The value of hormone receptor and human epidermal growth factor receptor 2 expression for predicting overall survival, distant relapse, and locoregional relapse was examined in patients with inflammatory breast cancer. Triple-negative disease was associated with worse outcomes, indicating the need for developing new locoregional and systemic treatment strategies for patients with this aggressive subtype.
Keywords: Inflammatory breast cancer, Estrogen receptor, Progesterone receptor, HER-2, Molecular subtypes
Abstract
Background.
Numerous studies have demonstrated that expression of estrogen/progesterone receptor (ER/PR) and human epidermal growth factor receptor (HER)-2 is important for predicting overall survival (OS), distant relapse (DR), and locoregional relapse (LRR) in early and advanced breast cancer patients. However, these findings have not been confirmed for inflammatory breast cancer (IBC), which has different biological features than non-IBC.
Methods.
We retrospectively analyzed the records of 316 women who presented to MD Anderson Cancer Center in 1989–2008 with newly diagnosed IBC without distant metastases. Most patients received neoadjuvant chemotherapy, mastectomy, and postmastectomy radiation. Patients were grouped according to receptor status: ER+ (ER+/PR+ and HER-2−; n = 105), ER+HER-2+ (ER+/PR+ and HER-2+; n = 37), HER-2+ (ER−/PR− and HER-2+; n = 83), or triple-negative (TN) (ER−PR−HER-2−; n = 91). Kaplan–Meier and Cox proportional hazards methods were used to assess LRR, DR, and OS rates and their associations with prognostic factors.
Results.
The median age was 50 years (range, 24–83 years). The median follow-up time and median OS time for all patients were both 33 months. The 5-year actuarial OS rates were 58.7% for the entire cohort, 69.7% for ER+ patients, 73.5% for ER+HER-2+ patients, 54.0% for HER=2+ patients, and 42.7% for TN patients (p < .0001); 5-year LRR rates were 20.3%, 8.0%, 12.6%, 22.6%, and 38.6%, respectively, for the four subgroups (p < .0001); and 5-year DR rates were 45.5%, 28.8%, 50.1%, 52.1%, and 56.7%, respectively (p < .001). OS and LRR rates were worse for TN patients than for any other subgroup (p < .0001–.03).
Conclusions.
TN disease is associated with worse OS, DR, and LRR outcomes in IBC patients, indicating the need for developing new locoregional and systemic treatment strategies for patients with this aggressive subtype.
Introduction
Breast cancer is increasingly recognized as a heterogeneous disease in which various subsets have distinctly different responses to treatment and outcomes [1]. Gene expression profiling has led to the discovery of four molecular subtypes of breast cancer [2–6]. Technical limitations associated with microarray analysis of paraffin-embedded tissue samples led to the use of estrogen or progesterone receptor (ER/PR) status and human epidermal growth factor receptor (HER)-2 expression as surrogates to define four subtypes of breast cancer: ER+ (ER+/PR+ and HER-2−), ER+HER-2+ (ER+/PR+ and HER-2+), HER-2+ (ER−/PR− and HER-2+), and triple negative (TN) (ER−PR−HER-2−) [7]. The prognostic value of this surrogate subtyping has been confirmed for patients with locally advanced noninflammatory breast cancer [8] and for those with early-stage disease [9, 10]. However, this subtyping approach has not been evaluated in patients with inflammatory breast cancer (IBC). Although IBC is considered one of the most aggressive forms of breast cancer, subcategories of IBC can be distinguished by the same molecular subtypes defined for non-IBC [11, 12]. Information on the influence of ER/PR and HER-2 status and breast cancer subtype on clinical outcome in IBC would aid in management decision making and counseling patients about anticipated outcomes.
Although most studies of patients with non-IBC receiving systemic treatment tend to focus on endpoints such as distant relapse (DR) and overall survival (OS), several reports have demonstrated that ER/PR and HER-2 status can also predict locoregional recurrence (LRR). For example, data from the Danish 82b trial revealed that ER/PR negativity and HER-2 positivity were associated with a higher LRR rate after postmastectomy radiation [8]. Studies from The University of Texas MD Anderson Cancer Center (hereafter, MD Anderson) and Harvard Massachusetts General Hospital of patients with early-stage breast cancer showed that HER-2 positivity and ER/PR negativity were predictive of a higher LRR rate after breast-conserving therapy [9, 13]. However, the potential influence of ER/PR and HER-2 status on locoregional control has not been evaluated in patients with IBC. Including locoregional control as an endpoint is important because it may help identify patients who would benefit from new locoregional treatments such as accelerated hyperfractionation or radiosensitizers.
We retrospectively analyzed the impact of breast cancer subtypes, defined by ER/PR and HER-2 status, on the outcomes of 316 women treated at MD Anderson for newly diagnosed, nonmetastatic IBC. The primary endpoints were the 5-year LRR, DR, and OS rates.
