The various mechanism-based toxicities of targeted agents, including rash associated with epidermal growth factor receptor inhibitors and hypertension/hypothyroidism associated with antiangiogenic drugs, are reviewed with the aim of assessing their utility as potential proof of pharmacodynamic effects or as predictive biomarkers.
Keywords: Molecular targeted therapy, Toxicity, Biomarker, Efficacy
Abstract
In addition to being present in tumor cells, many targets of signal transduction inhibitors are also found in normal tissue. Side effects attributable to the mechanism of action of molecular targeted agents thus represent “on-target” modulation in normal tissues. These mechanism-based toxicities can be pharmacodynamic effects of pathway inhibition and, in tumors depending on the inhibited pathway for proliferation, might be biomarkers of efficacy. The development of rash with tyrosine kinase inhibitors or monoclonal antibodies targeting the epidermal growth factor receptor is associated with superior outcomes in lung, head and neck, colorectal, and pancreatic cancer studies. Correlated with superior efficacy in retrospective analyses of large studies in advanced colorectal, breast, and renal cell carcinoma, arterial hypertension as an adverse event of antiangiogenic agents may also be a marker of effective target inhibition. An association between hypothyroidism and the activity of multitargeted tyrosine kinase inhibitors has been identified in renal cell carcinoma patients. Tumor growth addiction to the specific pathway that is effectively targeted may be the link between a mechanism-based toxicity and efficacy. The biological basis for this correlation can be pharmacological, with higher drug exposure being associated with greater toxicity and antitumor activity, and can also be genetic, because single nucleotide polymorphisms play an important role in drug pharmacokinetic and pharmacodynamic processes. Investigators have proposed that interpatient differences and associated toxicities can be exploited for dose selection and titration, and clinical trials are currently exploring intrapatient “dosing-to-toxicity” strategies. Ultimately, the predictive value of a side effect of molecular targeted therapies requires validation in prospective trials.
Introduction
Targeted therapies represent the most promising therapeutic approach currently being developed for the treatment of cancer. They are designed to selectively inhibit a molecular target, and dose recommendations should thus be based not only on toxicities but also on pharmacokinetic (PK) and pharmacodynamic (PD) variables. Consequently, clinicians are facing the challenge of codeveloping biomarkers to assist drug development [1, 2]. The PD effects of a targeted therapy can be molecular (pathway inhibition) or clinical (activity or toxicity). Biomarkers associated with the drug's effect are called PD markers and correlate with proof of mechanism of the agent, not necessarily with response or survival. Biological correlative studies may therefore serve to derive the optimal dose and schedule of a given agent and to determine whether or not it is inducing the intended biological effect. On the other hand, to predict clinical benefit, biomarkers need to have a validated, robust, and reproducible assessment methodologies in addition to clinical qualification in randomized trials that include the selected patient population [2].
Targeted biological agents may or may not have predictable effects on normal tissue. It has been demonstrated that targeted therapies are not exempt from toxicities and oncologists have had to adapt to manage novel side effects, such as rash, hypertension, hypothyroidism, hyperglycemia, and dyslipidemia. Some of these toxicities may be “off-target” effects of the drug, and therefore ideally something to prevent. Others may relate to the drug's pharmacological properties (e.g., hypersensitivity and infusional reactions with monoclonal antibodies [mAbs]) and alternative drugs can be designed if this hinders treatment. Other toxicities are related to the “on-target” effects of the drug and its inhibition of the pathway. This reflects target engagement and is directly related to the drug's mechanism of action. Such toxicities are termed mechanism-based toxicities (MBTs) [3].
Neutropenia is the classical MBT of conventional cytotoxic agents targeting DNA replication. Here, we review various MBTs of targeted agents, including rash associated with epidermal growth factor receptor (EGFR) inhibitors and hypertension/hypothyroidism associated with antiangiogenic drugs. The main interest in reviewing available data for such toxicities lies in their potential use as proof of PD effects in the clinic, and in some cases as predictive markers of efficacy. An understanding of these and other MBTs could aid in the decision-making process of drug development (“go versus no-go” decisions), dosing (“dosing-to-toxicity” studies), side-effect management (should we avoid these toxicities or should we learn how to manage them?), and trial design [2].
