Gastric cancer patients undergoing curative-intent surgical resection were evaluated to assess the prognostic value of human epidermal growth factor receptor 2 overexpression/amplification. Human epidermal growth factor receptor 2 was not found to be an independent prognostic factor.
Keywords: Gastric cancer, HER-2, Prognosis, IHC, FISH
Abstract
Background.
Opinions regarding the impact of human epidermal growth factor receptor (HER)-2 overexpression or HER-2 amplification on the prognosis of gastric cancer patients are mixed. The present study attempted to clarify this issue by investigating a large cohort of surgical patients.
Methods.
We investigated 1,036 gastric cancer patients undergoing curative-intent resection. Their surgical specimens were evaluated for HER-2 expression by immunohistochemistry (IHC), and those with HER-2 expression levels of 2+ were additionally subjected to fluorescence in situ hybridization (FISH). Data on demographic and clinicopathological features and relevant prognostic factors in these patients were analyzed.
Results.
HER-2 positivity was noted in 64 (6.1%) of 1,036 gastric cancer patients, including 46 patients whose HER-2 expression level was 3+ on IHC and 18 patients whose FISH results were positive. On univariate analysis, HER-2 positivity was more often associated with differentiated histology, intestinal type, and negative resection margins, whereas only differentiated histology was independently associated with HER-2 positivity in a logistic regression model. For stage I–IV gastric cancer, HER-2 was not a prognostic factor. In a subpopulation study, although HER-2 positivity emerged as a favorable prognostic factor for stage III–IV gastric cancer on univariate analysis, it failed to be an independent prognostic factor after multivariate adjustment.
Conclusions.
The prevalence of HER-2 positivity, determined using standardized assays and scoring criteria in a large cohort of gastric cancer patients after resection, was 6.1%. HER-2 positivity was phenotypically associated with differentiated histology. HER-2 is not an independent prognostic factor for gastric cancer.
Introduction
The overall survival outcome of patients with gastric cancer continues to improve since the introduction of multidisciplinary approaches and identification of novel targeted agents; however, gastric cancer remains the fourth most commonly diagnosed cancer and the second leading cause of cancer-related deaths worldwide [1, 2]. Most patients presenting with inoperable advanced or metastatic disease require palliative treatment. Combination chemotherapy regimens, including fluoropyrimidine-based and platinum-based regimens with or without a third drug such as docetaxel or epirubicin, are the most widely used and the most effective therapeutic agents for advanced gastric cancer, which afford a median survival duration of 8–11 months [3, 4].
Human epidermal growth factor receptor (HER)-2 (also known as ErbB-2) encodes a 185-kDa transmembrane tyrosine kinase receptor. It is involved in tumor cell proliferation, adhesion, migration, apoptosis, and differentiation [5]. Overexpression of HER-2 has been found to promote tumor growth and play a role in the pathogenesis of several human cancers [6]. HER-2 is generally accepted to be a poor prognostic marker in breast cancer [7]. However, the impact of HER-2 status on the prognosis of gastric cancer patients remains controversial [5, 8–11]. Furthermore, the reported rates of HER-2 positivity in gastric cancer range widely [11, 12]. The aims of this study were to elucidate the percentage of HER-2 positivity in Taiwanese gastric cancer patients, to perform a detailed analysis of the association between HER-2 and clinicopathological factors, and to determine the impact of HER-2 status on the survival of gastric cancer patients undergoing curative-intent resection.
Materials and Methods
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) studies were performed on the tissue samples of 1,036 gastric cancer patients who underwent curative-intent resection at Chang Gung Memorial Hospital, Taoyuan, Taiwan, between January 1999 and May 2007.
Clinicopathological Variables
Data on clinicopathological parameters such as patients' demographic characteristics, tumor location, type of operation, pathological findings, and tumor stage were obtained from the medical records of the patients. The tumors were staged according to the sixth edition of American Joint Committee on Cancer staging system [13]. Clinical outcome was evaluated from the date of surgery to the date of the last follow-up (December 31, 2008). Cases of surgical mortality were excluded from the survival analysis.