Materials and Methods
Patients
This retrospective chart review was approved by the institutional review board of MD Anderson. Chart review of patients presenting to MD Anderson in January 1974 through December 2008 identified 433 patients with IBC whose ER, PR, and HER-2 status was available and who had no distant metastases at the time of diagnosis (stage IIIB–IIIC). These included two consecutive cohorts of patients, with the first one presenting before April 2005 [14]. All the pathologic specimens from those patients were prospectively reviewed at MD Anderson by pathologists specializing in breast cancer [14]. The second cohort came from an institutional registry of patients diagnosed in May 2005 through December 2008. Patients who had recurrent disease at their first presentation to MD Anderson were excluded, leaving 316 patients diagnosed in June 1989 to December 2008 for the present study. For all patients, the diagnosis of IBC was made by a multidisciplinary team.
Pathology Methods
All cancer diagnoses were confirmed by core biopsy. ER/PR status was obtained by immunohistochemical staining of paraffin-embedded tissues with monoclonal antibodies (6F11 for ER and 1A6 for PR; Novacastra Laboratories Ltd., Newcastle Upon Tyne, U.K.). Nuclear staining of ≥10% of invasive cells was considered positive. Before 1993, ER and PR status were determined by a dextran-coated charcoal ligand-binding method. HER-2 status was evaluated by immunohistochemical staining or fluorescence in situ hybridization. HER-2 positivity was defined as 3+ receptor overexpression (strong membranous staining in ≥30% of cells) or gene amplification (a gene copy ratio of HER-2/CEP-17 >2.0).
Treatment and Follow-Up
The evolution of treatment for nonmetastatic IBC at MD Anderson over the past four decades has been described elsewhere [15]. Most patients received neoadjuvant chemotherapy, modified radical mastectomy, and postmastectomy radiation to the chest wall and draining lymphatics. Neoadjuvant chemotherapy consisted of 5-fluorouracil, doxorubicin, and cyclophosphamide, with taxanes introduced in 1994. Tamoxifen or aromatase inhibitors were used for patients with hormone receptor–positive disease, and HER-2–directed therapy (trastuzumab or lapatinib) was used since 1999 for HER-2+ cancer. Regarding radiation, most patients received 51 Gy in 1.5-Gy fractions delivered twice daily to the chest wall and draining lymphatics, followed by a 15-Gy boost, also in 1.5-Gy fractions delivered twice daily, bringing the total dose to 66 Gy. Many patients also received adjuvant chemotherapy.
Patients were followed on a regular basis after completion of treatment (every 6 months for 5 years and then yearly). From 1989 to mid-2006, follow-up studies included physical examination, biopsy, sonography, computed tomography (CT) scan, bone scan, and (after October 2006) positron emission tomography imaging for the diagnosis of LRR. Tests used for suspected distant metastasis (DM) included CT scanning, bone scanning, liver function tests, and alkaline phosphatase level measurements.
Statistical Analysis
The primary endpoints in this study were the LRR, DR, and OS rates. LRR was defined as any recurrence within the ipsilateral chest wall or regional lymphatics including axillary, supraclavicular, and internal mammary nodes. Recurrences in the contralateral breast were considered distant if contralateral nodes were involved; otherwise locoregional was distinguished from distant recurrence based on the clinical history and distribution of disease according to physical examinations and medical photography. Time to recurrence was computed from the date of diagnosis to the date of first local or distant disease recurrence. Patients without recurrence were censored at the last follow-up date. Patients who died without experiencing disease recurrence were censored at the date of death, except for the OS endpoint.
χ2 tests were used to compare the distribution of baseline characteristics among the four subgroups. Time to recurrence or death was estimated by the Kaplan–Meier method [16, 17] and compared between groups with log-rank tests. Univariate and multivariate analyses of time to event were performed using a Cox proportional hazards model. Only variables with a p-value <.25 on univariate analysis, except for hormone receptor status and hormone therapy because of their high linear correlation with molecular subtypes, were entered into the multivariate analysis model, and backward sequencing was used [18]. The variables analyzed were age at diagnosis (<45 years versus ≥45 years), menopausal status (premenopausal versus perimenopausal versus postmenopausal), lymphovascular invasion (present or absent), tumor nuclear grade (grade 3 versus grade 1–2), hormone receptor expression (positive or negative), HER-2 expression (positive or negative), percentage of positive nodes (≥20% versus <20%), extracapsular extension (ECE) (present or absent), margin status (close or positive versus negative), pathologic complete response (pCR) (yes versus no), neoadjuvant chemotherapy (yes versus no), taxane (yes versus no), hormone therapy (yes versus no), HER-2–directed therapy as initial treatment (yes versus no), and radiation (yes versus no). An identical method was used for time to OS, LRR, and DR. All statistical tests were two-sided, with p-values <.05 considered significant. All calculations were performed using Stata/MP 11.1 statistical software (StataCorp, College Station, TX).