Defining MBTs
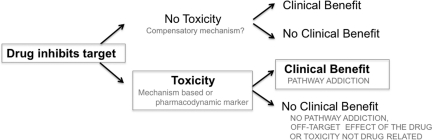
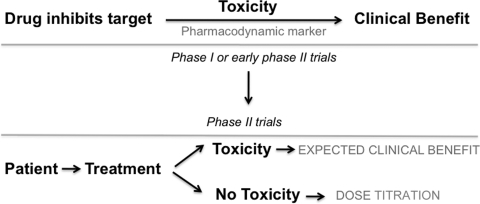
MBT is a side effect attributable to an agent's mechanism of action. It is always a clinical PD effect or marker of a given drug. When analyzing a possible MBT, one should keep in mind the various clinical situations that could occur, as shown in Figure 1. Initially, a targeted therapy with high selectivity and adequate potency to sufficiently inhibit the target is required. Any biological/molecular effect can then be used as surrogate of target inhibition (PD marker). These effects could include toxicities if sufficient rationale and observational data support the relationship and if no other confounding factors are present (i.e., not a result of off-target effects or a toxicity occurring in patients not receiving the drug). When a PD marker is associated with a certain (mechanism-based) toxicity, clinical decisions can be made based on the presence or absence of this event. Further clinical trials could use this marker as a tool for dose titration, as shown in Figure 2.
Figure 1.
Defining mechanism-based toxicity. Toxicities attributable to the mechanism of action of molecular targeted agents represent on-target modulation in normal tissues. These mechanism-based toxicities can be correlated with clinical benefit when the drug has high selectivity and adequate potency to hit the target and the tumor is addicted to the inhibited pathway.
Figure 2.
Translation of mechanism-based toxicities to clinical trials. When a mechanism-based toxicity is strongly associated with a pharmacodynamic marker in the early phases of clinical development, phase II trials could test this biomarker as a tool for dose titration.
In summary, the presence of an MBT can be used as evidence of PD effects if it reflects with certainty pathway inactivation, and therefore assumes sufficient target engagement. It can also be used as a predictive marker in diseases for which pathway inhibition is sufficient to determine clinical activity. Importantly, a clear relationship between the levels of target inhibition in a surrogate tissue and target inhibition in the tumor tissue is lacking for most molecular targeted therapies. Nevertheless, multiple early-phase clinical studies have shown that the development of on-target effects in normal tissues can be directly correlated with pathway inhibition in tumors. It is also critical to state that MBTs can only be used as predictors for outcome after initiating treatment. Therefore, they can be taken as surrogates for further clinical benefit of patients who continue therapy, which is not the perfect scenario. In the following sections, we review current data on side effects that are potential PD and predictive markers as well as the determinants of classical MBTs of molecular targeted agents.
Rash as an MBT of EGFR Inhibitors
EGFR is a tyrosine kinase receptor that is widely expressed in epithelial tumors. Its stimulation leads to activation of multiple pathways involved in cell proliferation and survival. EGFR was one of the first receptors to be proposed as a target for cancer therapy and several anti-EGFR agents have been approved for use, including the tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib and the mAbs cetuximab and panitumumab [4]. These agents have been shown to have efficacy in different clinical scenarios. The most impressive benefits have been found with gefitinib and erlotinib in non-small cell lung cancer (NSCLC) patients, chemotherapy combined with cetuximab in head and neck cancer patients, and cetuximab or panitumumab in colorectal cancer (CRC) patients. Treatment with EGFR inhibitors is frequently associated with an acneiform rash characterized by inflammatory papules and pustules on the scalp, face, neck, and upper trunk. The incidence is in the range of 50%–100%, depending on the agent and cancer type. The median onset is typically within 1–3 weeks of therapy initiation [5]. The skin toxicity of EGFR inhibitors (small molecules TKIs and mAbs) cannot be differentiated clinically in terms of profile or grading, likely representing a class effect.
The mechanism underlying the rash and its correlation with tumor response is still poorly understood. EGFR is highly expressed in normal tissues, including the skin. Rash may represent local receptor saturation, but other factors, such as drug exposure and immune status, may alter an individual's susceptibility. Skin biopsies from patients with drug-induced rash display a robust leukocyte infiltrate. However, systematic analysis of the type of infiltrating cells or its activation status has not yet been performed and is the objective of ongoing studies (ClinicalTrials.gov Identifier, NCT01137162) [5]. It appears that inhibition of EGFR homodimer signaling rather than EGFR–human epidermal growth factor receptor (HER)-2 heterodimers may be a key molecular event determining the skin toxicity of HER kinase–targeted therapies. Pertuzumab, an antibody interfering with functional HER heterodimerization, fails to block ligand-induced signaling in primary keratinocytes and is not associated with the characteristic acneiform rash [6].
