Abstract
Artery calcification reflects an admixture of factors such as ectopic osteochondral differentiation with primary host pathological conditions. We review how genetic factors, as identified by human genome-wide association studies, and incomplete correlations with various mouse studies, including knockout and strain analyses, fit into “pieces of the puzzle” in intimal calcification in human atherosclerosis, and artery tunica media calcification in aging, diabetes mellitus, and chronic kidney disease. We also describe in sharp contrast how ENPP1, CD73, and ABCC6 serve as “cogs in a wheel” of arterial calcification. Specifically, each is a minor component in the function of a much larger network of factors that exert balanced effects to promote and suppress arterial calcification. For the network to normally suppress spontaneous arterial calcification, the “cogs” ENPP1, CD73, and ABCC6 must be present and in working order. Monogenic ENPP1, CD73, and ABCC6 deficiencies each drive a molecular pathophysiology of closely related but phenotypically different diseases (generalized arterial calcification of infancy (GACI), pseudoxan-thoma elasticum (PXE) and arterial calcification caused by CD73 deficiency (ACDC)), in which premature onset arterial calcification is a prominent but not the sole feature.
Keywords: ENPP1, CD73, ABCC6, generalized arterial calcification of infancy, pseudoxanthoma elasticum
A. How Heritable Is Human Artery Calcification?
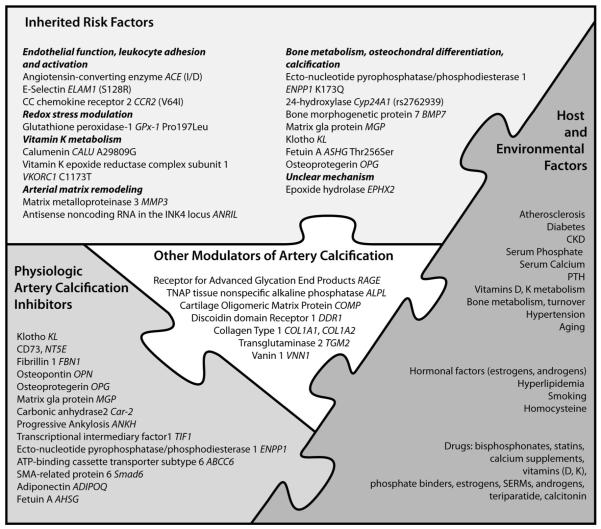
In complex disorders, such as arterial calcification, multiple genes predispose to the phenotype, but each gene typically has small effects on the phenotype. Genetic factors, including some that mediate osteochondral differentiation, vascular cell growth, and inflammation, fit into “pieces of the puzzle” in intimal calcification in atherosclerosis and artery tunica media calcification in chronic kidney disease (CKD), diabetes, and aging (Figure 1). Among the limitations of studies on genetics in arterial calcification are methodologies in assaying vascular calcification, especially distinguishing intimal from medial calcification using current techniques, such as electron beam computed tomography. Also, because coronary artery calcification (CAC) is also a marker for plaque burden, genetic associations might also represent effects of genes underlying atherosclerotic disease independent of calcification.
Figure 1. Pieces of the “puzzle”: Host, environmental, and genetic factors contributing to human arterial calcification.
Although the inhibitory role of some of the specific factors with regard to arterial calcification has been proven, others may have promoting or direct or indirect “regulatory” effects. Some of the factors, including COMP171 and TGM2,172 are not explicitly discussed in the text.
In the Multiethnic Study of Atherosclerosis (MESA), traditional cardiovascular risk factors were associated with risk of incident coronary calcium deposition and increases in existing calcification,1 but family CAD history was associated with CAC prevalence and amount in all ethnic groups in this cohort.2 Accordingly, CAC prevalence and amount were heavily influenced by ethnicity in this cohort.3,4 Forty-nine percent (P<0.001) of overall variance in abdominal aortic calcification was due to heritability in 1119 extended pedigrees from the original Framingham Heart Study.5 For CAC quantified by electron beam CT in 698 asymptomatic white adults, 43.5% of variation in CAC quantity was attributed to genetic factors.6 In coronary angiograms of 882 siblings with CAD from 401 German families, heritability of CAC was 0.51 (SEM, 0.17; P=0.001), comparable to heritability of proximal stenoses in left main CAD (h2=0.49; SEM, 0.12; P=0.001).7 Similarly, in 478 asymptomatic Amish adults, heritability for CAC quantity, adjusted for age and sex, was 0.42 (P=0.0001),8 and CAC was linked with lipid levels. It must be emphasized that heritability has been examined only for abdominal aortic5 and coronary atherosclerotic calcification.6-8 Moreover, the imaging methods implemented cannot discriminate between atherosclerotic and media calcification. Thus, heritability for calcification of the artery intima and media may differ.
B. Specific Methods to Identify Genetic Factors Contributing to Arterial Calcification
1. Genome-Wide Association Studies
Four independent consortia identified a common locus on chromosome 9p21.3 as a major risk allele for coronary artery disease (CAD).9-11 One of these consortia9 used DNA samples from the Dallas Heart Study (DHS), in which electron-beam CT-measured CAC was quantified12 for validation of their findings, and the risk allele was associated with both CAC and premature atherosclerosis.9 In the more recent multi-ethnic Atherosclerotic Disease, Vascular Function and Genetic Epidemiology (ADVANCE) study, high-risk alleles were associated with increased CAC in all non-African race/ethnicity groups, with magnitude of these associations by racial/ethnic group closely mirroring magnitude for clinical CAD.13 The identified 58kb region on chromosome 9p21.3 adjacent to the tumor suppressor genes CDKN2A and CDKN2B does not contain any annotated gene or microRNAs,9 but a conserved sequence within the 9p21.3 locus has functional enhancer activity in smooth muscle cells (SMCs). RNA expression of the short variants of ANRIL, an antisense noncoding RNA in the INK4 locus, was increased by 2.2-fold, whereas expression of the long ANRIL variant was decreased by 1.2-fold in healthy subjects homozygous for the risk allele. Expression of these variants correlated with that of cyclin-dependent kinase inhibitor CDKN2B (p15) and TDGF1 (Cripto), respectively. Hence, the risk allele may promote atherosclerosis (and CAC) by modulating cell proliferation.14 In this respect, ANRIL regulates gene silencing by functionally coordinating with chromatin-associated factors that modify and interact with histone H3 lysine 27 methylation.15
CAC was also linked with chromosomal regions 6p21.3 and 10q21.3.16 LOD scores (log10 odds in favor of linkage) were 2.22 (P=0.00070) for 6p21.3 (map position 76.4cM between markers D6S1053 and D10S2327) and 3.24 (P=0.000057) for 10q21.3 (map position 91.8 cM between markers D10S1423 and D10S2327). The region on chromosome 6 close to the marker DS1053 contains a group of collagen genes, BMP5, and IL17. The marker D10S189 at map position 19.0 cM on chromosome 10p14 gave the strongest signal (P=0.0012); candidate genes within this region include CALML5 (calmodulin-like 5) and CALML3 (calmodulin-like 3), which encode calcium-binding proteins.17
Genome-Wide Association Studies (GWAS) limitations for CAC are evident, as in other chronic diseases. Pinpointing genetic loci that confer susceptibility to artery calcification probably will require large-scale sample sizes and subdivision of precise phenotypes (ie, intimal and media calcification) to lessen heterogeneity. The next generation of human genetics studies for arterial calcification should include systems biology approaches. Genetic analyses of micro-RNAs probably will be a fruitful approach. Epigenetic approaches to arterial calcification are also ripe for development. The concept that there are racial differences between African Americans and other ethnic groups in gene expression profiles contributing to differential risks of artery calcification, merits further study.18
2. Candidate Gene-Based Association Studies
Candidate gene studies screening for small numbers of SNPs in cases and control subjects have been informative, though how some SNPs affect gene expression or protein function to modulate artery calcification remains unclear. Specific candidate genes have been selected due to influence on endothelial function and leukocyte adhesion and activation (CC chemokine receptor 2 CCR2, angiotensin-converting enzyme ACE, E-selectin ELAM1, epoxide hydrolase EPHX2), redox stress modulation (glutathione peroxidase-1 GPx-1), arterial extracellular matrix remodeling (matrix metalloproteinase 3 MMP3), bone metabolism (bone morphogenetic protein 7 BMP-7), and vitamin K metabolism that modulates γ-carboxylation of certain proteins in osteochondral differentiation such as matrix Gla protein (MGP) and osteocalcin (calumenin CALU, vitamin K epoxide reductase complex subunit 1 VKORC1).
Chemokine (C-C Motif) Receptor 2
The most studied chemokine (C-C motif) receptor 2 (CCR2) SNP is V64I, but there is both evidence of V64I effect on CCR function19 and lack of effect on CCR2 expression.20 The SNP was linked with reduced CAC in one study21 but not in others.22,23
Angiotensin-Converting Enzyme
One study on patients with documented CAD and coronary calcification suggests a role for the ACE insertion/deletion (I/D) polymorphism in intron 16 of the ACE gene in arterial calcification.24 Patients with the DD genotype had significantly more calcified lesions. Another study on the ACE I/D polymorphism and coronary calcification was negative.25
Epoxide Hydrolase 2
Epoxide hydrolases catalyze degradation of vasoactive epoxyeicosatrienoic acids into their corresponding dihydroxyeicosatrienoic acids.26 Epoxyeicosatrienoic acids and dihy-droxyeicosatrienoic acids promote vasorelaxation of small arteries and display anti-inflammatory properties through the inhibition of nuclear factor-κB activation. Polymorphisms in EPHX2, the gene encoding soluble epoxide hydrolase, were associated with CAC in some27,28 but not all studies.29 Because of the low allele frequency for most of the SNPs29 false-positive association is likely. Overall, effect size probably is small.
E-Selectin
The S128R E-selectin (ELAM1) polymorphism in the EGF-like domain of the protein can functionally alter leukocyte-endothelial interactions.30 Association between E-selectin S128R polymorphism and CAC was reported in women 50 years of age or younger.31 However, no relationship was observed for men or older women.