Results
Patient Characteristics
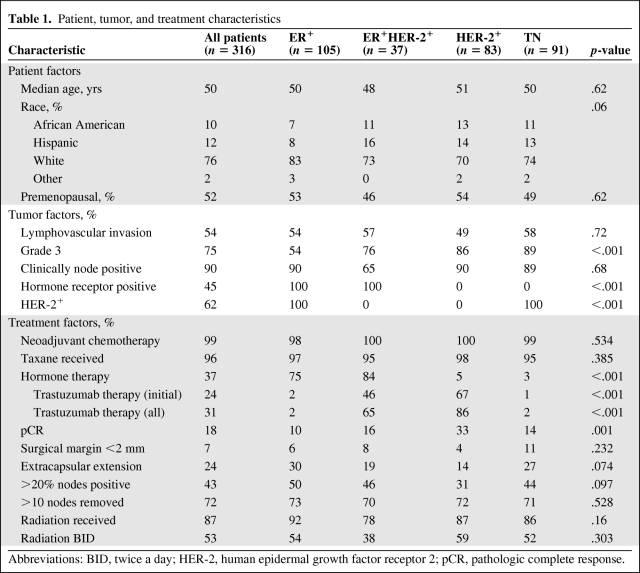
As shown in Table 1, 105 patients (33%) had ER+ disease, 37 (12%) had ER+HER-2+ disease, 83 (26%) had HER-2+ disease, and 91 (29%) had TN disease. The median age was 50 years (range, 24–83 years). Three quarters of the patients were white and roughly half were premenopausal. The four subgroups did not differ in age, race, or menopausal status. As for tumor-related factors, 54% of the tumors showed lymphovascular invasion (LVI) and 90% of the patients had clinically node-positive disease; these characteristics were well balanced among the four subgroups. Subgroups did differ in nuclear grade, hormone receptor positivity, and HER-2 positivity.
Table 1.
Patient, tumor, and treatment characteristics
Abbreviations: BID, twice a day; HER-2, human epidermal growth factor receptor 2; pCR, pathologic complete response.
Nearly all patients received neoadjuvant chemotherapy and most (96%) received a taxane. More than 80% of patients with hormone receptor–positive disease received hormone therapy, and those who did not were either unable to complete the therapy or had disease progression before completing definitive treatment. In HER-2+ patients, 62% received trastuzumab or lapatinib under a clinical trial as part of their initial systemic therapy (46% for ER+HER-2+ disease and 67% for HER-2+ disease). Those who did not receive HER-2–directed therapy did not because the treatment was unavailable at the time their disease was diagnosed. About 87% of patients received radiation therapy with curative intent, and two thirds of these received twice-daily treatment.
The pCR rate to neoadjuvant systemic therapy was 18%, and was highest in the HER-2+ group (33% overall, 37% for those who received HER-2–directed therapy, and 16% for those who did not). Overall, 7% of patients had close or positive surgical margins, and 24% had ECE. About 43% of the patients had disease in >20% of the removed lymph nodes. The HER-2+ subgroup was least likely to have positive margins, ECE, or nodal disease, probably because of a favorable response to neoadjuvant treatment.
After a median follow-up time of 38 months for living patients (range, 2–164 months) and 33 months for all patients (range, 2–185 months), 55 patients had had an LRR, 129 had had a DR, and 116 had died. Thirty-eight patients experienced both an LRR and a DR (19 synchronously and seven within a month of one another; eight patients had DM after LRR [interval, 4–14 months] and four patients had DM before LRR [interval, 3–18 months]). Six of those patients had an LRR after a previous DR.
OS
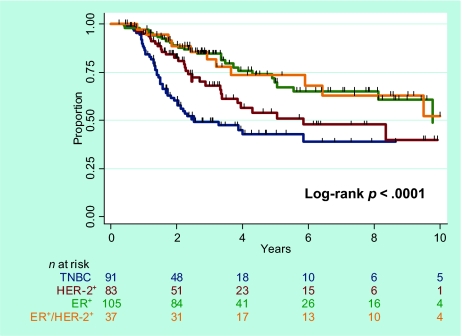
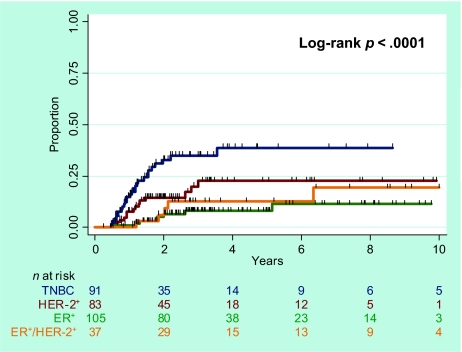
The median OS times were 33 months for the entire cohort (range, 2–185 months), 38 months for the ER+ subgroup, 40 months for the ER+HER-2+ subgroup, 29 months for the HER-2+ subgroup, and 24 months for the TN subgroup. The 5-year actuarial survival rates (Fig. 1) were as follows: for the entire study cohort, 58.7% (95% confidence interval [CI], 51.8%–64.9%); for the ER+ subgroup, 69.7% (95% CI, 57.0%–79.3%); for the ER+HER-2+ subgroup, 73.5% (95% CI, 53.3%–86.1%); for the HER-2+ subgroup, 54.0% (95% CI, 39.4%–66.5%); and for the TN subgroup, 42.7% (95% CI, 30.9%–54.1%) (p < .0001, log-rank tests). Pairwise comparisons showed that survival was worse for the TN subgroup than for the other three subgroups (p < .0001-.03). Survival in the HER-2+ subgroup was different from that in the ER+ subgroup (p = .03). No difference in survival was observed between the ER+ and ER+HER-2+ subgroups (p = .961).