Rash as a Biomarker of Response to EGFR Inhibitors
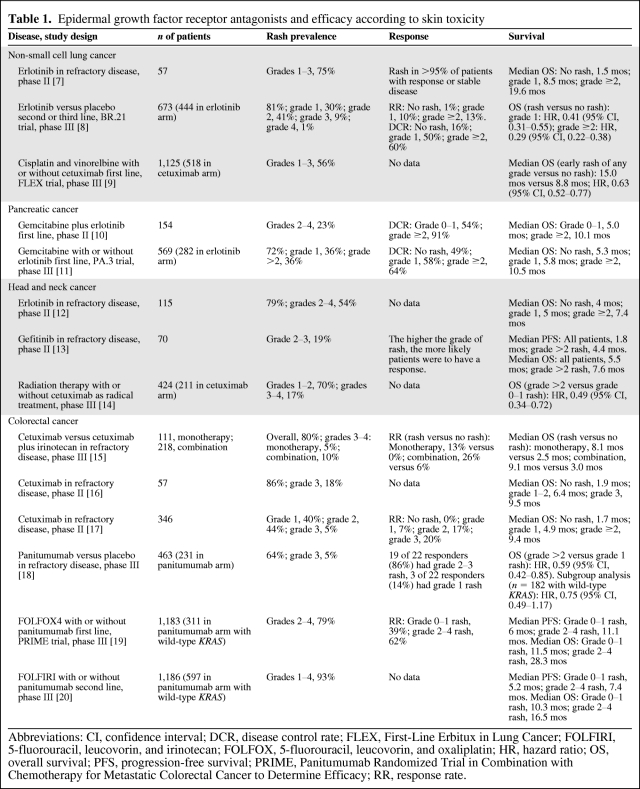
Several studies have suggested that the development of a rash with TKIs or mAbs against EGFR is associated with a better outcome in different tumor types, including NSCLC [7–9], pancreatic cancer [10, 11], head and neck cancer [12–14], CRC [15–20], and ovarian cancer [21]. Response rates (RRs) and median progression-free survival (PFS) and overall survival (OS) times are greater in patients developing rash in response to anti-EGFR targeted agents, as shown in Table 1.
Table 1.
Epidermal growth factor receptor antagonists and efficacy according to skin toxicity
Abbreviations: CI, confidence interval; DCR, disease control rate; FLEX, First-Line Erbitux in Lung Cancer; FOLFIRI, 5-fluorouracil, leucovorin, and irinotecan; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PRIME, Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy; RR, response rate.
The most convincing data for the association between survival and occurrence and severity of rash come from retrospective analyses of phase III trials conducted in NSCLC and CRC patients. For lung cancer, early development of rash during treatment with the TKI erlotinib as a single agent or the mAb cetuximab in combination with chemotherapy is associated with remarkably better survival outcome [8, 9]. A similar relationship between rash and clinical outcomes was reported in studies of the mAbs cetuximab and panitumumab in CRC patients in different advanced disease scenarios [15, 18–20]. In addition, in a phase III trial of combined cetuximab and radiotherapy in patients with localized head and neck cancer, the development of grade ≥2 rash was associated with a twice as long OS duration [14].
Determinants of Rash with EGFR Inhibitors
Clinicopathological Factors
Comparisons of baseline characteristics have been performed to attempt to predict, before initiating therapy, which patients are likely to develop a rash. In NSCLC patients treated with erlotinib, rash was more frequent in those with a good performance status, who had never smoked, and with tumors harboring EGFR amplification by fluorescence in situ hybridization (FISH) analysis. However, even in patients whose tumors had normal EGFR gene copy numbers, the incidence of rash was >70% [8]. In addition, rash was predictive of a better outcome regardless of a positive or negative EGFR status, whether measured as protein expression by immunohistochemistry or gene copy number by FISH [11, 12]. Importantly, the development of a rash in patients whose tumors did not present an activating EGFR mutation has also been associated with better outcomes [8].
Pharmacokinetic Factors
One of the possible explanations for the association of rash and clinical benefit with EGFR inhibitors is the PK activity of the drug, with patients with higher drug plasma levels having greater toxicity and antitumor response. Some studies suggest a correlation between drug exposure and rash [22, 23]. There is wide variability in drug exposure resulting from high variability in the metabolizing cytochrome P450 (CYP)3A4 system, and this might influence toxicity and clinical outcome. In addition, CYP1A2 induction by smoking increases erlotinib clearance, which could explain the lesser clinical activity of erlotinib in these patients [22, 24]. However, there is no clear association between drug exposure and the development of a rash. Prospective trials exploring whether or not increasing the dose of anti-EGFR agents can improve treatment outcomes are needed to address this question. “Dosing-to-rash” trials with EGFR inhibitors have been undertaken to account for the interpatient variability in rash development.