Matrix Metalloproteinase 3
Matrix metalloproteinases (MMP) that degrade components of the extracellular matrix (ECM) including collagen and elastin, can modulate artery calcification, probably not only by calcification-promoting effects on ECM, but also, partly by regulating migration, growth and apoptosis of vascular cells. A MMP3 genotype was recently linked with higher promoter activity and CAC.32
Vitamin D 24-Hydroxylase
Circulating 1,25 (OH)2D is biologically inactivated through a series of reactions beginning with 24-hydroxylation.33 The vitamin D 24-hydroxylase is encoded by vitamin D 24-hydroxylase (CYP24A1), and CYP24A1 is pivotal in vitamin D homeostasis. Deletion of Cyp24a1 in mice causes 1,25(OH)2D excess and hypercalcemia with severe bone mineralization defects and ectopic calcification (renal calcium deposition) after chronic treatment with 1,25(OH)2D.34 On the other hand, transgenic rats that constitutively express Cyp24a1 develop atherosclerotic lesions in the aorta, which greatly progress with high-fat and high-cholesterol feeding.35 A recent study found a common variant in the CYP24A1 gene associated with CAC quantity in 3 independent populations.36 Interplay of transcriptional intermediary factor 1α (Tif1α) with vitamin D receptor target genes in mice, an essential native inhibitory system for mouse artery calcification,37 is discussed below.
Bone Morphogenetic Protein 7
Mice unable to express the inhibitor of bone morphogenetic protein (BMP)signaling Smad6 develop arterial calcification.38 BMP-7 appears anabolic for bone and protective against arterial calcification.39,40 In vitro studies suggest that BMP7 promotes the vascular SMC phenotype41 and that receptors for the BMPs are expressed in vascular SMCs.42,43 In type II diabetes, SNPs in BMP-7 were associated with arterial calcification.44 Interestingly, this association was inversely correlated with bone mineral density. Defective BMP-7 functionality could lead to reduced osteoblastic differentiation in bone and perturbation of the bone-vascular axis.44
Calumenin
The functions of calumenin (CALU), a protein encoded by the gene CALU, are connected with Ca2+-dependent processes. Calumenin endogenously regulates the activity of the γ-carboxylation system, consisting of vitamin K1 2,3-epoxide reductase (VKOR), and γ-glutamyl carboxylase.45 Calumenin may have a role in atherogenesis, since it was localized in atherosclerotic plaques.46 The CALU A29809G polymorphism was linked with CAC and prognosis in non-ST-elevation acute coronary syndrome.47 The CALU 29809G allele was suggested to be protective for arterial calcification, contributing to better prognosis of carriers.
Vitamin K Epoxide Reductase Complex Subunit 1
Vitamin K epoxide reductase (VKOR) mediates recycling of vitamin K epoxide to vitamin K hydroquinone, an essential cosubstrate for γ-carboxylation of vitamin K-dependent proteins such as MGP and osteocalcin.48 The inhibition of VKOR by warfarin results in undercarboxylation of MGP and subsequent medial calcification of the arterial vessel wall.49 Numerous polymorphisms were identified in the vitamin K epoxide reductase complex subunit 1 (VKORC1) gene,50 with one (1173C>T) associated with aortic calcification.51
Glutathione Peroxidase 1
Glutathione peroxidase-1 (GPx-1) protects against reactive-oxygen-species (ROS)-induced oxidative stress and to a lesser extent to reactive nitrogen species (RNS) in vivo.52 Alterations of GPx-1 have been linked to the etiology of cardiovascular disease and diabetes.53 One study reported the common variant Pro197Leu of GPx-1 to be associated with 40% decreased activity.54 This polymorphism is associated with CAC in type II diabetes.55
3. Genetics of Murine Arterial Calcification
Mice are increasingly used to model arterial calcification and study risk factors. The degree of calcification differs among different strains, ranging from no aortic calcification in MRL substrains to 50% in DBA/2J strains on a chow diet. Even on atherogenic diet, incidence of aortic calcification in MRL mice (0%) was significantly lower than in C57BL/6J mice (24%). Hence, size and properties of murine atherosclerotic lesions, and susceptibility to calcification, are genetically determined.56 The presence of aortic cartilaginous metaplasia within atherosclerotic lesions in the “susceptible” C57BL/6J strain but not the C3H/HeJ strain was informative because analysis of a genetic cross between these strains revealed a recessive inheritance pattern for this abnormality.57
Age-related spontaneous dystrophic cardiac calcinosis (DCC) occurs in several susceptible inbred strains of mice, including BALB/c, DBA/2, and C3H.58 Susceptibility to DCC was also observed after a standardized myocardial freeze-thaw injury, suggesting a common genetic basis.59 By quantitative trait analysis, the gene determining DCC, designated dyscalc, was mapped to mouse chromosome 7.58 The causal gene for dystrophic calcification was identified to be Abcc6,60,61 whose implication in PXE is discussed below. Using an F2 mouse cross with 2 inbred strains of mice, C3/HeJ and C57/BL6, both null for the apoE allele as the breeders for the F1 generation, quantitative clinical trait loci (cQTL) causal for medial disruption and medial calcification were mapped to chromosome 7.62 The most likely candidate gene on this locus was Abcc6, subsequently confirmed to be causative for the phenotype by transgenic complementation.62 Also in this study, the hepatic expression quantitative trait locus (eQTL) for annexin A4 correlated with the cQTL for both medial calcification and medial disruption. Thus, annexin A4, a calcium and acidic phospholipid binding protein, which is a prominent component of mineralizing matrix vesicles from bone or vascular smooth muscle cells may also play a role in artery media calcification.63
4. Identification of Candidate Genes for Human Arterial Calcification by Analyzing Natural Occurring Mouse Mutants or Targeted Mouse Mutants: Mixed Lessons for Human Artery Calcification
Characterization of targeted or naturally occurring mouse mutations of a variety of cartilage and bone proteins has led to the identification of 13 inhibitors of arterial calcification in vivo (Table). These studies have proven the concept that arterial calcification is normally inhibited by physiological function of resident arterial cells. Deficient expression of even one inhibitor of arterial calcification is sufficient to trigger the calcification process.64-66 It is noteworthy that polymorphisms in the corresponding human gene of some of these inhibitors have been used as candidates for association studies in various populations, mostly with inconclusive results.
Table. List of Genes, Targeted Mutations of Which Lead to Artery Calcification in Mice With Respective Association Studies in Humans.
| Gene Symbol, Human Protein Name, [OMIM Entry No.], Human Gene Map Locus |
Mouse Model | Major Phenotypic Features | SNP-Based Association Studies in Humans |
References |
|---|---|---|---|---|
|
MGP, matrix Gla protein, [154870], 12p13.1-p12.3 |
mgp−/− | Arterial and cartilage calcification, tracheobronchial stenosis. Human correlate: Keutel syndrome |
Increased CAC in men | 73, 121, 122, 170 |
|
ENPP1, Ectonucleototide pyrophosphatase/ phosphodiesterase1, [173335], 6q22-q23 |
ttw/ttw enpp1−/− |
Articular cartilage calcification, hyperostosis, spine and peripheral joint fusion, arterial calcification. Human correlate: Generalized arterial calcification of infancy |
Increased aortic arch calcification in patients with type II diabetes Higher coronary calcification scores in ESRD patients |
92, 93, 153, 154 |
|
ANKH, Progressive ankylosis [605145], 5p15.2-p14.1 |
ank/ank | Articular cartilage calcification, hyperostosis, spine and peripheral joint fusion, arterial calcification. Human correlate: Craniometaphysial dysplasia, Chondrocalcinosis-2 |
97 | |
|
OPG, Osteoprotegerin, [602643], 8q24 |
opg−/− | Osteoporosis, vascular calcification | Increased risk for coronary artery disease |
83-86 |
|
Smad6/Madh6, SMA- and MAD-related protein 6 [602931], 15q21-q22 |
madh6-mutant | Endocardial cushion defects, aortic ossification | 38, 102 | |
| FBN1, Fibrillin1 [134797], 15q21.1 | mgΔ/mgΔ, mgR/mgR |
Aortic aneurysm, long bone overgrowth, artery media calcification. Human correlate: Marfan syndrome |
103 | |
|
CA2, Carbonic anhydrase2 [259730], 8q22 |
car-2−/− | Osteopetrosis, renal tubular acidosis, calcification of media of small arteries. Human correlate: Carbonic anhydrase II deficiency |
99 | |
| KL, Klotho [604824], 13q12 | klotho−/− | Vascular calcification, accelerated aging | Early onset of coronary artery disease |
81, 82 |
|
AHSG, α2-HS-glycoprotein/fetuin, [138680], 3q27 |
ahsg−/− | Widespread but mild vascular calcification | Coronary artery calcified plaques in type II diabetes patients |
79, 171 |
|
SPP1, Osteopontin, [166490], 4q21-q25 |
opn−/− | Enhanced calcification of cardiac valve implants |
72, 113 | |
|
ABCC6, ATP-binding cassette transporter subtype 6 [264800], 16p13.1 |
abcc6−/− | Myocardial necrosis and calcification, arterial calcification after freeze/thaw injury. Human correlate: Pseudoxanthoma elasticum |
58, 60, 61 | |
|
TIF1α, transcriptional intermediary factor 1 [603406], 7q32-q34 |
Tif1α−/− | Calcification of arterioles, arteries, vibrissae and lungs |
37 | |
|
ADIPOQ, Adiponectin [605441], 3q27 |
adiponectin−/− | Calcification of the aortic media in 30-week-old mice on chow diet |
108, 110 |
Matrix Gla Protein
Biochemical and genetic studies have established matrix Gla protein (MGP) as an inhibitor of pathological calcification in cartilage and blood vessels,67 as discussed elsewhere in this series of reviews. In mice, targeted deletion of the Mgp gene causes extensive ectopic chondrogenesis and lethal calcification of arteries.67 In humans, in vitro studies suggest that polymorphisms in MGP are associated with altered promoter activity.68,69 In addition, there is some evidence that MGP SNPs are associated with arterial calcification,70,71 though these results are not consistent.68,72 In one study, significant associations of polymorphisms in MGP and CAC were found in men only.73
Fetuin-A