Figure 1.
Overall survival according to breast cancer subtype.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
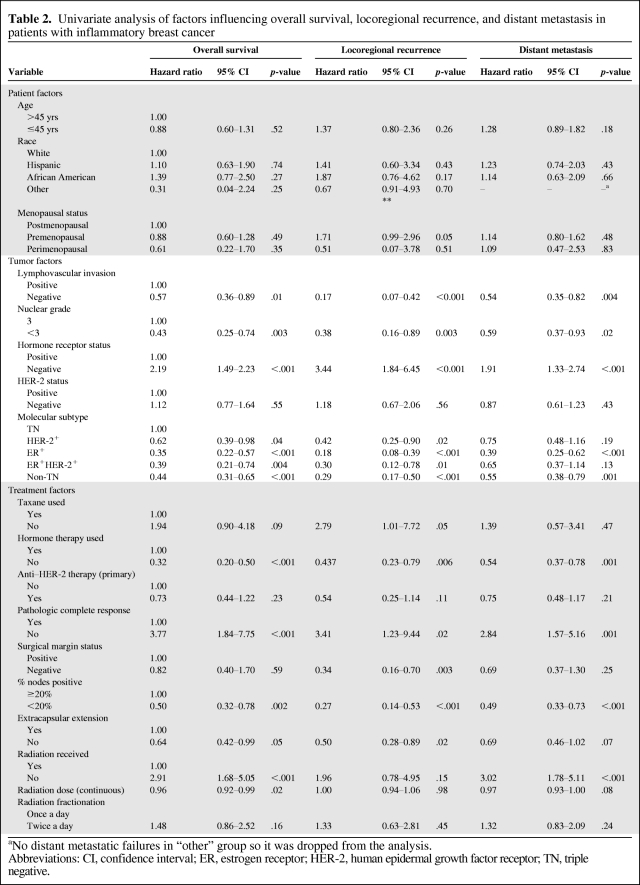
On univariate analysis, using TN as the baseline (Table 2), all three of the other subtypes were associated with a better survival outcome, with unadjusted hazard ratios (HRs) of 0.35 for ER+ patients (p < .001), 0.39 for ER+HER-2+ patients (p = .004), and 0.62 for HER-2+ patients (p = .04). When the ER+HER-2+ subgroup was used the baseline, ER+ patients did not have a statistically different survival outcome from that of ER+HER-2+ patients. Other factors prognostic for a poor OS outcome included having LVI, nuclear grade 3, no hormone receptor expression, no hormone therapy, no pCR, ECE, ≥20% of nodes positive, and no or low-dose radiation.
Table 2.
Univariate analysis of factors influencing overall survival, locoregional recurrence, and distant metastasis in patients with inflammatory breast cancer
aNo distant metastatic failures in “other” group so it was dropped from the analysis.
Abbreviations: CI, confidence interval; ER, estrogen receptor; HER-2, human epidermal growth factor receptor; TN, triple negative.