Tissue Microarrays and IHC
Formalin-fixed and paraffin-embedded tissue samples were arrayed using an automated tissue-arraying instrument (Beecher ATA-27; Beecher Instruments, Sun Prairie, WI). Three representative areas of each tumor were selected and marked on the tissue slide on which hematoxylin-eosin staining was performed, and the corresponding tissue block for each tumor was sampled. The designated zone from each donor block was punched with a tissue cylinder of 1-mm diameter, and the sample was transferred to a recipient block. The arrayed tissue sections were used for IHC studies of HER-2 (1:200, A485; Dako, Carpinteria, CA). Tissue sections of 3-μm thickness were deparaffinized in xylene and rehydrated in a graded ethanol series. The IHC study was performed using an automated immunostainer (Bond-Max™; Leica, Wetzlar, Germany). The following scoring system was used: score 0, no membrane staining or staining in <10% of cells; 1+, incomplete membrane staining in >10% of cells; 2+, weak to moderate complete membrane staining in >10% of cells; and 3+, strong and complete membrane or laterobasal staining in >10% of cells [14]. An appropriate positive control (HER-2–overexpressing breast carcinoma) was included in each run, and HER-2 staining was analyzed by two senior pathologists.
FISH
Gastric cancer specimens with a HER-2 expression level of 2+ were further subjected to FISH study. FISH for HER-2 amplification was performed using the PathVysion HER-2 DNA Probe Kit (Abbott Molecular, Des Plaines, IL). Tissue sections of 4-μm thickness were placed onto coated slides, air dried, and baked overnight at 56°C. Sections were deparaffinized in xylene three times for 10 minutes and then immersed in 100% ethanol twice for 5 minutes. After air drying, the slides were treated in paraffin pretreatment solution (Paraffin Pretreatment Kit II; Abbott Molecular/Vysis) for 10 minutes at 80°C, washed with distilled water for 3 minutes at room temperature, and treated with protease solution for 15 minutes at 37°C. Slides were subsequently washed in distilled water for 3 minutes; dehydrated in a series of 70%, 85%, and 100% ethanol; and allowed to dry in air. Next, 10 μL of the probe mixture was applied to the slides in an approximately 4-cm2 area selected from a pure-tumor population. Slides were denatured for 5 minutes at 75°C and hybridized for 16 hours at 37°C in a ThermoBrite™ hybridizer (Abbott Molecular). Excess probe was washed away using 2× saline sodium citrate buffer/0.3% NP-40 at 72°C for 2 minutes, and the nuclei were counterstained with 4′,6′-diamidino-2-phenylindole dihydrochloride/Vectashield (Vector Labs, Burlingame, CA). Slides were analyzed using a multifiltered fluorescence microscope (Olympus BX60; Olympus, Southall, Middlesex, UK) and DP controller software (Olympus) according to the standard procedure. A minimum of 60 cells was independently scored for the presence of signals by two pathologists in a blinded fashion. HER-2 status was scored as the ratio of HER-2 signals to chromosome 17 centromere signals. A ratio >2.0 indicated HER-2 amplification.
Statistical Analysis
Data are presented as mean ± standard deviation for numeric parameters. Clinical records were compared using either Fisher's exact test or Pearson's χ2 test, as appropriate. Clinicopathological variables associated with HER-2 positivity considered to be statistically significant in the univariate analysis (p < .05) were thereafter enrolled in the multivariate analysis using a logistic regression model. Prognostic factors deemed to be of potential importance in the univariate analysis (p < .05) were included in the multivariate analysis using a Cox regression model. A p-value < .05 was considered significant. Statistical analyses were performed with SPSS for Windows, version 13 (SPSS, Inc., Chicago, IL).
Results
Prevalence of HER-2 Overexpression and HER-2 Amplification
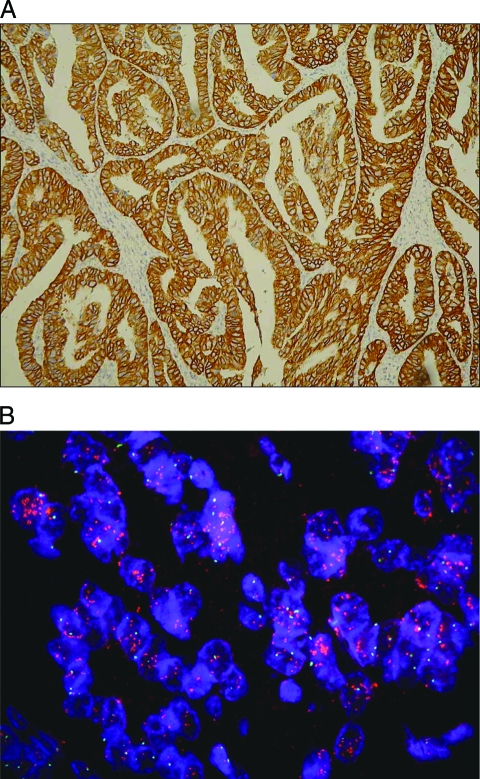
The HER-2 status of protein expression in the 1,036 gastric cancer tissue samples was determined by IHC. Among the samples, 929 (89.7%), 61 (5.9%), and 46 (4.4%) were assigned scores of 0 or 1+, 2+, and 3+, respectively (Fig. 1A). FISH analysis was further performed in samples with HER-2 expression levels of 2+ (n = 61). Among these 61 tumors, 18 (29.5%) were positive for HER-2 amplification (Fig. 1B). Thus, the total HER-2-positivity rate of 1,036 patients with gastric cancer after gastrectomy was 6.1%.