Fetuin-A (AHSG), a major systemic inhibitor of calcification, is discussed in the context of CKD in this series of reviews. Distinct AHSG1 and AHSG2 alleles are linked to different serum fetuin-A levels.74 For example, in healthy Japanese volunteers, the different AHSG alleles were associated with decreased fetuin-A and increased free Pi levels.75 Some polymorphisms in AHSG have been associated with decreased serum fetuin-A levels76 and with different factors affecting arterial calcification, such as type II diabetes,77 obesity,78 and dyslipidemia.77 Moreover, 4 different AHSG polymorphisms were associated with extent of coronary artery calcified plaque in type II diabetes.79 Patients with end-stage renal disease (ESRD) carrying AHSG polymorphism T256S had lower fetuin-A levels compared with patients without this variant. Additionally, inflammation had an inhibitory effect on fetuin-A levels on patients carrying the T256S polymorphism. Thus, patients with ESRD and a genetic propensity for lower fetuin-A levels, for example, patients carrying the T256S polymorphism, have been hypothesized to have higher risk of cardiovascular premature death if subjected to chronic inflammation.80
Klotho
Klotho (KL) is a β-glucuronidase that has a variety of effects, including regulation of insulin sensitivity and endothelial NO production. Klotho circulating levels decline in aging and in renal impairment. Defects in kl gene expression in mice result in a syndrome that is similar to human aging, including arteriosclerosis, osteoporosis, infertility, emphysema, and ectopic calcification,81 associated with increased production of vitamin D. Indeed, dietary vitamin D restriction inhibits development of arterial disease and premature aging in these mice. Studies in humans have identified functional variants of KL with CAD, HDL levels, systolic blood pressure, risk of stroke, and bone density, but no association was found in a study of arterial calcification.82
Osteoprotegerin
Osteoprotegerin (OPG) is discussed elsewhere in this review series, and Opg knockout mice show severe osteoporosis with calcification in the aorta and renal arteries.83-85 OPG gene polymorphisms were associated with increased risk for CAD and osteoporotic fracture,86 but another study found no association with arterial calcification and CAD.87 In contrast, polymorphisms in the OPG ligand RANKL were found to be associated with arterial calcification of the aorta in Korean women.88
Transcriptional Intermediary Factor 1α and the Calcium-Sensing Receptor
Mice carrying an activating mutation of calcium-sensing receptor (Casr) demonstrate arterial calcification involving the media in arterioles and medium-sized arteries.89 Transcriptional intermediary factor 1α (Tif1α) knockout mice develop similarly distributed spontaneous vascular calcifications37 and Casr expression and demonstrate heightened renal expression of other vitamin D receptor (VDR) direct target genes (Car2, Cyp24a1, Trpv5, Trpv6, Calb1, S100g, Pthlh, and Spp1).37 Therefore, TIF1α and the renal CASR and VDR pathway form a major physiological regulatory system that normally suppresses arterial calcification. CASR gene polymorphisms (A990G, C1011G) were not linked with cardiac valve calcification in one study.90
ENPP1 (ttw/ttw) and ANK (ank/ank) Phenotypes
The tiptoe-walking mouse (ttw/ttw) had, for many years, been a model to study pathological calcification of perispinal ligaments. Okawa et al were able to identify a naturally occurring truncation mutation in Enpp1 (npps) encoding for ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1)91 as the cause of the phenotype. The discovery of ENPP1 mutations in the human disease GACI by our group92 led to reappraisal of arterial media calcifications in this mouse model.93 Enpp1 knockout mice recapitulate the phenotype of ttw/ttw mice and also share features of human GACI, including spontaneous periarticular soft tissue calcifications and artery media calcification adjacent to the internal elastic lamina in early life. However, the combination of perispinal and periarticular soft tissue ankylosis is lethal in Enpp1 knockout and ttw/ttw mice, whereas GACI is variably associated with scattered periarticular calcifications. Also, GACI is associated with frequently lethal myointimal proliferation with associated calcifications in large and mediumsized arteries, as opposed to nonlethal artery calcification as a less dominant feature of the phenotype in Enpp1-deficient mice.94,95 ENPP1 is a major physiological generator of extracellular PPi, a potent inhibitor of hydroxyapatite crystal formation and growth, and a molecule that exerts a variety of other, unexpected effects on cell differentiation and function.96 A phenotype remarkably similar to the ttw/ttw mouse is found in the ank/ank mouse, carrying a C-terminal truncation mutation in the ANK protein, a transporter of PPi, predominantly from the intracellular to the extracellular space.97 Both the ttw/ttw and ank/ank mouse models have spurred insight into the role of PPi metabolism in the context of arterial calcification (see below).
Carbonic Anhydrase-2
Carbonic anhydrase-2 (CA2) provides protons and bicarbonate ions to the local microenvironment and allows for robust acid secretion that leads to degradation of the organic matrix in bones.98 Mice deficient in Car2 develop age-dependent medial calcification in the arterioles and smaller arteries in numerous organs. In male car2−/− mice, the genital tract revealed most extensive arterial calcifications, and male mice were affected more than females, suggesting an effect of sex on vascular calcification in this mouse model.99 In human beings, loss of function mutations in CA2 encoding carbonic anydrase-2 lead to osteopetrosis, renal tubular acidosis, and cerebral calcification.100,101 However, arterial calcifications have not been linked with CA2 mutations in human beings.
SMA-Related and MAD-Related Protein 6
SMA-related and MAD-related protein 6 (SMAD6) plays a pivotal role in negative regulation of transforming growth factor-β (TGFβ) family signaling as a feedback molecule as well as a mediator of cross-talk with other pathways. Mice lacking Madh6, the mouse homologue of SMAD6, develop cartilaginous metaplasia and trabeculated bone within the arterial wall, recapitulating the complete process of endochondral ossification.38 In a recent association study, the SMAD6 SNPs rs16950046, rs16950516 and rs266336 were significantly associated with sudden cardiac death.102
Fibrillin-1
Fibrillin-1 (FBN1) appears to be essential for homeostasis of established elastic fibers and for cell adhesion involved in remodeling the artery ECM,103 as shown in fibrillin-1 knockout mice. These mice reveal a predictable sequence of abnormalities including elastic fiber degeneration, excessive deposition of extracellular matrix proteins, elastolysis, intimal hyperplasia, and calcification.103 Interestingly, vascular SMCs from fibrillin-1-deficient mice show an abnormal synthetic repertoire in early vascular lesions including elastin, among other matrix elements, and MMP-9, a known mediator of elastinolysis.104 In this respect, elastin degradation is related to onset and progression of elastin calcification,105 and this process is mediated by MMPs that include MMP-2 and MMP-9.106
Fibrillin-1 mutations result in the pleiotropic manifestations of Marfan syndrome in humans, which is associated with joint hypermobility, aortic aneurysm formation and calcification, and long bone overgrowth.107 To our knowledge, associations of FBN1 polymorphisms with other human arterial calcification phenotypes have not been defined.
Adiponectin
Adiponectin (ADIPOQ)108 is an adipocyte derived hormone with antidiabetic, anti-inflammatory, and protective effects for the vasculature.109 Adiponectin-deficient mice develop mild aortic calcification after being fed with a chow diet for 30 weeks.108 The inhibitory effect of adiponectin on osteoblastic differentiation of vascular SMCs is mediated through the adiponectin receptor 1/p38 signaling pathway.108 In a most recent study in 2847 participants of the MESA study cohort,1 African Americans with ADIPOQ genotypes AG/GG of rs2241767 had 36% greater (95% confidence interval [CI], 16% to 59%, P=0.0001) CAC prevalence. Also, in African Americans, genotypes AG/AA of rs1063537 were associated with a 35% (95% CI, 14% to 59%; P=0.0005) greater CAC prevalence.110
Osteopontin (SPP1)
Osteopontin (OPN) is a major noncollagenous matrix protein of bone and a constitutive component of normal elastic fibers in the skin and aorta regulating chronic inflammation and vascular calcification.111 OPN, like PPi, potently inhibits hydroxyapatite crystal deposition and calcification by SMCs in vitro.112 Although unchallenged Opn-knockout mice are grossly normal, enhanced calcification of implanted glutaral-dehyde-fixed aortic valve leaflets has been demonstrated.113 Likewise, increased arterial medial calcification was observed in Opn−/−Mgp−/− double knockout mice, supporting the concept that OPN is an inducible inhibitor of arterial media calcification.
Furthermore, in apoE−/−/Opn-deficient mice vascular calcification was increased 2.5-fold in comparison to apoE−/− mice.115 However, a study of the common T443C and Asp94Asp single nucleotide polymorphisms of SPP1 in human patients with coronary artery calcification failed to detect any statistically significant association.72
5. Knockout Mouse Models Modulating Arterial Calcification Through Primary Deficiency of a Native Artery Calcification Regulator
The propensity of certain genes to promote arterial calcification has come to light also by studying certain mouse models, with knockout of different genes, which manifest artery calcification. Such genes include vanin-1 (VNN1), discoidin domain receptor-1 (DDR1), and RAGE.
Vanin-1
Vanin-1 (VNN1) is a glycosylphosphatidylinositol-anchored plasma membrane ecto-enzyme involved in cysteine and glutathione (GSH) metabolism. Vanin-1 promotes ectopic chondrogenesis in vitro, mediated by decreased cellular GSH stores.116 Increased chondrogenesis of ank/ank bone marrow stromal cells and increased chondrogenic transdifferentiation and calcification by ank/ank aortic smooth muscle cells and explants were corrected by Vanin-1 knockout in ank/ank Vnn1−/− cross-breeding studies.