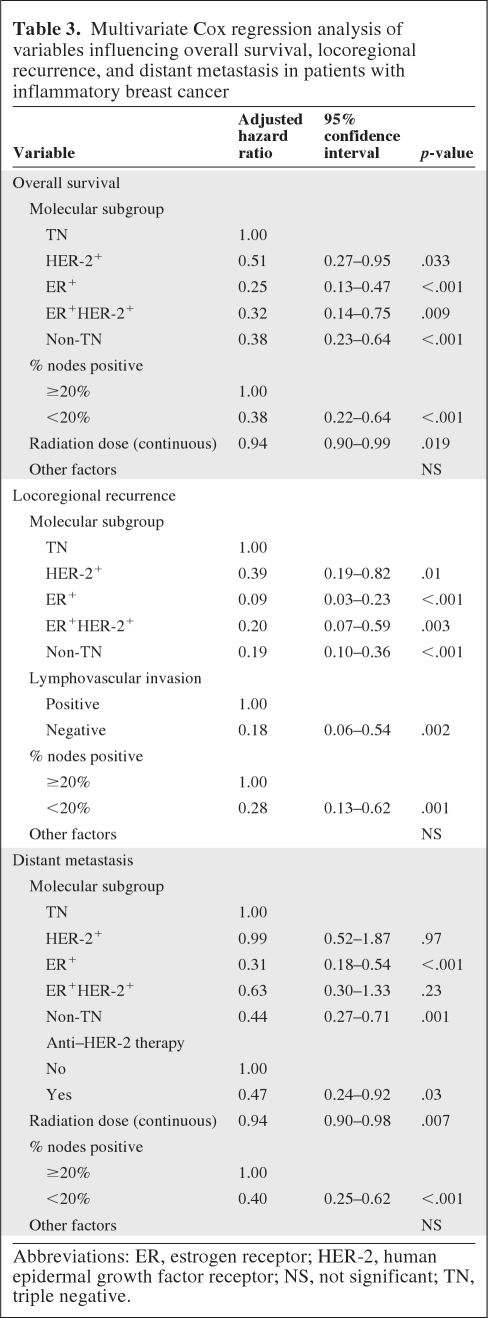
On multivariate analysis after adjusting for prognostic factors, the TN subgroup was found to have a worse survival outcome than the other three subtypes: adjusted hazard ratios (AHRs) for death were 0.25 for ER+ patients (p < .001); 0.32 for ER+HER-2+ patients (p = .009), and 0.51 for HER-2+ patients (p = .033). When the non-TN subtypes (ER+, ER+HER-2+, and HER-2+ combined) were compared with the TN subgroup, patients with the non-TN subtypes had a better survival outcome (AHR, 0.38; p < .001). In the final model, the percentage of positive lymph nodes (<20% versus ≥20%) after neoadjuvant chemotherapy and radiation dose remained prognostic for survival (Table 3).
Table 3.
Multivariate Cox regression analysis of variables influencing overall survival, locoregional recurrence, and distant metastasis in patients with inflammatory breast cancer
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor; NS, not significant; TN, triple negative.
LRR
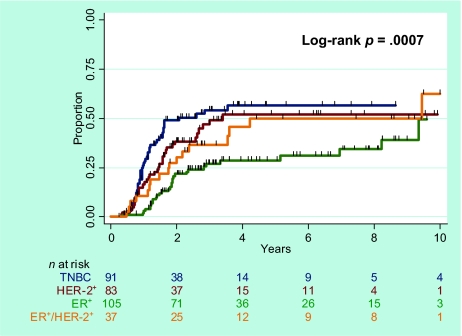
At a median follow-up time of 33 months (range, 2–184 months) for the 316 patients, 55 had had an LRR, and 38 of those patients had also experienced a DR. The median time to LRR was 36 months for the ER+ subgroup, 35 months for the ER+HER-2+ subgroup, 26 months for the HER-2+ subgroup, and 19 months for the TN subgroup. The 5-year LRR rates (Fig. 2) were 20.3% (95% CI, 15.7%–26.1%) for all patients, 8.0% (95% CI, 3.8%–16.1%) for ER+ patients, 12.6% (95% CI, 4.9%–30.1%) for ER+HER-2+ patients, 22.6% (95% CI, 13.5%–36.5%) for HER-2+ patients, and 38.6% (95% CI, 27.3%–52.6%) for the TN subgroup (p < .0001, log-rank test). Pairwise comparisons showed a higher LRR rate in the TN subgroup than in the other three subgroups (p < .0001–.023), but the LRR rate was no different in the ER+ and ER+HER-2+ subgroups (p = .393).
Figure 2.
Rate of locoregional recurrence according to breast cancer subtype.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
On Cox univariate analysis using TN disease as the baseline (Table 2), the ER+, ER+HER-2+, and HER-2+ subtypes were associated with a lower LRR rate (unadjusted HRs of 0.18 [p < .001], 0.30 [p = .01], and 0.42 [p = .02], respectively). When the ER+HER-2+ subgroup was used as the baseline, the LRR rate of ER+ patients was not statistically different from that of ER+HER-2+ patients. Other factors prognostic of a higher LRR rate included premenopausal status, having LVI, nuclear grade 3, no hormone receptor expression or hormone therapy, no taxane or radiation, no pCR, a positive surgical margin, ≥20% of nodes positive, and ECE.
Multivariate analysis after adjusting for prognostic factors also showed the TN subgroup to have the highest LRR rate (AHR, 0.09 for the ER+ subgroup, 0.20 for the ER+HER-2+ subgroup, and 0.39 for the HER-2+ subgroup). When non-TN (ER+, ER+HER-2+, and HER-2+ combined) patients were compared with the TN subgroup, patients with non-TN subtypes had a lower LRR rate (AHR, 0.19; p < .001). In the final model, LVI and ≥20% of nodes positive were prognostic for a worse LRR outcome (Table 3).