For NSCLC, erlotinib dose escalation appeared feasible but efficacy was not greater than with the standard dose [25]. With regard to this, it is important to mention that TKI-associated diarrhea is the other important and frequent adverse event with gefitinib and erlotinib. Nevertheless, patients with skin rash do not necessarily have a higher incidence of diarrhea and vice versa. Although it is known that diarrhea is also related to the expression of EGFR in the intestines, there are no data correlating intrapatient receptor expression in the skin and digestive system. For advanced CRC, the Evaluation of Various Erbitux® Regimens by means of Skin Tumor biopsies study evaluated whether or not cetuximab dose escalation in patients with no rash or slight rash in the first weeks of treatment induces a more pronounced rash and greater activity [26]. The study evaluated 89 patients randomized to the standard regimen or dosing-to-rash. A higher RR, 46% versus 21%, was observed in wild-type KRAS patients with escalating cetuximab doses. In patients with tumors carrying a KRAS mutation, higher cetuximab doses did not lead to a higher RR [26]. This strategy is currently under evaluation in a larger trial (ClinicalTrials.gov Identifier, NCT01251536). For advanced pancreatic cancer, a dose escalation-to-rash study of erlotinib combined with gemcitabine is currently recruiting patients (ClinicalTrials.gov Identifier, NCT00652366). More important than inducing rash, it has to be proven that inducing rash with higher doses translates into superior survival endpoints without excessive toxicities.
Pharmacogenetic Factors
The most attractive explanation for the correlation of rash and efficacy relates to genetic differences among individuals. Several studies tested the hypothesis that genetic variations in the EGFR gene shared between normal tissue and malignant tumors could explain the observed clinical association.
The EGFR gene contains a highly polymorphic sequence in intron 1, which consists of a variable number of cytosine-adenosine (CA) dinucleotide repeats, in the range of 9–23. This microsatellite polymorphism has been associated with EGFR expression, with the repeat length of CA dinucleotides inversely correlating with EGFR mRNA and protein levels, as well as erlotinib sensitivity in vitro [27–29]. EGFR intron-1 CA repeats <17 and ≥17 define short (S) and long (L) alleles, respectively. Germline EGFR intron-1 S/S status resulting in EGFR upregulation in normal tissues may trigger this molecular predisposing condition for skin toxicity. Graziano et al. [30] assessed 110 patients with refractory advanced CRC treated with combined irinotecan and cetuximab and found EGFR intron-1 S/S to be significantly associated with skin toxicity, treatment response, and a favorable OS outcome, compared with EGFR intron-1 L/L. Rudin et al. [23] reported a prospective trial investigating pharmacogenomic and PK determinants of skin rash and diarrhea among 80 patients with NSCLC, head and neck cancer, and ovarian cancer treated with erlotinib. Variability in skin rash was best explained by a multivariate logistic regression model incorporating the trough erlotinib plasma concentration and the EGFR intron 1 polymorphism [23]. In contrast, Klinghammer et al. [31] detected no significant interaction between intron-1 CA repeat polymorphism and skin toxicity or PFS or OS outcomes in head and neck cancer patients. Because all studies evaluating the role of EGFR intron-1 CA repeats had a single arm, a control arm without an EGFR inhibitor is needed to definitively clarify this issue. In addition, one argument against the hypothesis that the EGFR intron-1 germline polymorphism affects gefitinib- or erlotinib-associated skin toxicity is the fact that it has not been shown to be an important predictor of the clinical activity of these drugs.
Single nucleotide polymorphisms (SNPs) in the essential promoter region of EGFR are associated with greater gene expression. The variant −216G/T was reported to be associated with gefitinib response and toxicity in lung cancer patients [32]. In addition, polymorphisms in the genes involved in the PK properties of EGFR inhibitors, such as metabolizing enzymes and drug transporters, can lead to considerable interindividual and interracial differences in toxicity. CYP3A4 and CYP3A5 polymorphisms determining enzyme expression and activity levels demonstrated marginal associations with erlotinib PK parameters and skin toxicity [23]. Of note, germline polymorphisms of CYP3A4 or CYP3A5 cannot explain the substantial interindividual variability in the enzymatic system, and the relatively rare gene mutations that may have clinical relevance are difficult to find. A clear understanding of the basis of variability in toxicity to EGFR inhibitors may guide the use of the currently available agents at optimal doses and in patients most likely to benefit.
Hypertension as an MBT of Antiangiogenic Agents
Targeting angiogenesis is an attractive approach for cancer treatment, and many antiangiogenic agents are now in use in the clinic, including mAbs and TKIs of the vascular endothelial growth factor (VEGF) pathway. Prior attempts to identify biomarkers of antiangiogenic activity have been unsuccessful in predicting efficacy, including plasma VEGF levels and tumor-derived traits (e.g., VEGF expression), on-treatment changes in circulating factors (e.g., endothelial cells), and imaging parameters (e.g., diffusion contrast-enhanced magnetic resonance imaging) [33].