Figure 1.
Evaluation of HER-2 overexpression and HER-2 amplification. (A): HER-2 overexpression evaluated by IHC in well-differentiated intestinal-type gastric carcinoma (IHC score, 3+). (B): FISH targeting HER-2 in gastric cancer specimen. Red-labeled probes targeting HER-2 and green-labeled probes targeting chromosome 17 centromere are stained with DAPI (FISH +). Abbreviations: DAPI, 4′,6′-diamidino-2-phenylindole dihydrochloride; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Association of HER-2 Status with Clinicopathological Variables
Table 1 shows the clinicopathological features of gastric cancer patients classified by HER-2 status. Compared with the HER-2− group, the HER-2+ group had a higher proportion of differentiated tumors (70.3% versus 38.4%; p < .0001), Lauren's intestinal-type cancers (76.6% versus 48.5%; p ≤ .001), and negative resection margins (100.0% versus 91.5%; p = .015). No significant differences were found between the groups in terms of the following factors: age, gender, type of gastrectomy, tumor location, tumor size, depth of tumor invasion, nodal status, stage of disease, extent of lymphadenectomy, perineural invasion, and Helicobacter pylori infection. After logistic regression modeling, only differentiated histology turned out to be independently associated with HER-2 positivity (odds ratio, 3.8; p < .0001) (Table 1).
Table 1.
Clinicopathological variables of gastric cancer patients stratified by HER-2 status
Values in parentheses are percentages, unless otherwise stated.
aThe maximum likelihood estimation does not exist when complete separation of the factor occurs.
bNot all data were available in HER-2− cases.
Abbreviations: CI, confidence interval; EC, esophagocardia; HER-2, human epidermal growth factor receptor 2; HP, Helicobacter pylori; LN ratio, ratio of metastatic to retrieved lymph nodes.
Impact of HER-2 Status on Survival in Gastric Cancer
Regarding stage I–IV gastric cancer patients, univariate analysis followed by multivariate adjustment revealed that age, tumor size, Lauren's classification, nodal status, resection margins, vascular invasion, and Helicobacter pylori infection were independent prognostic factors, whereas HER-2 status was not (Table 2). For the subpopulation study, univariate analysis showed that the following factors significantly affected the prognosis of patients with stage III–IV gastric cancer: type of gastrectomy, tumor location, tumor size, histological differentiation, Lauren's classification, nodal status, pathological staging, ratio of metastatic to retrieved lymph nodes, resection margins, existence of lymphatic, vascular, and perineural invasion, presence of Helicobacter pylori infection, and HER-2 status (p = .025), whereas HER-2 failed to be an independent prognostic factor for stage III–IV gastric cancer patients after multivariate adjustment (p = .399) (Table 3).
Table 2.
Univariate and multivariate analyses of prognostic factors in patients with stage I–IV gastric cancer
aExcluding T1N0M0 cases.
Abbreviations: CI, confidence interval; EC, esophagocardia; HER-2, human epidermal growth factor receptor 2; HP, Helicobacter pylori; LN ratio, ratio of metastatic to retrieved lymph nodes.
Table 3.
Univariate and multivariate analyses of prognostic factors in patients with stage III–IV gastric cancer
Abbreviations: CI, confidence interval; EC, esophagocardia; HER-2, human epidermal growth factor receptor 2; HP, Helicobacter pylori; LN ratio, ratio of metastatic to retrieved lymph nodes; NA, not available.
