creatine kinases: preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol Pharmacol. 2002;61:516–23. doi: 10.1124/mol.61.3.516. [DOI] [PubMed] [Google Scholar]
- [51].Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–41. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387–97. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- [53].Wang L, Ma W, Markovich R, Chen JW, Wang PH. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res. 1998;83:516–22. doi: 10.1161/01.res.83.5.516. [DOI] [PubMed] [Google Scholar]
- [54].Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem. 2003;278:4705–12. doi: 10.1074/jbc.M211616200. [DOI] [PubMed] [Google Scholar]
- [55].Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343–52. doi: 10.1007/s00280-002-0522-7. [DOI] [PubMed] [Google Scholar]
- [56].Poizat C, Puri PL, Bai Y, Kedes L. Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol. 2005;25:2673–87. doi: 10.1128/MCB.25.7.2673-2687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101:6975–80. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].L’Ecuyer T, Sanjeev S, Thomas R, Novak R, Das L, Campbell W, et al. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol. 2006;291:H1273–80. doi: 10.1152/ajpheart.00738.2005. [DOI] [PubMed] [Google Scholar]
- [59].Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem. 2005;273:25–32. doi: 10.1007/s11010-005-5905-8. [DOI] [PubMed] [Google Scholar]
- [60].Kim Y, Ma AG, Kitta K, Fitch SN, Ikeda T, Ihara Y, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol. 2003;63:368–77. doi: 10.1124/mol.63.2.368. [DOI] [PubMed] [Google Scholar]
- [61].Lou H, Danelisen I, Singal PK. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;288:H1925–30. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- [62].Thandavarayan RA, Watanabe K, Sari FR, Ma M, Lakshmanan AP, Giridharan VV, et al. Modulation of doxorubicin-induced cardiac dysfunction in dominant-negative p38alpha mitogen-activated protein kinase mice. Free Radic Biol Med. 2010;49:1422–31. doi: 10.1016/j.freeradbiomed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [63].Mercier I, Vuolo M, Madan R, Xue X, Levalley AJ, Ashton AW, et al. ARC, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo- and radiation-resistance. Cell Death Differ. 2005;12:682–6. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- [64].An J, Li P, Li J, Dietz R, Donath S. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med. 2009;87:401–10. doi: 10.1007/s00109-008-0434-z. [DOI] [PubMed] [Google Scholar]
- [65].Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, et al. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–9. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- [66].Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–9. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Das J, Ghosh J, Manna P, Sil PC. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol. 2011;81:891–909. doi: 10.1016/j.bcp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- [68].d’Anglemont de Tassigny A, Berdeaux A, Souktani R, Henry P, Ghaleh B. The volume-sensitive chloride channel inhibitors prevent both contractile dysfunction and apoptosis induced by doxorubicin through PI3kinase, Akt and Erk 1/2. Eur J Heart Fail. 2008;10:39–46. doi: 10.1016/j.ejheart.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [69].Yan C, Ding B, Shishido T, Woo CH, Itoh S, Jeon KI, et al. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ Res. 2007;100:510–9. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000;102:572–8. doi: 10.1161/01.cir.102.5.572. [DOI] [PubMed] [Google Scholar]
- [71].Niu J, Azfer A, Wang K, Wang X, Kolattukudy PE. Cardiac-targeted expression of soluble fas attenuates doxorubicin-induced cardiotoxicity in mice. J Pharmacol Exp Ther. 2009;328:740–8. doi: 10.1124/jpet.108.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nitobe J, Yamaguchi S, Okuyama M, Nozaki N, Sata M, Miyamoto T, et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003;57:119–28. doi: 10.1016/s0008-6363(02)00646-6. [DOI] [PubMed] [Google Scholar]
- [73].Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, et al. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol. 2002;34:1595–607. doi: 10.1006/jmcc.2002.2110. [DOI] [PubMed] [Google Scholar]
- [74].Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–39. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–40. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li H, Gu H, Sun B. Protective effects of pyrrolidine dithiocarbamate on myocardium apoptosis induced by adriamycin in rats. Int J Cardiol. 2007;114:159–65. doi: 10.1016/j.ijcard.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [77].Nozaki N, Shishido T, Takeishi Y, Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation. 2004;110:2869–74. doi: 10.1161/01.CIR.0000146889.46519.27. [DOI] [PubMed] [Google Scholar]
- [78].Jang YM, Kendaiah S, Drew B, Phillips T, Selman C, Julian D, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–90. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- [79].Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005;270:13–9. doi: 10.1007/s11010-005-2541-2. [DOI] [PubMed] [Google Scholar]