Implicated in many physiological processes, VEGF pathway inhibition can lead to on-target side effects, such as hypertension, proteinuria, thromboembolic events, or impaired wound healing [34]. Hypertension is the best documented toxicity of both mAbs (bevacizumab) and multitargeted TKIs (sunitinib, sorafenib, pazopanib, vandetanib, axitinib, cediranib, etc.). The incidence of drug-induced hypertension is in the range of 15% with sorafenib (grade 1–2, 10%; grade 3–4, 5%) to 25% with sunitinib (grade 1–2, 15%; grade 3–4, 10%) [35–37], and up to 35% with bevacizumab (grade 1–2, 25%; grade 3–4, 10%) [38, 39]. When antiangiogenic drugs are used in combination, the incidence of hypertension rises, reaching 65% in patients treated with sorafenib plus bevacizumab (grade 3–4, 35%) [40] and up to 90% in patients treated with sunitinib plus bevacizumab (grade 3–4, 55%) [41, 42]. Lower rates of severe hypertension have been reported in more recent trials, which could be explained by greater awareness of the problem and more aggressive management for early hypertension. Onset usually occurs during the first weeks of treatment, and some studies have shown an increase in blood pressure within hours of treatment initiation [43]. Nevertheless, in some long-term treatment studies with bevacizumab, such as an adjuvant CRC trial, a constant increase in the rate of hypertension has been shown even after 6 months on treatment [44]. Considering the long half-life of bevacizumab, which reaches its steady state at approximately 3–4 months of treatment, the incidence of hypertension appears to be higher in patients who are on therapy for a longer time. In addition, higher doses are associated with a higher risk for hypertension [38, 39].
The biological mechanism of antiangiogenic-induced hypertension is not fully understood. It has been demonstrated that the adrenergic system and the renin–angiotensin–aldosterone axis do not play a role in this process. Similarly, changes in VEGF levels do not correlate with hypertension [45]. On the other hand, inhibition of the VEGF pathway produces a decrease in nitric oxide levels, leading to vasoconstriction. This finding correlates with a rapid increase in blood pressure under anti-VEGF therapy [43]. Additionally, sustained VEGF pathway inhibition induces endothelial cell apoptosis, a possible mechanism for continued hypertension. This reduces the number of capillaries, increasing global vascular resistance and ultimately resulting in increased blood pressure [43]. Capillary rarefaction has been observed in patients treated with antiangiogenic drugs such as bevacizumab [46], sunitinib [47], and telatinib [48]. This appears to be reversible 2 weeks after discontinuing antiangiogenic therapy [49, 50].
Hypertension as a Biomarker of Response to Antiangiogenic Agents
Several studies examining hypertension as a PD biomarker of target inhibition have correlated its development with clinical outcome in patients treated with antiangiogenic agents. As shown in Table 2, many are retrospective analyses of single-arm trials of chemotherapy plus bevacizumab or multitargeted TKIs in different tumor types [51–58]. These series have shown a greater RR, PFS time, or even OS time in patients developing hypertension during treatment. Hurwitz et al. [59] recently presented a retrospective analysis of six randomized phase III trials with bevacizumab in different types of metastatic cancer, analyzing the correlation between hypertension and efficacy. For metastatic CRC, the results of the AVF2107 and NO16966 trials have been analyzed. In the AVF2107 study (first-line bolus irinotecan, 5-fluorouracil, and leucovorin chemotherapy with or without bevacizumab), the development of hypertension predicted better PFS and OS results. In contrast, hypertension was not predictive of benefit with the addition of bevacizumab to oxaliplatin-based chemotherapy in the NO16966 trial [59].
Table 2.
Antiangiogenics and efficacy according to hypertension development
Abbreviations: 5-FU, 5-fluorouracil; AVADO, Avastin and Docetaxel; AVAiL, Avastin in Lung Cancer; AVOREN, Avastin and Roferon in Renal Cell Carcinoma; BP, blood pressure; CALGB, Cancer and Leukemia Group B; DBP, diastolic blood pressure; DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; FOLFIRI, 5-fluorouracil, leucovorin, and irinotecan; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; HTN, hypertension; IFL, irinotecan, leucovorin, and 5-fluorouracil; IFN, interferon; LV, leucovorin; NSCLC, non-small cell lung cancer; OS, overall survival; PC, paclitaxel plus carboplatin; PCB, paclitaxel, carboplatin, and bevacizumab; PCC, paclitaxel, carboplatin, and cediranib; PFS, progression-free survival; RIBBON, Regimens in Bevacizumab for Breast Oncology; RR, response rate; SaiL, Safety of Avastin in Lung Cancer; SBP, systolic blood pressure; TTP, time to progression; XELIRI, capecitabine and irinotecan; XELOX, capecitabine and oxaliplatin.