Discussion
In the present single-center study, we investigated the HER-2 status of the surgical specimens of 1,036 gastric cancer patients using standardized assays and scoring criteria for HER-2 analysis [9, 14]. To the best of our knowledge, this is one of the largest relevant studies worldwide (Table 4). As shown in Table 4, there are large discrepancies in reported HER-2 positivity rates, ranging from 6.1% to 22.6%, even though these data were all published in 2005 or later. The possible reasons for this include geographic variations, tumor heterogeneity, and, most importantly, the employment of different antibodies, interobserver variation, and different scoring systems. In the present study, the rate of HER-2 positivity, which was defined by a HER-2 3+ score on IHC or a HER-2 2+ score on IHC and FISH positivity, was 6.1%, which was lower than in other reports [8, 10, 12, 14–16]. However, there are some pitfalls that suggest these results should be interpreted cautiously. Most investigators regard IHC scores of 2+ and 3+ as HER-2 positivity [5, 10, 12, 16]. For example, Begnami et al. [15] reported a 12% HER-2 positivity rate among 221 gastric cancer samples, which included cases with HER-2 IHC expression levels of 2+ (9%) and 3+ (3%). Obviously, if they had employed our scoring criteria as a definition of HER-2 positivity, the rate of HER-2 positivity would be similar to ours. Furthermore, Hoffman et al. [14] demonstrated a 13.6% (23 of 168) HER-2 positivity rate interpreted by an amended HercepTest™ scoring system using surgical specimens derived from Germany, China, and Mexico. Taken together, we speculate that the reasonable prevalence of HER-2 positivity in gastric cancer using standardized assays and scoring systems is ∼6%–15%.
Table 4.
Literature review of HER-2 overexpression and HER-2 amplification in gastric cancer
Abbreviations: FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; IHC, immunohistochemistry.
Several lines of evidence have shown that HER-2 overexpression or HER-2 amplification is associated with histological differentiation, Lauren's classification, and tumor location [8, 10, 12, 14–16]. The association of this oncogene with a specific histological phenotype indicates that certain characteristics may be expressed preferentially. For example, HER-2 amplification associates inversely with E-cadherin mutations [17], whereas E-cadherin mutations are typical for diffuse gastric cancer but rare in intestinal-type gastric cancer. Our large-scale study reconfirms the observations [5, 10, 12, 15] that differentiated (10.7% versus 3.0%; p < .0001) and intestinal-type (9.4% versus 2.0%; p < .0001) gastric cancers do exhibit a higher rate of HER-2 positivity than their counterparts on univariate analysis; nevertheless, after logistic regression, we identified, for the first time, that differentiated tumor was the only independent pathological variable associated with HER-2 positivity. Interestingly, we observed that HER-2+ tumors exhibited a lower incidence of positive resection margins than HER-2− tumors on univariate analysis (p = .015). The reasonable explanation is that cases with either less histological differentiation or a diffuse type are more liable to have submucosal infiltration of cancer cells and result in microscopically positive resection margins [18]. Meanwhile, less differentiated and diffuse-type tumors exhibited a lower rate of HER-2 positivity. Taken together, we could clinically observe that patients with HER-2+ tumors (usually differentiated and/or intestinal-type tumors) had a lower incidence of positive resection margins.
The tumor–node–metastasis stage is the most important prognostic factor for gastric cancer [13]. However, prognosis varies among patients with the same stage. Therefore, additional classification parameters need to be defined in order to better identify biological subsets in this disease. Biological prognostic factors are often derived from genetic signaling, which represents a crucial step in gastric cancer. The role of HER-2 as a prognostic factor in gastric cancer has been controversial [5, 11, 19, 20]. As shown in Table 4, most investigators reported that HER-2 overexpression or HER-2 amplification indicates a poor survival outcome. However, again, these data should be carefully interpreted. In most circumstances, either the number of patients in the series was too small to be enrolled into a logistic regression model or the definition of HER-2 positivity did not adhere to that of the HercepTest™ scoring system. For example, in the study of Begnami et al. [15], the survival time of gastric cancer patients with HER-2+ tumors was shorter than in those with HER-2− tumors (17 months versus 40 months; p = .023); however, the number of HER-2+ gastric cancer patients was only 11. Our results showed that HER-2 failed to be a prognostic factor for stage I–IV gastric cancer patients undergoing resection. In a subpopulation study, although HER-2 positivity emerged as a favorable prognostic factor for stage III–IV gastric cancer on univariate analysis, it failed to be an independent prognostic factor after multivariate adjustment. Of note, despite the fact that our surgical series was rather large, it was hardly to evaluate the prognostic effect of HER-2 because only 6.1% of the population was HER-2+ in this study. In the future, large consortia worldwide are needed to evaluate the prognostic role of HER-2 in depth.