Discoidin Receptor 1
Discoidin receptor 1 (DDR1) is a receptor tyrosine kinase, which is activated by several subtypes of triple helical collagens. Through DDR1 signaling, type I collagen regulates proliferation, migration, and differentiation of many cell types. DDR1 serves as a positive regulator of neointimal formation and plays a pivotal role in regulating vascular inflammation and matrix accumulation, mediating the responses of macrophages and vascular SMCs. Mice deficient in Ddr1 and LDL receptor (Ldlr) demonstrate significantly reduced calcium content in the aortic arch, and attenuated SMC-mediated calcification of the extracellular matrix; SMCs derived from Ddr−/− mice showed increased ENPP1 expression.117
Receptor for Advanced Glycation End Products RAGE (AGER)
RAGE serves as a cognate receptor not only for the advanced glycation end-products that accumulate in diabetic and aging tissues, and also for multiple S100 calgranulins known to be increased in diseased arteries. RAGE promotes atherosclerosis and diabetic vascular and neurological complications, and mediates chondrocyte maturation and osteoclast function, as well as calcification by SMCs. Aortic explants from Enpp1−/− mice demonstrated decreased inorganic phosphate (Pi)-stimulated release of the RAGE inhibitor sRAGE, as well as increased calcification and intra-arterial chondrogenic differentiation, which was suppressed by Rage knockout in Enpp1−/−Rage−/− mice.118 In the apoE knockout mouse model of atherosclerosis and artery calcification, selective SMC expression of the RAGE ligand S100A12 increased medial calcification by 2- to 6-fold in the proximal aorta and innominate arteries, respectively.119 The pro-osteogenic propensity of the S100A12 transgene required conditioned media from lipid-challenged apoE null macrophages.120 In this model, the actions of S100A12 were dependent on RAGE and oxidative stress signaling, since both recombinant sRAGE and NADPH oxidase inhibition reduced osteogenic programming and calcification.119 Evidence suggests a role for the human paracrine S100/RAGE axis in enhancing vascular calcification, and given that RAGE conveys arterial osteo-chondrogenic signals activated by PPi deficiency118 and by oxidative stress,119 RAGE probably is involved in an amplification loop of arterial osteochondral differentiation in calcification.118,120
6. Fitting Human Puzzle Pieces Together From Mouse Models of Arterial Calcification
Effects of knockout of specific mouse genes are not fully comparable to sequence variants of the same human genes. This is exemplified by the severe phenotype observed in Mgp-knockout mice, which show a generalized ossification of the whole arterial tree,121 whereas in the human correlate, Keutel syndrome, arterial calcifications are almost absent.122 One factor that probably plays a major role in regulatory differences between mouse and human arterial calcification is the relative hyperphosphatemia of normal mice compared with normal humans, for example, serum Pi levels of ≈8 mg/dL in mice, as compared with 2.5 to 4.5 mg/dL in normal humans.123 Some of these mouse genes have been used as candidates for SNP association studies in populations enriched for arterial calcification (see above). Most of these studies so far revealed conflicting results as exemplified by the K173Q polymorphism in ENPP1, which has been studied in multiple ethnic groups and was demonstrated to be associated with coronary artery calcification only indirectly (see below). However, from the data presented above, it became obvious that some of the mouse candidate genes have proven to be valuable for association studies with respect to CAC and cardiovascular risk prediction in human beings: For example, presence of MGP polymorphisms affect CAC risk, especially in males.73
7. Rare Human Monogenic Causes of Premature Onset Arterial Calcification
Positional cloning approaches and homozygosity mapping identified mutations in ABCC6 in pseudoxanthoma elasticum (PXE),124-129 mutations in ENPP1 in GACI,92 and, most recently, mutations in NT5E in another rare disease phenotype consisting of peripheral artery calcification with distal joint calcification.130
PXE and ABCC6 Mutations
PXE; OMIM 264800, 177850 is a rare autosomal recessive disorder characterized by dystrophic calcification of soft connective tissues including the dermis of the skin, the Bruch membrane of the eye, and the arterial media.131,132 Positional cloning approaches provided strong evidence for linkage to the short arm of chromosome 16, and the critical interval was eventually refined by the use of informative recombinants to consist of approximately 500 kb.125,126 Systematic sequencing of the candidate genes in this region resulted in identification of the ABCC6 gene as the one harboring mutations in PXE.124,127-129 Human PXE exhibits selective medial calcification and degeneration without atherosclerotic involvement.133 Accordingly, the Lusis group134 recently demonstrated that in mice on a apoE null background the Abcc6 genotype is a significant contributor specific to media calcification and media disruption but not to atherosclerotic calcification or atherosclerotic lesion size. These studies provide genetic evidence of the pathobiological differences between artery media and atherosclerotic intima vascular calcification.
ABCC6, which is primarily expressed in the liver encodes for MRP6, a putative transmembrane protein, which belongs to the family of ATP-binding cassette (ABC) transport proteins.135 Several studies were set out to identify the mechanisms how ABCC6 mutations lead to aberrant tissue mineralization. Based on previous studies demonstrating that activation of MGP by γ-carboxylation of glutamyl residues is critical for prevention of unwanted mineralization,136 Li et al found reduced γ-glutamyl carboxylation of MGP in the serum, in the liver and in various calcified tissues of Abcc6−/− mice.137 Accordingly, patients with PXE carrying biallelic mutations in ABCC6, characteristically show uncarboxylated MGP in PXE skin lesions.138 Whereas reduced γ-carboxylation of MGP is considered a major pathogenic factor contributing to the increased arterial mineralization in PXE, the physiological substrate of the MRP6 transport protein is still unknown. Ilias et al have shown that ABCC6 expressed in Sf9 insect cells was capable of transporting glutathione conjugates, including leukotriene C4 and N-ethylmaleimide S-glutathione (NEM-GS), and organic anions were postulated to be the primary substrates.139 However, studies in humans so far have not substantiated this hypothesis.
One school of thought is that absence of ABCC6 activity primarily in the liver results in the deficiency of circulating factors (including partial deficiency of fetuin), which leads to loss of physiological suppression of artery calcification under normal calcium and phosphate homeostatic conditions.140 In this context, overexpression of fetuin in Abcc6−/− mice was effective in reducing soft tissue mineralization in these animals.141 The finding that when muzzle skin from wild-type mice was grafted onto the back of knockout mice, mineralization of the vibrissae was observed within the subsequent 2 months, also supported this hypothesis.142 Furthermore, serum from PXE patients, when added to tissue culture medium of fibroblasts, has been shown to alter the expression of elastin.143 It was postulated that ABCC6 might participate in transmembrane transport and redistribution of vitamin KH2, an obligatory cofactor of γ-glutamyl carboxylase,144 especially when conjugated to glutathione. However, most recently it was proven that this was not the case.145
GACI and ENPP1 Mutations
In GACI, OMIM No. 208000, calcification of the internal elastic lamina is associated with myointimal proliferation in medium and large-sized arteries (Figure 2).146 The disease phenotype is often associated with periarticular calcification (Figure 2). Affected patients frequently die within the first weeks of life as the result of heart failure and myocardial infarction.147,148 Based on the observation that an affected child had a systemic deficiency of PPi,146 we found decreased plasma levels of the PPi generating enzyme ENPP1.149 In a candidate gene approach, we identified homozygosity or compound heterozygosity for “loss of function” mutations in ENPP1 as the cause for this rare disorder. To date, more than 40 different causative mutations have been identified in GACI patients, accounting for more than 75% of the affected cases.148 ENPP1 expression is regulated during atheromatous plaque calcification, suggesting a protective role as an inhibitor of vascular mineralization by generating extracellular PPi also in the context of atherosclerosis.150 On the other hand, as shown most recently by cross breeding mice deficient for both apoE and Enpp1, Enpp1 promotes atherosclerotic lesion progression,151 probably mediated in part by promotion of OPN expression.
Figure 2. Typical manifestations of generalized arterial calcification of infancy (GACI) caused by mutations in ENPP1.
A, Cardiac ultrasound (suprasternal long-axis view) of a 4-week-old boy; note increased echogenicity of the wall of the ascending aorta (arrow-heads). B, Radiograph of the left hand of a 2-week-old boy showing periarticular calcifications (arrow). C, Cross section of the left coronary artery from a boy who died of myocardial infarction at the age of 9 weeks, showing calcification at the level of the internal elastic lamina (arrows) and intimal proliferation (hematoxylin and eosin staining). Ao indicates aorta, LA; left atrium.
Hypophosphatemia can compensate for GACI, and it has been argued that this reflects a physiological compensation mechanism rather than a primary defect.148 However, several mutations in the ENPP1 gene result in a phenotype with complete dissociation between GACI and autosomal-recessive hypophosphatemic rickets (ARHR),152,153 suggesting a different pathway involved in the generation of ARHR linked to direct renal Pi-modulating functions of ENPP1.
Studies investigating an association of ENPP1 polymorphism K173Q with arterial calcification revealed conflicting results (linked with type II diabetes154 and ESRD155 but not in other patient cohorts156). A noteworthy confounder is that ENPP1 variants differentially modulate insulin receptor signaling157 and K173Q and other SNPs in ENPP1 may have an indirect effect on parameters affecting arterial calcification in certain populations.
Arterial Calcification and Distal Joint Calcification and NT5E Mutations
Recently, using a genome-wide homozygosity mapping approach, NT5E, the gene for familial peripheral arterial calcification and distal joint calcification (OMIM No. 211800), was identified in a large consanguineous pedigree.130 The phenotype, notably, has a relatively late onset in young adulthood, and the symptomatic arterial disease, with prominent calcification, affects large lower extremity arteries, but remarkably, spares the coronary circulation.130 The authors detected biallelic nonsense, missense, and single-nucleotide insertion-frameshift mutations in the ecto-5′-nucleotidase gene NT5E, encoding the GPI-linked plasma membrane CD73 ecto-enzyme, which generates extracellular adenosine, directly downstream of ENPP1 in the extracellular ATP-degradation pathway. In their study, reduction in extracellular adenosine levels enhanced tissue nonspecific alkaline phosphatase (TNAP) activity, and adenosine supplementation reversed the increase in TNAP activity in CD73-deficient cells. It is possible that loss of the capacity of adenosine to suppress vascular inflammation and neointima formation contributes to the arterial disease phenotype in arterial calcification and distal joint calcification (ACDC). Because CD73 deficiency contributes to arterial calcification only in specific vascular territories, namely iliac, femoropopliteal, and tibial arteries, it is tempting to speculate that the related family member CD39 and its isoforms may compensate in an imperfect, functionally redundant fashion in other vascular beds, for example, CDL39L2 in the heart.158,159
C. “Cogs in the Wheel”: Model of Unified Molecular Pathophysiology for 3 Heritable Monogenic Deficiency States of “Cogs” That Prevent Spontaneous Arterial Calcification
Primary monogenic deficiencies of ENPP1, CD73, and ABCC6 serve as “cogs in a wheel.” Specifically, each is a minor component in the function of a much larger network of factors (Figure 3) that exert finely balanced promotion and suppression of arterial calcification. For the network to suppress spontaneous arterial calcification, the “cogs” ENPP1, CD73, and ABCC6 must be present and in working order. Monogenic ENPP1, CD73, and ABCC6 deficiencies each drive a molecular pathophysiology of closely related but phenotypically different human diseases (GACI, PXE, and arterial calcification due to CD73 deficiency [ACDC]), in which spontaneous, premature onset of arterial calcification is a prominent but not sole feature.