DR
Distant failure occurred in 129 of the 316 patients. The median time to DR was 26 months for the entire cohort, 34 months for the ER+ subgroup, 31 months for the ER+HER-2+ subgroup, 22 months for the HER-2+ subgroup, and 19 months for the TN subgroup. The 5-year DR rates were 45.5% (95% CI, 39.4%–52%) for the whole cohort, 28.9% (95% CI, 20.4%–39.6%) for the ER+ subgroup, 50.1% (95% CI, 33.5%–69.4%) for the ER+HER-2+ subgroup, 52.1% (95% CI, 39.9%–65.5%) for the HER-2+ subgroup, and 56.7% (95% CI, 45.6%–68.3%) for the TN subgroup (p < .001, log-rank test) (Fig. 3). Pairwise comparisons showed a higher DR rate for the TN subgroup than for the ER+ subgroup (p < .0001) but no differences between the TN subgroup and the other two subgroups or between the ER+ subgroup and the ER+HER-2+ subgroup (p = .11).
Figure 3.
Rate of distant relapse according to breast cancer subtype.
Abbreviations: ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
On Cox univariate analysis using the TN subtype as the baseline, having ER+ disease was associated with a lower DR rate, with an unadjusted HR of 0.39 (p < .001). Although the DR rate seemed lower in the ER+HER-2+ and HER-2+ subgroups (HR, 0.75 and 0.65, respectively), this was not significant. However, the DR rate for non-TN patients (combined ER+, ER+HER-2+, and HER-2+) was significantly lower than that for TN patients (HR, 0.55; p = .001). When the ER+HER-2+ subtype was used as the baseline, the DR rate was not different for the ER+ and ER+HER-2+ subgroups. Other factors prognostic of a higher DR risk included nuclear grade 3, LVI, no hormone receptor expression or hormone therapy, no radiation, no pCR, and ≥20% of nodes positive (Table 2).
On multivariate analysis after adjusting for prognostic factors, the DR risk was higher for patients with the TN subtype than for those with the ER+ subtype (AHR, 0.31; p < .001). The DR risk was no different between the TN and ER+HER-2+ or HER-2+ subgroups. However, having non-TN (ER+, ER+HER-2+, or HER-2+) disease was associated with a lower DR risk (AHR, 0.44; p = .001). In the final model, anti–HER-2 therapy, percent nodes positive, and radiation dose were predictive for DR.
Discussion
The predictive and prognostic values of ER, PR, and HER-2 status have been reported for early and advanced breast cancer [8–10, 19, 20], but to our knowledge, this is the first report of the significance of these surrogate markers in IBC patients, with clear links between breast cancer subtype and OS and LRR outcomes. These findings are consistent with a study by Bertucci et al. [11] in which gene expression profiling was used to confirm the presence of molecular subtypes in IBC; however, that study had only 37 IBC patients and did not correlate subtype with outcome. Our results suggest that stratification of breast cancer subtypes through routine analysis of ER, PR, and HER-2 status is clinically useful for estimating survival and recurrence in patients with IBC.
Breast cancer subtyping, in addition to being predictive of survival and metastasis, is also prognostic for local recurrence in non-IBC patients [8, 9, 13, 20]. We showed that breast cancer subtypes can be prognostic for LRR in IBC patients; this finding suggests that, even though IBC is often considered a systemic disease, the risk for LRR remains an important clinical problem, particularly for patients with TN IBC. Therefore, locoregional treatment intensification should be considered on an individual basis. We previously found that accelerated hyperfractionated radiotherapy (66 Gy at 1.5 Gy per fraction delivered twice daily versus 60 Gy at 2 Gy per fraction once daily) led to better local control of IBC [21], but this came at the cost of late skin toxicity (29% versus 15%) [22], leading to the recommendation that patients at low risk for recurrence (e.g., aged >45 years, with taxane chemotherapy and a good response to neoadjuvant chemotherapy) be given conventionally fractionated radiation [22]. In this regard, a recent report from Memorial Sloan-Kettering Cancer Center showed a 5-year LRR rate of 13% using once-daily radiation for patients treated after 1995 [23], which is only slightly higher than the 8% LRR rate in patients treated at MD Anderson after 1994 [22]. The results of the current study provide the rationale for trials of conventional radiation treatment for selected ER+ subtypes, for patients with ER+HER-2+ disease receiving HER-2–directed therapy, and for intensifying radiation with or without a concurrent radiosensitizer in patients with TN cancer.