Studies have shown that trastuzumab (a monoclonal antibody against HER-2) has a remarkable tumor growth inhibition ability in human HER-2+ gastric cancer xenograft models [21]. Additional studies also suggested that trastuzumab plus chemotherapeutic drugs showed antitumor growth activity in a gastric cancer cell line with HER-2 amplification [22]. Recently, the Trastuzumab for Gastric Cancer trial showed that trastuzumab administration in combination with chemotherapy led to a significantly longer median survival time than with chemotherapy alone in patients with HER-2+, inoperable, locally advanced or metastatic gastric cancer (16.0 months versus 11.8 months; hazard ratio, 0.65; p = .036) [9]. This survival gain was statistically significant but was substantially limited when weighed by cost-to-effect analysis. We therefore suggest adopting strict HER-2 scoring criteria (IHC score of 3+ or 2+ and FISH positivity) for selecting gastric cancer candidates subjected to trastuzumab treatment.
In conclusion, the prevalence of HER-2 positivity, determined using standardized assays and scoring criteria in a large cohort of gastric cancer patients after resection, was 6.1%. HER-2 positivity was phenotypically associated with differentiated gastric cancer. HER-2 is not an independent prognostic factor for gastric cancer.
Acknowledgments
This work was partly supported by the Chang Gung Medical Research Program, Taiwan (CMRPG 380161) and grants from the Department of Health, Taiwan (DOH99-TD-C-111-006; PMRPG390071).
The authors thank Shu-Fang Huang for updating the database and performing data analysis.
Jun-Te Hsu and Tse-Ching Chen contributed equally to this manuscript.
Author Contributions
Conception/Design: Ta-Sen Yeh, Tse-Ching Chen
Provision of study material or patients: Ta-Sen Yeh, Jun-Te Hsu, Tse-Ching Chen, Tsann-Long Hwang, Cheng-Tang Chiu, Jeng-Hwei Tseng, Keng-Hao Liu, Chun-Nan Yeh, Yi-Yin Jan
Collection and/or assembly of data: Jun-Te Hsu, Tse-Ching Chen
Data analysis and interpretation: Ta-Sen Yeh, Jun-Te Hsu, Tse-Ching Chen
Manuscript writing: Jun-Te Hsu, Tse-Ching Chen
Final approval of manuscript: Ta-Sen Yeh, Jun-Te Hsu, Tse-Ching Chen, Tsann-Long Hwang, Cheng-Tang Chiu, Jeng-Hwei Tseng, Keng-Hao Liu, Chun-Nan Yeh, Yi-Yin Jan
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28:2430–2436. doi: 10.1200/JCO.2009.26.9654. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 6.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 8.Tanner M, Hollmén M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: Association with topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 9.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 10.Barros-Silva JD, Leitão D, Afonso L, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology. 2010;78:26–33. doi: 10.1159/000288295. [DOI] [PubMed] [Google Scholar]
- 12.Kim MA, Jung EJ, Lee HS, et al. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol. 2007;38:1386–1393. doi: 10.1016/j.humpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Greence FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Sixth Ed. New York: Springer; 2002. American Joint Committee on Cancer; pp. 99–103. [Google Scholar]
- 14.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 15.Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinoma: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;22:3030–3036. doi: 10.1200/JCO.2010.33.6313. [DOI] [PubMed] [Google Scholar]
- 16.Park DI, Yun JW, Park JH, et al. Her2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371–1379. doi: 10.1007/s10620-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 17.Berx G, Becker KF, Höfler H, et al. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Yeh CN, Lee HL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol. 2009;16:2738–2743. doi: 10.1245/s10434-009-0616-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee KE, Lee HJ, Kim YH, et al. Prognostic significance of p53, nm23, PCNA and cerbB-2 in gastric cancer. Jpn J Clin Oncol. 2003;33:173–179. doi: 10.1093/jjco/hyg039. [DOI] [PubMed] [Google Scholar]
- 20.Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: Their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331–1339. doi: 10.1007/s00432-009-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, et al. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59:795–805. doi: 10.1007/s00280-006-0337-z. [DOI] [PubMed] [Google Scholar]
- 22.Kim SY, Kim HP, Kim YJ, et al. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008;32:89–95. [PubMed] [Google Scholar]