Figure 3. Model of an SMC functional network revealed by 3 human monogenic diseases of artery calcification (GACI due to ENPP1 deficiency, PXE due to ABCC6 deficiency, and ACDC due to CD73 deficiency).
This review describes how ENPP1, CD73, and ABCC6 serve as “cogs in a wheel.” Specifically, each is a minor component in the function of a much larger network of factors, illustrated here, that exert balanced effects to promote and suppress arterial calcification. For the network to normally suppress spontaneous arterial calcification, the “cogs,” ENPP1, CD73, and ABCC6, must be present and in working order. Monogenic ENPP1, CD73, and ABCC6 deficiencies each drive a molecular pathophysiology of closely related but phenotypically different diseases (GACI, ACDC, and PXE, respectively), in which premature onset arterial calcification is a prominent but not sole feature. The transmembrane ectoenzyme ENPP1 generates AMP and PPi from ATP, which are hydrolyzed by the GPI-linked ecto-enzymes CD73 (5′exonucleotidase) and TNAP, to generate adenosine and PPi, respectively. PPi suppresses hydroxyapatite deposition and inhibits ectopic chondrogenesis (and modulates artery calcification by other effects). ENPP1 modulates RAGE expression, as discussed in the text, and PPi limits promineralizing type I collagen. The PPi transporter ANKH, as well as ENPP1, elevates extracellular PPi, and murine deficiency of this transporter is associated with arterial calcification, driven in part by vanin-1 pantetheinase expression, which depresses cellular GSH stores and promotes ectopic chondrogenesis. Adenosine signaling suppresses TNAP expression and inhibits vascular inflammation and neointima formation. ABCC6 has been hypothesized, though not yet proven, to modulate adenosine transport. Pi signals, in part through uptake, mediated the Na+-dependent Pi cotransporter173 to promote osteochondral differentiation, and Pi is a component of hydroxyapatite crystal deposition. In addition, systemic effects of hepatic ABCC6 deficiency, including circulating fetuin deficiency, appear to modulate arterial and skin features of PXE (illustration credit: Cosmocyte/Ben Smith).
Prior models for unified understanding of mechanisms of ectopic artery calcification focused on systematic regulatory influences of PPi and Pi metabolism, mediated by ANKH, ENPP1, and TNAP on expression of OPN.160 ENPP1, through PPi generation, promotes OPN expression161; OPN is proatherogenic111 and probably mediates proatherogenic effects of ENPP1.151 However, OPN, in part by suppressing hydroxyapatite crystal deposition, potently inhibits calcification by cultured SMCs and suppresses artery calcification in vivo.114,162 Figure 3 schematizes the linkages between PPi and Pi metabolism and adenosine signaling that appear to intimately link GACI due to ENPP1 deficiency, PXE due to ABCC6 deficiency, and ACDC due to CD73 deficiency. Other factors in this paradigm include secondary changes in TNAP effects,95 because TNAP upregulation in SMCs, and consequent degradation of the artery calcification inhibitor PPi and generation of the critical artery calcification promoter Pi, at sites of fibrillar type I collagen expression, plays a substantial role in driving a variety of models of artery calcification in vitro, and in experimental artery calcification in vivo.123,163-166 TNAP activity reduces PPi levels, and, in part, thereby antagonizes ENPP1 in the ectopic calcification process.94 Regulation by ENPP1 and PPi of OPN and type I collagen expression are central components in this functional system,161,167 and OPN might further promote artery calcification by suppressing ENPP1 and ANKH expression.168 TNAP generates procalcifying Pi by hydrolyzing both PPi and ATP, and TNAP is a central, physiological player in osteoblast and chondrocyte maturation and normal calcification of the skeleton.94 In this model, because the histopathologic phenotype of the arterial lesions with fragmented and broken elastic fibers in the arterial media of ACDC patients closely resembles the arterial lesion in PXE patients, it has been speculated that both PXE and ACDC share common pathogenetic pathways and that adenosine transport may be modulated by ABCC6.169
Conclusion
Genetic factors clearly contribute to variation in amounts of media and intimal arterial calcification; this review did not focus on genetics in aortic valve calcification. GWAS have substantially increased our knowledge on risk stratification in CAC and atherosclerosis. However, functional significance of genes contained in the identified loci remains uncertain in most cases, especially since mutations in the respective candidate genes have not yet been detected in the affected patients. Candidate gene studies based on SNPs have more or less failed to provide reproducible results confirming genetic risk factors of arterial calcification in different human populations. We believe that systems biology approaches are the natural next step in identifying further hereditable contributing factors, along with large scale whole exome sequencing studies in at risk populations.
The analysis of naturally occurring or targeted mutant mice has substantially enhanced our knowledge on pathophysiologic mechanisms of arterial calcification, but knockout of specific mouse genes is not fully comparable to sequence variants of the homologous human genes, with functionally variable consequences for the gene products. Some of the mouse candidate genes have proven to be valuable for association studies, with respect to coronary artery calcification and cardiovascular risk prediction in human beings, which holds true for MGP, ASHG, and RANKL.
Major insight has been provided through rare monogenic human disorders associated with spontaneous, premature artery media calcification, (GACI, PXE, ACDC). The underlying disease genes, ENPP1, ABCC6, and NT5E, respectively, appear to be natural “cogs” that prevent spontaneous calcification within an arterial molecular pathophysiology network modulated by ATP metabolism, PPi, adenosine, and Pi generation. Roles of ENPP1, ABCC6, and NT5E in the context of atherosclerosis are now emerging. Nevertheless, how genetic factors fit into the pathogenetic puzzle in most instances of artery intima and media calcification remains to be defined
Acknowledgments
Sources of Funding
F.R. and Y.N. are supported by a grant from the Interdisciplinary Center for Clinical Research (IZKF), Münster. R.T. was supported by the VA Research Service and the National Institutes of Health (HL087252, HL077360).
Non-standard Abbreviations and Acronyms
- ACDC
arterial calcification due to CD73 deficiency
- ACE
angiotensin-converting enzyme
- CAC
coronary artery calcification
- CAD
coronary artery disease
- CKD
chronic kidney disease
- DCC
dystrophic cardiac calcinosis
- ECM
extracellular matrix
- ESRD
end-stage renal disease
- GACI
generalized arterial calcification of infancy
- GWAS
genome-wide association studies
- Pi
inorganic phosphate
- PPi
inorganic pyrophosphate
- PXE
pseudoxanthoma elasticum
- SERMs
selective estrogen receptor modulators
- SMC
smooth muscle cells
- sRAGE
soluble RAGE
- TNAP
tissue nonspecific alkaline phosphatase
Footnotes
Disclosures
None.
References
- 1.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 2.Nasir K, Budoff MJ, Wong ND, Scheuner M, Herrington D, Arnett DK, Szklo M, Greenland P, Blumenthal RS. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 3.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 4.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell CJ, Chazaro I, Wilson PW, Fox C, Hannan MT, Kiel DP, Cupples LA. Evidence for heritability of abdominal aortic calcific deposits in the Framingham Heart Study. Circulation. 2002;106:337–341. doi: 10.1161/01.cir.0000022663.26468.5b. [DOI] [PubMed] [Google Scholar]
- 6.Peyser PA, Bielak LF, Chu JS, Turner ST, Ellsworth DL, Boerwinkle E, Sheedy PF. Heritability of coronary artery calcium quantity measured by electron beam computed tomography in asymptomatic adults. Circulation. 2002;106:304–308. doi: 10.1161/01.cir.0000022664.21832.5d. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, Broeckel U, Holmer S, Baessler A, Hengstenberg C, Mayer B, Erdmann J, Klein G, Riegger G, Jacob HJ, Schunkert H. Distinct heritable patterns of angiographic coronary artery disease in families with myocardial infarction. Circulation. 2005;111:855–862. doi: 10.1161/01.CIR.0000155611.41961.BB. [DOI] [PubMed] [Google Scholar]
- 8.Rampersaud E, Bielak LF, Parsa A, Shen H, Post W, Ryan KA, Donnelly P, Rumberger JA, Sheedy PF, Peyser PA, Shuldiner AR, Mitchell BD. The association of coronary artery calcification and carotid artery intima-media thickness with distinct, traditional coronary artery disease risk factors in asymptomatic adults. Am J Epidemiol. 2008;168:1016–1023. doi: 10.1093/aje/kwn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boer-winkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Assimes TL, Knowles JW, Basu A, Iribarren C, Southwick A, Tang H, Absher D, Li J, Fair JM, Rubin GD, Sidney S, Fortmann SP, Go AS, Hlatky MA, Myers RM, Risch N, Quertermous T. Susceptibility locus for clinical and subclinical coronary artery disease at chromosome 9p21 in the multi-ethnic ADVANCE study. Hum Mol Genet. 2008;17:2320–2328. doi: 10.1093/hmg/ddn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarinova O, Stewart AF, Roberts R, Wells G, Lau P, Naing T, Buerki C, McLean BW, Cook RC, Parker JS, McPherson R. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler Thromb Vasc Biol. 2009;29:1671–1677. doi: 10.1161/ATVBAHA.109.189522. [DOI] [PubMed] [Google Scholar]
- 15.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange LA, Lange EM, Bielak LF, Langefeld CD, Kardia SL, Royston P, Turner ST, Sheedy PF, Boerwinkle E, Peyser PA. Autosomal genome-wide scan for coronary artery calcification loci in sibships at high risk for hypertension. Arterioscler Thromb Vasc Biol. 2002;22:418–423. doi: 10.1161/hq0302.105721. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Lewis CE, Wagenknecht LE, Myers RH, Pankow JS, Hunt SC, North KE, Hixson JE, Jeffrey CJ, Shimmin LC, Borecki I, Province MA. Genome-wide admixture mapping for coronary artery calcification in African Americans: the NHLBI Family Heart Study. Genet Epidemiol. 2008;32:264–272. doi: 10.1002/gepi.20301. [DOI] [PubMed] [Google Scholar]