A similar disparity was shown in a retrospective analysis of phase III trials evaluating the addition of bevacizumab to chemotherapy in patients with advanced breast cancer. In the Eastern Cooperative Oncology Group (ECOG) 2100 trial (weekly paclitaxel with or without bevacizumab), the OS duration was significantly longer among patients presenting with hypertension during bevacizumab treatment [60]. Conversely, the recently published retrospective analysis of the AVADO (with docetaxel as standard first-line chemotherapy) and RIBBON-1 trials (with capecitabine, nab-paclitaxel, docetaxel, or anthracycline-based chemotherapy) showed no statistically significant correlation between bevacizumab-related hypertension and outcome [59].
Regarding nonsquamous NSCLC, the predictive value of hypertension with antiangiogenic agents is also uncertain. A retrospective analysis of the ECOG 4599 study (carboplatin plus paclitaxel with or without bevacizumab) showed better PFS and OS results in patients experiencing hypertension during treatment [61]. In an analysis of >2,000 patients treated with bevacizumab in combination with a variety of chemotherapies for advanced NSCLC, the median survival times were 18.8 months in those who developed hypertension and 12.9 months in those who did not [62]. On the other hand, subanalysis of the bevacizumab arm of the AVAiL trial (cisplatin plus gemcitabine with or without bevacizumab) did not show a survival benefit in patients with hypertension [59].
For renal cell carcinoma, data from the phase III trials evaluating the addition of bevacizumab to interferon-α are not conclusive. Whereas in the Cancer and Leukemia Group B 90206 trial a survival benefit was noted [63], subanalysis of the AVOREN trial showed no correlation between hypertension and outcome [59]. On the other hand, sunitinib-induced hypertension consistently correlated with RR, PFS, and OS benefits in a subset analysis of multiple trials [64, 65]. Hypertension during treatment with sorafenib was also positively associated with greater tumor shrinkage [57]. Additional retrospective series have found a positive correlation between hypertension and efficacy with other tumor types, such as sorafenib for hepatocellular carcinoma [58] and axitinib for melanoma and thyroid cancer [55].
As shown in Table 2, the threshold used to categorize patients into those experiencing and those not experiencing hypertension varies significantly across trials. In addition, information on the time course of the occurrence of hypertension is also lacking in some reports. In general, patients with early development of grade ≥1 hypertension (>150/100 mmHg or an increase in diastolic blood pressure >20 mmHg) were categorized as hypertensive. In a retrospective study by Hurwitz et al. [59], for example, the threshold used was an increase in systolic blood pressure >20 mmHg or in diastolic blood pressure >10 mmHg. In other studies, the subgroup of patients experiencing grade >3 hypertension (requiring more than one drug for control) was considered in the analysis [60]. Taking into consideration the different chemotherapy regimens and tumor types in bevacizumab trials and the various thresholds for definition of hypertension, a definitive conclusion regarding its value as a predictive biomarker cannot be made. On the other hand, it appears that a positive correlation was found mainly in studies that considered higher levels of hypertension and in tumors with clear addiction to angiogenic factors, such as renal cell carcinoma.
Unplanned retrospective evaluations have clear limitations in identifying biomarkers. In addition, analyses of single-arm studies and of subgroups of patients receiving the antiangiogenic agent in a randomized trial do not differentiate whether hypertension is predictive of benefit with the drug or simply has prognostic value. Retrospective analysis of both arms is mandatory, and prospective validations are the best way to determine the predictive value of a biomarker. The ongoing phase III study of axitinib as second-line treatment for renal cell carcinoma, for example, incorporates a dose-titration scheme based on patient tolerance and blood pressure (ClinicalTrials.gov Identifier, NCT00678392). Interesting exploratory data from the phase II randomized BR.24 trial were recently reported, examining the incidence and predictors of hypertension in patients receiving carboplatin plus paclitaxel with cediranib (a potent TKI of all VEGF receptors) or placebo for advanced NSCLC [66]. Although the sample size was small (296 patients), that study has landmark importance given that it evaluated hypertension as a potential surrogate biomarker in both arms of a randomized study. Treatment-emergent hypertension (new onset or worsening of pre-existing hypertension) was reported in 68% of patients receiving cediranib and 45% of those treated with chemotherapy. Treatment-emergent hypertension was associated with a longer survival time in both arms. For cediranib patients, developing hypertension reduced the risk for death by 38% (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.38–1.03; p = .06), whereas for placebo patients it reduced the risk for death by 51% (HR, 0.49; 95% CI, 0.30–0.80; p = .004). Surprisingly, a differential effect in terms of the RR and PFS interval was not observed, and the authors proposed that hypertension development is not a biomarker of efficacy but is a prognostic factor [66].