TN IBC, like its non-IBC counterpart, is particularly challenging to treat, given its aggressiveness and the ineffectiveness of endocrine and anti–HER-2 therapy. Efforts to identify subtype-specific potential targets have led to epidermal growth factor receptor (EGFR), Src, and Ras signaling [24] and patterns of kinase overexpression in ER− tumors; the latter study showed that tumors that overexpress a cluster of genes controlling the S6 kinase pathway were associated with an extremely poor prognosis [25]. Clinical trials of EGFR inhibitors for metastatic TN breast cancer are under way, and MD Anderson is initiating a phase II study with panitumumab as part of the preoperative chemotherapy regimen for patients with primary IBC without HER-2 overexpression. Other possible agents that may be tested for TN disease include Hedgehog inhibitors, the Src kinase inhibitor dasatinib, the mammalian target of rapamycin inhibitor everolimus [26], and inhibitors of nuclear factor-κB [27], cyclin E, and Skp2 [28].
Given the poor survival rates associated with IBC despite the advent of trimodality therapy (5-year OS, 59%), new IBC-specific molecular targets are being sought to improve the outcome of patients with IBC. Efforts undertaken to date revealed three potential markers that are overexpressed in IBC relative to non-IBC: RhoC, a GTPase involved in cytoskeletal reorganization [29]; WISP3, a tumor suppressor gene [29, 30, 31]; and eIF4G1, a translation-initiation factor that promotes IBC tumor cell survival and perhaps metastasis [32, 33]. These markers may eventually serve as IBC-specific therapeutic targets.
A limitation of our study is its retrospective nature and consequent patient and treatment selection bias and variability in prognostic factors. However, this limitation may be alleviated to some extent by the large sample size of our cohort and significant number of events. Recently, a multinational registry was created at MD Anderson for the intent of prospectively establishing risk factors and prognostic factors for IBC. Another limitation of our study is the classification of breast cancer using immunohistochemically based surrogate markers (ER, PR, and HER-2), which provides only an approximation of genotype-identified molecular subgroups. Recent studies have shown that newer immunohistochemically based markers, such as Ki-67 [2–4, 34], EGFR, and CD5/CD6 [19, 35], can be used to further refine breast cancer classification. Despite the shortcomings of using receptor status to classify subtypes, receptor status is readily obtained and much less expensive and time-consuming than gene profiling. At this time, it seems to yield clinically relevant information on prognosis that may help in guiding therapy. Another potential limitation was our use of 10% staining as a cutoff value for classifying ER expression. In 2010, the American Society of Clinical Oncology and College of American Pathologists guidelines were changed to recommend that <1% be used to define ER negativity. Thus, our findings may not necessarily represent patients with ER− disease diagnosed after 2010. Finally, at 38 months for living patients, our median follow-up time was fairly short, particularly given the tendency of ER+ breast cancers to recur >5 years after treatment. Because TN disease tends to recur within the first 5 years, the short follow-up may have influenced the differences in the LRR that we found. However, the median time to LRR for the two ER+ subgroups (ER+ and ER+HER-2+) were 36 months and 35 months, respectively, whereas the median time to LRR for the two ER− subgroups (HER-2+ and TN) were 26 months and 19 months, respectively. This difference strongly supports the argument that ER negativity predicts early LRR and may explain, in part, the higher 5-year LRR rate among ER− patients. Moreover, ER negativity seems to predict a higher rate of LRR at longer follow-up times as well. For example, at 8 years, the LRR rates in our study remained high for ER− patients (39% for TN patients, 23% for HER-2+ patients), compared with ER+ patients (13% for ER+ patients and 19% for ER+HER-2+ patients). One other observation that is worth commenting on is that the pCR rate in the TN cohort in our study was only 14%, despite 96% of patients receiving a taxane. This appears low relative to published literature, wherein the pCR rates for TN patients generally are >25% in non-IBC patients. The lower pCR rate in TN patients diagnosed with IBC likely reflects the aggressive nature of IBC and its resistance to therapy. This notion is supported by a study that included 40% IBC patients, in which the pCR rate was only 9.5% [36].
In summary, our findings indicate that breast cancer subtype, as approximated by the surrogate markers ER, PR, and HER-2, is significantly associated with OS and LRR outcomes in patients with IBC. The TN subtype consistently predicted the worst outcomes. This information may be useful in determining the aggressiveness of therapies aimed at controlling distant and locoregional disease. It also underscores the need to understand the biology underlying the aggressiveness of IBC and to identify new molecular targets specific to IBC, especially TN IBC.
Acknowledgments
We would like to thank Christine Wogan for her excellent editorial assistance.