- 18.Huang CC, Lloyd-Jones DM, Guo X, Rajamannan NM, Lin SM, Du P, Huang Q, Hou L, Liu K. Gene expression variation between African Americans and whites is associated with coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis. Physiol Genomics. 2011 April 26; doi: 10.1152/physiolgenomics.00243.2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama EE, Tanaka Y, Nagai Y, Iwamoto A, Shioda T. A CCR2-V64I polymorphism affects stability of CCR2A isoform. AIDS. 2004;18:729–738. doi: 10.1097/00002030-200403260-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Doranz BJ, Rana S, Yi Y, Mellado M, Frade JM, Martinez A, O'Brien SJ, Dean M, Collman RG, Doms RW. Influence of the CCR2-V64I polymorphism on human immunodeficiency virus type 1 coreceptor activity and on chemokine receptor function of CCR2b, CCR3, CCR5, and CXCR4. J Virol. 1998;72:7450–7458. doi: 10.1128/jvi.72.9.7450-7458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdes AM, Wolfe ML, O'Brien EJ, Spurr NK, Gefter W, Rut A, Groot PH, Rader DJ. Val64Ile polymorphism in the C-C chemokine receptor 2 is associated with reduced coronary artery calcification. Arterioscler Thromb Vasc Biol. 2002;22:1924–1928. doi: 10.1161/01.atv.0000038486.48400.e7. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez P, Alvarez R, Batalla A, Reguero JR, Alvarez V, Astudillo A, Cubero GI, Cortina A, Coto E. Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun. 2001;2:191–195. doi: 10.1038/sj.gene.6363760. [DOI] [PubMed] [Google Scholar]
- 23.Dow DJ, McMahon AD, Gray IC, Packard CJ, Groot PH. CCR2 and coronary artery disease: a WOSCOPS substudy. BMC Res Notes. 2010;3:31. doi: 10.1186/1756-0500-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfohl M, Athanasiadis A, Koch M, Clemens P, Benda N, Haring HU, Karsch KR. Insertion/deletion polymorphism of the angiotensin I-converting enzyme gene is associated with coronary artery plaque calcification as assessed by intravascular ultrasound. J Am Coll Cardiol. 31:987–991. doi: 10.1016/s0735-1097(98)00044-8. 199. [DOI] [PubMed] [Google Scholar]
- 25.Oei HH, Sayed-Tabatabaei FA, Hofman A, Oudkerk M, van Duijin CM, Witteman JC. The association between angiotensin-converting enzyme gene polymorphism and coronary calcification: the Rotterdam Coronary Calcification Study. Atherosclerosis. 2005;182:169–173. doi: 10.1016/j.atherosclerosis.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 27.Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2004;109:335–339. doi: 10.1161/01.CIR.0000109487.46725.02. [DOI] [PubMed] [Google Scholar]
- 28.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Jr, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Burdon KP, Lehtinen AB, Langefeld CD, Carr JJ, Rich SS, Freedman BI, Herrington D, Bowden DW. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diab Vasc Dis Res. 2008;5:128–134. doi: 10.3132/dvdr.2008.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida M, Takano Y, Sasaoka T, Izumi T, Kimura A. E-selectin polymorphism associated with myocardial infarction causes enhanced leukocyte-endothelial interactions under flow conditions. Arterioscler Thromb Vasc Biol. 2003;23:783–788. doi: 10.1161/01.ATV.0000067427.40133.59. [DOI] [PubMed] [Google Scholar]
- 31.Ellsworth DL, Bielak LF, Turner ST, Sheedy PF, Boerwinkle E, Peyser PA. Gender- and age-dependent relationships between the E-selectin S128R polymorphism and coronary artery calcification. J Mol Med. 2001;79:390–398. doi: 10.1007/s001090100235. [DOI] [PubMed] [Google Scholar]
- 32.Pollanen PJ, Lehtimaki T, Ilveskoski E, Mikkelsson J, Kajander OA, Laippala P, Perola M, Goebeler S, Penttila A, Mattila KM, Syrjakoski K, Koivula T, Nikkari ST, Karhunen PJ. Coronary artery calcification is related to functional polymorphism of matrix metalloproteinase 3: the Helsinki Sudden Death Study. Atherosclerosis. 2002;164:329–335. doi: 10.1016/s0021-9150(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 33.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 34.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 35.Kasuga H, Hosogane N, Matsuoka K, Mori I, Sakura Y, Shimakawa K, Shinki T, Suda T, Taketomi S. Characterization of transgenic rats con-stitutively expressing vitamin D-24-hydroxylase gene. Biochem Biophys Res Commun. 2002;297:1332–1338. doi: 10.1016/s0006-291x(02)02254-4. [DOI] [PubMed] [Google Scholar]
- 36.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O'Connell JR, Hixson JE, Kardia SL, Sun YV, Jhun MA, Wang X, Mehta NN, Li M, Koller DL, Hakonarson H, Keating BJ, Rader DJ, Shuldiner AR, Peyser PA, Reilly MP, Mitchell BD. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2010;30:2648–2654. doi: 10.1161/ATVBAHA.110.211805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ignat M, Teletin M, Tisserand J, Khetchoumian K, Dennefeld C, Chambon P, Losson R, Mark M. Arterial calcifications and increased expression of vitamin D receptor targets in mice lacking TIF1alpha. Proc Natl Acad Sci U S A. 2008;105:2598–2603. doi: 10.1073/pnas.0712030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 39.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 40.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Perez VA, Ali Z, Alastalo TP, Ikeno F, Sawada H, Lai YJ, Kleisli T, Spiekerkoetter E, Qu X, Rubinos LH, Ashley E, Amieva M, Dedhar S, Rabinovitch M. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J Cell Biol. 2011;192:171–188. doi: 10.1083/jcb.201008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman BI, Bowden DW, Ziegler JT, Langefeld CD, Lehtinen AB, Rudock ME, Lenchik L, Hruska KA, Register TC, Carr JJ. Bone morphogenetic protein 7 (BMP7) gene polymorphisms are associated with inverse relationships between vascular calcification and BMD: the Diabetes Heart Study. J Bone Miner Res. 2009;24:1719–1727. doi: 10.1359/JBMR.090501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajih N, Sane DC, Hutson SM, Wallin R. The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system: characterization of the system in normal and warfarin-resistant rats. J Biol Chem. 2004;279:25276–25283. doi: 10.1074/jbc.M401645200. [DOI] [PubMed] [Google Scholar]
- 46.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Romero D, Ruiz-Nodar JM, Marin F, Tello-Montoliu A, Roldan V, Mainar L, Perez-Andreu V, Anton AI, Bonaque JC, Valdes M, Vicente V, Gonzalez-Conejero R. CALU A29809G polymorphism in coronary atherothrombosis: implications for coronary calcification and prognosis. Ann Med. 2010;42:439–446. doi: 10.3109/07853890.2010.499131. [DOI] [PubMed] [Google Scholar]
- 48.Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 49.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–1407. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 50.Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hortnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Muller CR, Strom TM, Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 51.Teichert M, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, De Smet PA, Witteman JC, Stricker BH. Vitamin K epoxide reductase complex subunit 1 (VKORC1) polymorphism and aortic calcification: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2008;28:771–776. doi: 10.1161/ATVBAHA.107.159913. [DOI] [PubMed] [Google Scholar]
- 52.Fu Y, Porres JM, Lei XG. Comparative impacts of glutathione peroxidase-1 gene knockout on oxidative stress induced by reactive oxygen and nitrogen species in mouse hepatocytes. Biochem J. 2001;359:687–695. doi: 10.1042/0264-6021:3590687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnabel R, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Torzewski M, Lubos E, Bickel C, Cambien F, Tiret L, Munzel T, Blankenberg S. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 54.Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, Nanjo K. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- 55.Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, Fujimoto K, Sakuma T, Ohashi T, Fukuda K, Eto Y, Tajima N. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao JH, Xie PZ, Fishbein MC, Kreuzer J, Drake TA, Demer LL, Lusis AJ. Pathology of atheromatous lesions in inbred and genetically engi-neered mice: genetic determination of arterial calcification. Arterioscler Thromb. 1994;14:1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- 57.Qiao JH, Fishbein MC, Demer LL, Lusis AJ. Genetic determination of cartilaginous metaplasia in mouse aorta. Arterioscler Thromb Vasc Biol. 1995;15:2265–2272. doi: 10.1161/01.atv.15.12.2265. [DOI] [PubMed] [Google Scholar]
- 58.Ivandic BT, Qiao JH, Machleder D, Liao F, Drake TA, Lusis AJ. A locus on chromosome 7 determines myocardial cell necrosis and calcification (dystrophic cardiac calcinosis) in mice. Proc Natl Acad Sci U S A. 1996;93:5483–5488. doi: 10.1073/pnas.93.11.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunnert SR, Altman NH. Dystrophic cardiac calcinosis in mice: abnormal myocardial response to freeze-thaw injury. Lab Anim Sci. 1990;40:616–619. [PubMed] [Google Scholar]
- 60.Meng H, Vera I, Che N, Wang X, Wang SS, Ingram-Drake L, Schadt EE, Drake TA, Lusis AJ. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci U S A. 2007;104:4530–4535. doi: 10.1073/pnas.0607620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aherrahrou Z, Doehring LC, Ehlers EM, Liptau H, Depping R, Linsel-Nitschke P, Kaczmarek PM, Erdmann J, Schunkert H. An alternative splice variant in Abcc6, the gene causing dystrophic calcification, leads to protein deficiency in C3H/He mice. J Biol Chem. 2008;283:7608–7615. doi: 10.1074/jbc.M708290200. [DOI] [PubMed] [Google Scholar]
- 62.Wang SS, Martin LJ, Schadt EE, Meng H, Wang X, Zhao W, Ingram-Drake L, Nebohacova M, Mehrabian M, Drake TA, Lusis AJ. Disruption of the aortic elastic lamina and medial calcification share genetic determinants in mice. Circ Cardiovasc Genet. 2009;2:573–582. doi: 10.1161/CIRCGENETICS.109.860270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapustin A, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011 May 12; doi: 10.1161/CIRCRESAHA.110.238808. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Weissen-Plenz G, Nitschke Y, Rutsch F. Mechanisms of arterial calcification: spotlight on the inhibitors. Adv Clin Chem. 2008;46:263–293. [PubMed] [Google Scholar]