Determinants of Hypertension with Antiangiogenic Agents
Pharmacogenetic Factors
A molecular link between the development of hypertension and benefit with VEGF inhibitors is still lacking. There is wide interindividual variability in blood pressure response to these agents, which has not been directly linked to PK parameters such as drug exposure or maximum concentration [43, 67]. Given that these agents target a host-mediated process (i.e., angiogenesis), one logical explanation could be the presence of germline polymorphisms in critical players in the VEGF pathway. Such polymorphisms can have major effects on drug PK and PD profiles. Recent studies looked at SNPs within the gene encoding VEGF and its receptor and identified a VEGF genotype (VEGF-634 C/C) that protects against the development of hypertension in breast cancer patients receiving paclitaxel plus bevacizumab [60] and in renal cell carcinoma patients receiving sunitinib [68]. Moreover, additional SNPs were also positively related to hypertension and tumor shrinkage in the retrospective analysis of the sunitinib trial in renal cell carcinoma patients [69]. How these SNPs affect VEGF pathway signaling and their influence in antiangiogenic-induced hypertension are not well understood. As stated by Humphreys and Atkins, specific polymorphisms could alter VEGF transcription or translation with a net effect of rendering the patient less susceptible to VEGF inhibition, consequently resulting in less toxicity and efficacy with antiangiogenic agents [70]. Of note, in the breast cancer study with bevacizumab plus chemotherapy, the alleles associated with hypertension were different from those that predicted a favorable prognosis [60].
Hypothyroidism as an MBT of Multitargeted TKIs
Sunitinib and sorafenib inhibit tyrosine kinase activity of the VEGF receptor, the platelet-derived growth factor receptor, the stem cell factor receptor (a cytokine receptor), the fms-like tyrosine kinase 3 receptor, and the protein product of the RET oncogene. Both agents can induce subclinical or overt hypothyroidism in up to 80% and 20% of patients, respectively [71–73]. Explanations for TKI-induced hypothyroidism include reduction in fenestration number and enhancement of capillary regression in the thyroid vascular system (via VEGF inhibition), direct inhibition of thyroid cell growth (with reduced iodine uptake and peroxidase activity), and inhibition of the RET oncogene protein product [74–76]. Symptoms possibly attributable to hypothyroidism, such as fatigue, constipation, cold intolerance, and dry skin, are reported in many patients receiving these agents, and it can be difficult to distinguish whether they are a result of thyroid gland dysfunction or are a direct toxicity of the drug.
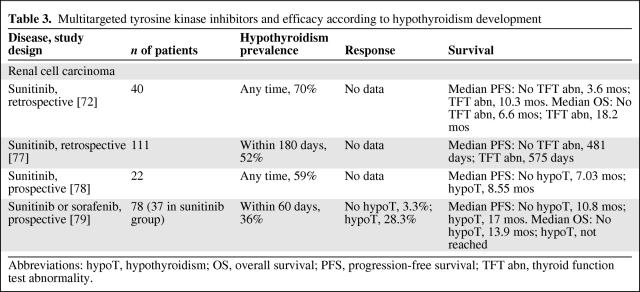
Prospective series have demonstrated that patients with advanced renal cell carcinoma developing hypothyroidism during treatment with sunitinib or sorafenib have a significantly greater probability of responding to treatment and surviving longer, as shown in Table 3 [72, 77–79]. Hypothyroidism has been observed 1–6 months after therapy initiation and its incidence usually increases with prolonged treatment [71, 72, 79]. Importantly, because these studies evaluated hypothyroidism occurring at any time during treatment with multitargeted TKIs, patients who survived longer were more likely to develop the adverse event because of length-biased sampling.
Table 3.
Multitargeted tyrosine kinase inhibitors and efficacy according to hypothyroidism development
Abbreviations: hypoT, hypothyroidism; OS, overall survival; PFS, progression-free survival; TFT abn, thyroid function test abnormality.
The mechanisms by which hypothyroidism may be associated with a longer survival time are not entirely understood. In addition to the hypothesis of on-target toxicity already discussed, a direct action of thyroid hormone on cancer cell proliferation, cancer cell growth, and angiogenesis has also been proposed. In animal models, hypothyroidism inhibited the growth of diverse tumors [80, 81]. Moreover, clinical studies have shown that hypothyroidism was associated with better outcomes in patients with head and neck cancer, glioblastoma, and metastatic breast cancer [82–84]. Thus, hypothyroidism could be involved in slowing tumor growth, thus leading to longer survival times. Its occurrence may reflect differences in individual doses and affinity to receptor tyrosine kinases, but no PK correlative data are currently available. Hypothyroidism may lead to modulation of paracrine growth factors and reduced angiogenesis [85, 86]. Therefore, if induction of hypothyroidism is part of the mode of action of sunitinib and sorafenib and a surrogate of efficacy, then levothyroxine replacement therapy could undermine the antitumor activity of these agents. In some cohorts, levothyroxine treatment did not impact outcome; however, most of the patients remained in a hypothyroid state despite replacement therapy [79]. Other authors reported that hypothyroid patients with systematic hormonal replacement did not have a significantly different survival outcome from patients without hypothyroidism [77]. Further studies in this field are required [87].