- [80].Bruynzeel AM, Abou El Hassan MA, Torun E, Bast A, van der Vijgh WJ, Kruyt FA. Caspase-dependent and -independent suppression of apoptosis by monoHER in Doxorubicin treated cells. Br J Cancer. 2007;96:450–6. doi: 10.1038/sj.bjc.6603598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bae S, Siu PM, Choudhury S, Ke Q, Choi JH, Koh YY, et al. Delayed activation of caspase-independent apoptosis during heart failure in transgenic mice overexpressing caspase inhibitor CrmA. Am J Physiol Heart Circ Physiol. 2010;299:H1374–81. doi: 10.1152/ajpheart.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006;281:22943–52. doi: 10.1074/jbc.M601025200. [DOI] [PubMed] [Google Scholar]
- [83].Zhang J, Clark JR, Jr., Herman EH, Ferrans VJ. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J Mol Cell Cardiol. 1996;28:1931–43. doi: 10.1006/jmcc.1996.0186. [DOI] [PubMed] [Google Scholar]
- [84].Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, et al. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–43. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- [85].Miyata S, Takemura G, Kosai K, Takahashi T, Esaki M, Li L, et al. Anti-Fas gene therapy prevents doxorubicin-induced acute cardiotoxicity through mechanisms independent of apoptosis. Am J Pathol. 2010;176:687–98. doi: 10.2353/ajpath.2010.090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- [87].Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32:342–53. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- [88].Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H, Kawamura S, et al. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res. 2000;45:642–50. doi: 10.1016/s0008-6363(99)00271-0. [DOI] [PubMed] [Google Scholar]
- [89].Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, 2nd, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108:3036–41. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- [90].Schlecht-Bauer D, Antier D, Machet MC, Hyvelin JM. Short- and long-term cardioprotective effect of darbepoetin-alpha: role of Bcl-2 family proteins. J Cardiovasc Pharmacol. 2009;54:223–31. doi: 10.1097/FJC.0b013e3181b04d01. [DOI] [PubMed] [Google Scholar]
- [91].Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117:2692–701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A. 2004;101:769–74. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhu L, Yu Y, Chua BH, Ho YS, Kuo TH. Regulation of sodium-calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J Mol Cell Cardiol. 2001;33:2135–44. doi: 10.1006/jmcc.2001.1476. [DOI] [PubMed] [Google Scholar]
- [94].Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free Radic Biol Med. 2008;45:1723–8. doi: 10.1016/j.freeradbiomed.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- [96].Zhu W, Shou W, Payne RM, Caldwell R, Field LJ. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr Res. 2008;64:488–94. doi: 10.1203/PDR.0b013e318184d732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Aihara Y, Kurabayashi M, Tanaka T, Takeda SI, Tomaru K, Sekiguchi KI, et al. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/threonine kinase-dependent pathways. J Mol Cell Cardiol. 2000;32:1401–14. doi: 10.1006/jmcc.2000.1173. [DOI] [PubMed] [Google Scholar]
- [98].Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, et al. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997;272:22800–8. doi: 10.1074/jbc.272.36.22800. [DOI] [PubMed] [Google Scholar]
- [99].Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121:675–83. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–92. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- [102].Cusack BJ, Musser B, Gambliel H, Hadjokas NE, Olson RD. Effect of dexrazoxane on doxorubicin pharmacokinetics in young and old rats. Cancer Chemother Pharmacol. 2003;51:139–46. doi: 10.1007/s00280-002-0544-1. [DOI] [PubMed] [Google Scholar]
- [103].Li J, Gwilt PR. The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol. 2003;51:395–402. doi: 10.1007/s00280-002-0554-z. [DOI] [PubMed] [Google Scholar]
- [104].Sanchis D, Mayorga M, Ballester M, Comella JX. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–86. doi: 10.1038/sj.cdd.4401267. [DOI] [PubMed] [Google Scholar]
- [105].Burgess DH, Svensson M, Dandrea T, Gronlund K, Hammarquist F, Orrenius S, et al. Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ. 1999;6:256–61. doi: 10.1038/sj.cdd.4400489. [DOI] [PubMed] [Google Scholar]
- [106].Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–46. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Madden SD, Donovan M, Cotter TG. Key apoptosis regulating proteins are down-regulated during postnatal tissue development. Int J Dev Biol. 2007;51:415–23. doi: 10.1387/ijdb.062263sm. [DOI] [PubMed] [Google Scholar]
- [108].Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–61. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wiseman LR, Spencer CM. Dexrazoxane. A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs. 1998;56:385–403. doi: 10.2165/00003495-199856030-00009. [DOI] [PubMed] [Google Scholar]
- [110].Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Walker UA. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol. 2007;151:771–8. doi: 10.1038/sj.bjp.0707294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837–46. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- [112].Armstrong SC. Anti-oxidants and apoptosis: attenuation of doxorubicin induced cardiomyopathy by carvedilol. J Mol Cell Cardiol. 2004;37:817–21. doi: 10.1016/j.yjmcc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [113].Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–62. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- [114].Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [115].Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–4. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]