- 65.Rutsch F, Terkeltaub R. Deficiencies of physiologic calcification inhibitors and low-grade inflammation in arterial calcification: lessons for cartilage calcification. Joint Bone Spine. 2005;72:110–118. doi: 10.1016/j.jbspin.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Rutsch F, Terkeltaub R. Parallels between arterial and cartilage calcification: what understanding artery calcification can teach us about chondrocalcinosis. Curr Opin Rheumatol. 2003;15:302–310. doi: 10.1097/00002281-200305000-00019. [DOI] [PubMed] [Google Scholar]
- 67.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi N, Kitazawa R, Maeda S, Schurgers L, Kitazawa S. T-138C polymorphism of matrix gla protein promoter alters its expression but is not directly associated with atherosclerotic vascular calcification. Kobe J Med Sci. 2004;50:69–81. [PubMed] [Google Scholar]
- 69.Farzaneh-Far A, Davies JD, Braam LA, Spronk HM, Proudfoot D, Chan SW, O'Shaughnessy KM, Weissberg PL, Vermeer C, Shanahan CM. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem. 2001;276:32466–32473. doi: 10.1074/jbc.M104909200. [DOI] [PubMed] [Google Scholar]
- 70.Herrmann SM, Whatling C, Brand E, Nicaud V, Gariepy J, Simon A, Evans A, Ruidavets JB, Arveiler D, Luc G, Tiret L, Henney A, Cambien F. Polymorphisms of the human matrix gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20:2386–2393. doi: 10.1161/01.atv.20.11.2386. [DOI] [PubMed] [Google Scholar]
- 71.Brancaccio D, Biondi ML, Gallieni M, Turri O, Galassi A, Cecchini F, Russo D, Andreucci V, Cozzolino M. Matrix GLA protein gene polymorphisms: clinical correlates and cardiovascular mortality in chronic kidney disease patients. Am J Nephrol. 2005;25:548–552. doi: 10.1159/000088809. [DOI] [PubMed] [Google Scholar]
- 72.Taylor BC, Schreiner PJ, Doherty TM, Fornage M, Carr JJ, Sidney S. Matrix Gla protein and osteopontin genetic associations with coronary artery calcification and bone density: the CARDIA study. Hum Genet. 2005;116:525–528. doi: 10.1007/s00439-005-1258-3. [DOI] [PubMed] [Google Scholar]
- 73.Crosier MD, Booth SL, Peter I, Dawson-Hughes B, Price PA, O'Donnell CJ, Hoffmann U, Williamson MK, Ordovas JM. Matrix Gla protein polymorphisms are associated with coronary artery calcification in men. J Nutr Sci Vitaminol (Tokyo) 2009;55:59–65. doi: 10.3177/jnsv.55.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox DW, Andrews BJ, Wills DE. Genetic polymorphism of alpha 2HS-glycoprotein. Am J Hum Genet. 1986;38:699–706. [PMC free article] [PubMed] [Google Scholar]
- 75.Osawa M, Tian W, Horiuchi H, Kaneko M, Umetsu K. Association of alpha2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Hum Genet. 2005;116:146–151. doi: 10.1007/s00439-004-1222-7. [DOI] [PubMed] [Google Scholar]
- 76.Jung JY, Hwang YH, Lee H, Ro H, Lee H, Chung W, Chae DW, Joo KW, Ahn C, Oh KH. Association of AHSG gene polymorphisms and aortic stiffness in peritoneal dialysis patients. Am J Nephrol. 2010;31:510–517. doi: 10.1159/000309789. [DOI] [PubMed] [Google Scholar]
- 77.Andersen G, Burgdorf KS, Sparso T, Borch-Johnsen K, Jorgensen T, Hansen T, Pedersen O. AHSG tag single nucleotide polymorphisms associate with type 2 diabetes and dyslipidemia: studies of metabolic traits in 7,683 white Danish subjects. Diabetes. 2008;57:1427–1432. doi: 10.2337/db07-0558. [DOI] [PubMed] [Google Scholar]
- 78.Lavebratt C, Wahlqvist S, Nordfors L, Hoffstedt J, Arner P. AHSG gene variant is associated with leanness among Swedish men. Hum Genet. 2005;117:54–60. doi: 10.1007/s00439-005-1286-z. [DOI] [PubMed] [Google Scholar]
- 79.Lehtinen AB, Burdon KP, Lewis JP, Langefeld CD, Ziegler JT, Rich SS, Register TC, Carr JJ, Freedman BI, Bowden DW. Association of alpha2-Heremans-Schmid glycoprotein polymorphisms with subclinical atherosclerosis. J Clin Endocrinol Metab. 2007;92:345–352. doi: 10.1210/jc.2006-0429. [DOI] [PubMed] [Google Scholar]
- 80.Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, Barany P, Lindholm B, Jogestrand T, Heimburger O, Holmes C, Schalling M, Nordfors L. Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney Int. 2005;67:2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 81.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;16(309):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jo SH, Kim SG, Choi YJ, Joo NR, Cho GY, Choi SR, Kim EJ, Kim HS, Kim HJ, Rhim CY. KLOTHO gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in a Korean population. Int Heart J. 2009;50:23–32. doi: 10.1536/ihj.50.23. [DOI] [PubMed] [Google Scholar]
- 83.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/ osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 85.Orita Y, Yamamoto H, Kohno N, Sugihara M, Honda H, Kawamata S, Mito S, Soe NN, Yoshizumi M. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol. 2007;27:2058–2064. doi: 10.1161/ATVBAHA.107.147868. [DOI] [PubMed] [Google Scholar]
- 86.Soufi M, Schoppet M, Sattler AM, Herzum M, Maisch B, Hofbauer LC, Schaefer JR. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J Clin Endocrinol Metab. 2004;89:3764–3768. doi: 10.1210/jc.2003-032054. [DOI] [PubMed] [Google Scholar]
- 87.Rhee EJ, Oh KW, Jung CH, Lee WY, Oh ES, Yun EJ, Baek KH, Kang MI, Kim SW. The relationship between four single nucleotide polymorphisms in the promoter region of the osteoprotegerin gene and aortic calcification or coronary artery disease in Koreans. Clin Endocrinol (Oxf) 2006;64:689–697. doi: 10.1111/j.1365-2265.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 88.Rhee EJ, Yun EJ, Oh KW, Park SE, Park CY, Lee WY, Park SW, Kim SW, Baek KH, Kang MI. The relationship between Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL) gene polymorphism and aortic calcification in Korean women. Endocr J. 2010;57:541–549. doi: 10.1507/endocrj.k10e-015. [DOI] [PubMed] [Google Scholar]
- 89.Hough TA, Bogani D, Cheeseman MT, Favor J, Nesbit MA, Thakker RV, Lyon MF. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci U S A. 2004;101:13566–13571. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turkmen F, Ozdemir A, Sevinc C, Eren PA, Demiral S. Calciumsensing receptor gene polymorphisms and cardiac valvular calcification in patients with chronic renal failure: a pilot study. Hemodial Int. 2009;13:176–180. doi: 10.1111/j.1542-4758.2009.00333.x. [DOI] [PubMed] [Google Scholar]
- 91.Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 92.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 93.Hosoda Y, Yoshimura Y, Higaki S. A new breed of mouse showing multiple osteochondral lesions-twy mouse. Ryumachi. 1981;21(Suppl):157–164. [PubMed] [Google Scholar]
- 94.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase andplasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson K, Polewski M, van ED, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1-/- mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 96.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 97.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 98.Blair HC. How the osteoclast degrades bone. Bioessays. 1998;20:837–846. doi: 10.1002/(SICI)1521-1878(199810)20:10<837::AID-BIES9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 99.Spicer SS, Lewis SE, Tashian RE, Schulte BA. Mice carrying a CAR-2 null allele lack carbonic anhydrase II immunohistochemically and show vascular calcification. Am J Pathol. 1989;134:947–954. [PMC free article] [PubMed] [Google Scholar]
- 100.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985;313:139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- 101.Roth DE, Venta PJ, Tashian RE, Sly WS. Molecular basis of human carbonic anhydrase II deficiency. Proc Natl Acad Sci U S A. 1992;89:1804–1808. doi: 10.1073/pnas.89.5.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tseng ZH, Vittinghoff E, Musone SL, Lin F, Whiteman D, Pawlikowska L, Kwok PY, Olgin JE, Aouizerat BE. Association of TGFBR2 polymorphism with risk of sudden cardiac arrest in patients with coronary artery disease. Heart Rhythm. 2009;6:1745–1750. doi: 10.1016/j.hrthm.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res. 2001;88:37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- 105.Bailey M, Pillarisetti S, Jones P, Xiao H, Simionescu D, Vyavahare N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovasc Pathol. 2004;13:146–155. doi: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- 106.Vyavahare N, Jones PL, Tallapragada S, Levy RJ. Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol. 2000;157:885–893. doi: 10.1016/S0002-9440(10)64602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee B, Godfrey M, Vitale E, Hori H, Mattei MG, Sarfarazi M, Tsipouras P, Ramirez F, Hollister DW. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991;352:330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- 108.Luo XH, Zhao LL, Yuan LQ, Wang M, Xie H, Liao EY. Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J Bone Miner Res. 2009;24:1461–1468. doi: 10.1359/jbmr.090227. [DOI] [PubMed] [Google Scholar]
- 109.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 110.Wassel CL, Pankow JS, Rasmussen-Torvik LJ, Li N, Taylor KD, Guo X, Goodarzi MO, Palmas WR, Post WS. Associations of SNPs in ADIPOQ and Subclinical Cardiovascular Disease in the Multi-Ethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2011;19:840–847. doi: 10.1038/oby.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 112.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 113.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–1034. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 116.Johnson KA, Yao W, Lane NE, Naquet P, Terkeltaub RA. Vanin-1 pantetheinase drives increased chondrogenic potential of mesenchymal precursors in ank/ank mice. Am J Pathol. 2008;172:440–453. doi: 10.2353/ajpath.2008.070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmad PJ, Trcka D, Xue S, Franco C, Speer MY, Giachelli CM, Bendeck MP. Discoidin domain receptor-1 deficiency attenuates atherosclerotic calcification and smooth muscle cell-mediated mineralization. Am J Pathol. 2009;175:2686–2696. doi: 10.2353/ajpath.2009.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cecil DL, Terkeltaub RA. Arterial calcification is driven by RAGE in Enpp1-/- Mice. J Vasc Res. 2011;48:227–235. doi: 10.1159/000318805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Towler DA. Vascular calcification: it's all the RAGE! Arterioscler Thromb Vasc Biol. 2011;31:237–239. doi: 10.1161/ATVBAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Maadawy S, Kaartinen MT, Schinke T, Murshed M, Karsenty G, McKee MD. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44(Suppl 1):272–278. [PubMed] [Google Scholar]