The study was supported in part by funds from the State of Texas for “Rare and Aggressive Diseases.” The MD Anderson Breast Cancer Management System is supported in part by the Nellie B. Connally Breast Cancer Research Fund.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo, Tse K. Yu, Wendy A. Woodward
Provision of study material or patients: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo, Tse K. Yu, Wendy A. Woodward, Naoto T. Ueno, Anthony Lucci, Savitri Krishnamurthy, Yun Gong, Melissa L. Bondy, Wei Yang, Jie S. Willey, Massimo Cristofanilli, Vicente Valero
Collection and/or assembly of data: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo, Jie S. Willey, Massimo Cristofanilli, Vicente Valero
Data analysis and interpretation: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo, Pamela K. Allen, Naoto T. Ueno, Yun Gong, Massimo Cristofanilli
Manuscript writing: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo
Final approval of manuscript: Thomas A. Buchholz, Jing Li, Ana M. Gonzalez-Angulo, Pamela K. Allen, Tse K. Yu, Wendy A. Woodward, Naoto T. Ueno, Anthony Lucci, Savitri Krishnamurthy, Yun Gong, Melissa L. Bondy, Wei Yang, Jie S. Willey, Massimo Cristofanilli, Vicente Valero
References
- 1.Huber KE, Carey LA, Wazer DE. Breast cancer molecular subtypes in patients with locally advanced disease: Impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol. 2009;19:204–210. doi: 10.1016/j.semradonc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 8.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. [DOI] [PubMed] [Google Scholar]
- 12.Van Laere SJ, Van den Eynden GG, Van der Auwera I, et al. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- 13.Albert JM, Gonzalez-Angulo AM, Guray M, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 2010;77:1296–1302. doi: 10.1016/j.ijrobp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Angulo AM, Hennessy BT, Broglio K, et al. Trends for inflammatory breast cancer: Is survival improving? The Oncologist. 2007;12:904–912. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 15.Bristol IJ, Buchholz TA. Inflammatory breast cancer: Current concepts in local management. Breast Dis. 2005;22:75–83. doi: 10.3233/bd-2006-22109. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:547–581. [Google Scholar]
- 17.Thames HD, Buchholz TA, Smith CD. Frequency of first metastatic events in breast cancer: Implications for sequencing of systemic and local-regional treatment. J Clin Oncol. 1999;17:2649–2658. doi: 10.1200/JCO.1999.17.9.2649. [DOI] [PubMed] [Google Scholar]
- 18.Cox DR, Oakes N. Analysis of Survival Data. New York: Chapman and Hall; 1984. pp. 1–110. [Google Scholar]
- 19.Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 20.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 21.Liao Z, Strom EA, Buzdar AU, et al. Locoregional irradiation for inflammatory breast cancer: Effectiveness of dose escalation in decreasing recurrence. Int J Radiat Oncol Biol Phys. 2000;47:1191–1200. doi: 10.1016/s0360-3016(00)00561-7. [DOI] [PubMed] [Google Scholar]
- 22.Bristol IJ, Woodward WA, Strom EA, et al. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:474–484. doi: 10.1016/j.ijrobp.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damast S, Ho AY, Montgomery L, et al. Locoregional outcomes of inflammatory breast cancer patients treated with standard fractionation radiation and daily skin bolus in the taxane era. Int J Radiat Oncol Biol Phys. 2010;77:1105–1112. doi: 10.1016/j.ijrobp.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Bild AH, Parker JS, Gustafson AM, et al. An integration of complementary strategies for gene-expression analysis to reveal novel therapeutic opportunities for breast cancer. Breast Cancer Res. 2009;11:R55. doi: 10.1186/bcr2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speers C, Tsimelzon A, Sexton K, et al. Identification of novel kinase targets for the treatment of estrogen receptor-negative breast cancer. Clin Cancer Res. 2009;15:6327–6340. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurebayashi J. Possible treatment strategies for triple-negative breast cancer on the basis of molecular characteristics. Breast Cancer. 2009;16:275–280. doi: 10.1007/s12282-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi N, Ito T, Azuma S, et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci. 2009;100:1668–1674. doi: 10.1111/j.1349-7006.2009.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voduc D, Nielsen TO, Cheang MC, et al. The combination of high cyclin E and Skp2 expression in breast cancer is associated with a poor prognosis and the basal phenotype. Hum Pathol. 2008;39:1431–1437. doi: 10.1016/j.humpath.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 29.van Golen KL, Davies S, Wu ZF, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- 30.Houchens NW, Merajver SD. Molecular determinants of the inflammatory breast cancer phenotype. Oncology (Williston Park) 2008;22:1556–1561. discussion 1561, 1565–1568, 1576. [PubMed] [Google Scholar]
- 31.Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110–R115. doi: 10.1186/bcr755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvera D, Arju R, Darvishian F, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 33.Kleer CG, van Golen KL, Braun T, et al. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2001;14:458–464. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- 34.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 36.Gogas H, Pectasides D, Kostopoulos I, et al. Paclitaxel and carboplatin as neoadjuvant chemotherapy in patients with locally advanced breast cancer: A phase II trial of the Hellenic Cooperative Oncology Group. Clin Breast Cancer. 2010;10:230–237. doi: 10.3816/CBC.2010.n.031. [DOI] [PubMed] [Google Scholar]