- 122.Meier M, Weng LP, Alexandrakis E, Ruschoff J, Goeckenjan G. Tracheobronchial stenosis in Keutel syndrome. Eur Respir J. 2001;17:566–569. doi: 10.1183/09031936.01.17305660. [DOI] [PubMed] [Google Scholar]
- 123.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van SS, Baas F, ten Brink JB, de Jong PT. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 125.Cai L, Struk B, Adams MD, Ji W, Haaf T, Kang HL, Dho SH, Xu X, Ringpfeil F, Nancarrow J, Zach S, Schaen L, Stumm M, Niu T, Chung J, Lunze K, Verrecchia B, Goldsmith LA, Viljoen D, Figuera LE, Fuchs W, Lebwohl M, Uitto J, Richards R, Hohl D, Ramesar R. A 500-kb region on chromosome 16p13.1 contains the pseudoxanthoma elasticum locus: high-resolution mapping and genomic structure. J Mol Med. 2000;78:36–46. doi: 10.1007/s001090000079. [DOI] [PubMed] [Google Scholar]
- 126.Le Saux O, Urban Z, Goring HH, Csiszar K, Pope FM, Richards A, Pasquali-Ronchetti I, Terry S, Bercovitch L, Lebwohl MG, Breuning M, van den Berg P, Kornet L, Doggett N, Ott J, de Jong PT, Bergen AA, Boyd CD. Pseudoxanthoma elasticum maps to an 820-kb region of the p13.1 region of chromosome 16. Genomics. 1999;62:1–10. doi: 10.1006/geno.1999.5925. [DOI] [PubMed] [Google Scholar]
- 127.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali-Ronchetti I, Pope FM, Richards A, Terry S, Bercovitch L, de PA, Boyd CD. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25:223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 128.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Struk B, Cai L, Zach S, Ji W, Chung J, Lumsden A, Stumm M, Huber M, Schaen L, Kim CA, Goldsmith LA, Viljoen D, Figuera LE, Fuchs W, Munier F, Ramesar R, Hohl D, Richards R, Neldner KH, Lindpaintner K. Mutations of the gene encoding the transmembrane transporter protein ABC-C6 cause pseudoxanthoma elasticum. J Mol Med. 2000;78:282–286. doi: 10.1007/s001090000114. [DOI] [PubMed] [Google Scholar]
- 130.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ringpfeil F, Pulkkinen L, Uitto J. Molecular genetics of pseudoxanthoma elasticum. Exp Dermatol. 2001;10:221–228. doi: 10.1034/j.1600-0625.2001.100401.x. [DOI] [PubMed] [Google Scholar]
- 132.Miksch S, Lumsden A, Guenther UP, Foernzler D, Christen-Zach S, Daugherty C, Ramesar RK, Lebwohl M, Hohl D, Neldner KH, Lind-paintner K, Richards RI, Struk B. Molecular genetics of pseudoxanthoma elasticum: type and frequency of mutations in ABCC6. Hum Mutat. 2005;26:235–248. doi: 10.1002/humu.20206. [DOI] [PubMed] [Google Scholar]
- 133.Mendelsohn G, Bulkley BH, Hutchins GM. Cardiovascular manifestations of Pseudoxanthoma elasticum. Arch Pathol Lab Med. 1978;102:298–302. [PubMed] [Google Scholar]
- 134.Wang SS, Martin LJ, Schadt EE, Meng H, Wang X, Zhao W, Ingram-Drake L, Nebohacova M, Mehrabian M, Drake TA, Lusis AJ. Disruption of the aortic elastic lamina and medial calcification share genetic determinants in mice. Circ Cardiovasc Genet. 2009;2:573–582. doi: 10.1161/CIRCGENETICS.109.860270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, Ramsay M, Viljoen D, Terry PF, de PA, Uitto J, Bercovitch LG. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- 137.Li Q, Jiang Q, Schurgers LJ, Uitto J. Pseudoxanthoma elasticum: reduced gamma-glutamyl carboxylation of matrix gla protein in a mouse model (Abcc6−/−) Biochem Biophys Res Commun. 2007;364:208–213. doi: 10.1016/j.bbrc.2007.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Le BG, Labreze C, Croue A, Schurgers LJ, Chassaing N, Wittkampf T, Rutsch F, Martin L. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet A. 2010;152A:118–123. doi: 10.1002/ajmg.a.33162. [DOI] [PubMed] [Google Scholar]
- 139.Ilias A, Urban Z, Seidl TL, Le SO, Sinko E, Boyd CD, Sarkadi B, Varadi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 140.Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang Q, Dibra F, Lee MD, Oldenburg R, Uitto J. Overexpression of fetuin-a counteracts ectopic mineralization in a mouse model of pseudoxanthoma elasticum (abcc6(−/−)) J Invest Dermatol. 2010;130:1288–1296. doi: 10.1038/jid.2009.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–354. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Le Saux O, Bunda S, VanWart CM, Douet V, Got L, Martin L, Hinek A. Serum factors from pseudoxanthoma elasticum patients alter elastic fiber formation in vitro. J Invest Dermatol. 2006;126:1497–1505. doi: 10.1038/sj.jid.5700201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 145.Jiang Q, Li Q, Grand-Pierre AE, Schurgers LJ, Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (−/−) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–707. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rutsch F, Schauerte P, Kalhoff H, Petrarulo M, August C, Diekmann L. Low levels of urinary inorganic pyrophosphate indicating systemic pyrophosphate deficiency in a boy with idiopathic infantile arterial calcification. Acta Paediatr. 2000;89:1265–1269. doi: 10.1080/080352500750027682. [DOI] [PubMed] [Google Scholar]
- 147.Moran JJ. Idiopathic arterial calcification of infancy: a clinicopathologic study. Pathol Annu. 1975;10:393–417. [PubMed] [Google Scholar]
- 148.Rutsch F, Boyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, Weissen-Plenz G, Fischer RJ, Mughal Z, Gregory JW, Davies JH, Loirat C, Strom TM, Schnabel D, Nurnberg P, Terkeltaub R. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nitschke Y, Hartmann S, Torsello G, Horstmann R, Seifarth H, Weissen-Plenz G, Rutsch F. Expression of NPP1 is regulated during atheromatous plaque calcification. J Cell Mol Med. 2009 December 8; doi: 10.1111/j.1582-4934.2009.00988.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nitschke Y, Weissen-Plenz G, Terkeltaub R, Rutsch F. Npp1 promotes atherosclerosis in ApoE knockout mice. J Cell Mol Med. 2011 April 8; doi: 10.1111/j.1582-4934.2011.01327.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee JE, Choi YK, Seo HA, Jeon JH, Jeong JY, Moon SS, Kim JG, Kim BW, Kim SW, Min Y, Kim JY, Lee IK. Impact of ENPP1 and MMP3 gene polymorphisms on aortic calcification in patients with type 2 diabetes in a Korean population. Diabetes Res Clin Pract. 2010;88:87–96. doi: 10.1016/j.diabres.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 155.Eller P, Hochegger K, Feuchtner GM, Zitt E, Tancevski I, Ritsch A, Kronenberg F, Rosenkranz AR, Patsch JR, Mayer G. Impact of ENPP1 genotype on arterial calcification in patients with end-stage renal failure. Nephrol Dial Transplant. 2008;23:321–327. doi: 10.1093/ndt/gfm566. [DOI] [PubMed] [Google Scholar]
- 156.Jeong DJ, Lee DG, Kim HJ, Cho EH, Kim SW. ENPP1 K121Q genotype not associated with coronary artery calcification in Korean patients with type 2 diabetes mellitus. Korean Diabetes J. 2010;34:320–326. doi: 10.4093/kdj.2010.34.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanyolac S, Mahley RW, Hodoglugil U, Goldfine ID. Gender differences in the relationship of ENPP1/PC-1 variants to obesity in a Turkish population. Obesity (Silver Spring) 2008;16:2468–2471. doi: 10.1038/oby.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yeung G, Mulero JJ, McGowan DW, Bajwa SS, Ford JE. CD39L2, a gene encoding a human nucleoside diphosphatase, predominantly expressed in the heart. Biochemistry. 2000;39:12916–12923. doi: 10.1021/bi000959z. [DOI] [PubMed] [Google Scholar]
- 159.Rucker B, Almeida ME, Libermann TA, Zerbini LF, Wink MR, Sarkis JJ. E-NTPDases and ecto-5′-nucleotidase expression profile in rat heart left ventricle and the extracellular nucleotide hydrolysis by their nerve terminal endings. Life Sci. 2008;82:477–486. doi: 10.1016/j.lfs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 160.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson K, Goding J, van ED, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millan JL, Terkeltaub R. Linked deficiencies in extracellular PP (I) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- 162.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 163.Huang MS, Sage AP, Lu J, Demer LL, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun. 2008;374:553–558. doi: 10.1016/j.bbrc.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 165.O'Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25:187–191. doi: 10.1093/ndt/gfp362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Villa-Bellosta R, Wang X, Millan JL, Dubyak GR, O'Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2011 April 13; doi: 10.1152/ajpheart.01020.2010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Polewski MD, Johnson KA, Foster M, Millan JL, Terkeltaub R. Inorganic pyrophosphatase induces type I collagen in osteoblasts. Bone. 2010;46:81–90. doi: 10.1016/j.bone.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millan JL. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J Bone Miner Res. 2006;21:1377–1386. doi: 10.1359/jbmr.060619. [DOI] [PubMed] [Google Scholar]
- 169.Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, Chaganti K, Nussbaum RL, Boehm M, Gahl WA. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Munroe PB, Olgunturk RO, Fryns JP, Van ML, Ziereisen F, Yuksel B, Gardiner RM, Chung E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 171.Jahnen-Dechent W, Schinke T, Trindl A, Muller-Esterl W, Sablitzky F, Kaiser S, Blessing M. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 172.Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu CJ, Zhang T, Xu Q, Zhu Y, Ake O, Qi Y, Tang C, Kong W, Wang X. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ Res. 2011;108:917–928. doi: 10.1161/CIRCRESAHA.110.234328. [DOI] [PubMed] [Google Scholar]
- 173.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: emerging role of the sodium-dependent phosphate co-transporter PiT-1. Thromb Haemost. 2010;104:464–470. doi: 10.1160/TH09-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]