Conclusions
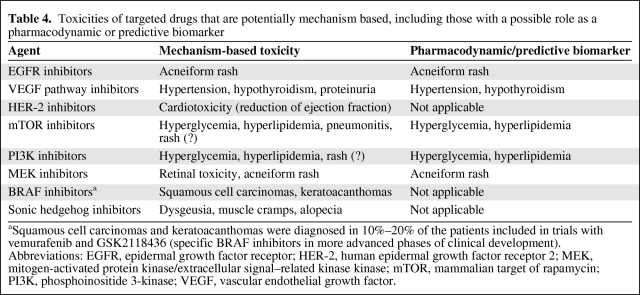
The interest in using biomarkers to aid clinical decisions in treatment with targeted therapies is obvious. The exponential increase in economic treatment costs is pushing the need for reliable predictive factors to identify patients (or tumors) more likely to benefit from targeted therapies. Many attempts to find tissue-based, imaging, or blood biomarkers can be found in the literature. However, the validation of biomarkers through clinical research, leading to successful implementation in clinical practice, remains a challenge [88]. Many marker assessment methods have limitations when it comes to reliability and reproducibility, costs, and the feasibility of obtaining specimens. Targeted biological agents that induce MBTs may reduce the need for surrogate tissue and therefore represent an alternative. In addition to rash, hypertension and hypothyroidism, which are extensively reviewed in the literature, the hypertriglyceridemia and hyperglycemia seen with phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin inhibitors [89] are likely PD effects of pathway inhibition, and their value as markers of efficacy needs to be evaluated. Table 4 summarizes the different on-target side effects of targeted drugs with potential as molecular PD markers or predictive biomarkers. Some toxicities might be mechanism based but cannot be used as predictive biomarkers. Diarrhea is a common adverse event of various targeted therapies. It seems to be mediated by inhibition of multiple different targets. With EGFR antagonists, it has been described more frequently and at a higher grade with small molecule TKIs than with mAbs, for example. Nevertheless, diarrhea has been consistently described with other multitargeted TKIs, such as sorafenib and sunitinib, that do not inhibit EGFR. Therefore, the use of diarrhea as a predictive biomarker would be compromised by the multifactorial nature of the toxicity.
Table 4.
Toxicities of targeted drugs that are potentially mechanism based, including those with a possible role as a pharmacodynamic or predictive biomarker
aSquamous cell carcinomas and keratoacanthomas were diagnosed in 10%–20% of the patients included in trials with vemurafenib and GSK2118436 (specific BRAF inhibitors in more advanced phases of clinical development).
Abbreviations: EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor 2; MEK, mitogen-activated protein kinase/extracellular signal–related kinase kinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; VEGF, vascular endothelial growth factor.
Because rash and hypertension usually occur within 4 weeks of therapy initiation, in the absence of reliable predictive factors, future studies to address the importance of toxicities as biomarkers of better outcomes may require a 1-month lead-in period of a targeted agent to identify patients who develop the anticipated toxicity. Patients could then be stratified according to whether toxicity occurs and randomized to different therapies or doses of the agent. Absence of the predictive side effects could represent an important prediction for lack of activity and ultimately suggest an early change in the treatment strategy. In addition to the RR, studies may need to evaluate survival endpoints. Drawbacks of this approach include the possibility of chronic toxic effects, such as serious vascular injuries with antiangiogenics, as well as a higher incidence of other clinically significant adverse events, such as diarrhea with EGFR inhibitors. It is important to develop effective strategies to manage these toxicities while continuing therapy.
Interindividual differences in pharmacological parameters and inherited variations in genes coding for drug targets may be connections between mechanism-based adverse events and efficacy. Importantly, the pathway being targeted must have a central role as a driver of tumor progression for the related MBT to be a predictive marker. As an example, renal cancer has been linked to von Hippel Lindau gene mutations, which increase hypoxia inducible factor 1α levels that subsequently elevate VEGF expression. Treatment with a VEGF receptor TKI such as sunitinib is likely to produce responses in this setting and the mechanism-based hypertension may be a predictive marker of efficacy. On the other hand, sunitinib-associated hypertension may be a PD marker but not a predictive marker in diseases in which activation of angiogenesis may not be the driving oncogenic event, such as breast cancer.
It is currently too early for clinicians to select patients to continue or not with molecular targeted agents based on early MBTs. However, if a patient develops a toxicity that potentially predicts a better outcome, concentrated efforts should be made to adequately control such events to allow targeted therapy to be continued. The clinical value of the possible association between an MBT and efficacy remains to be established in future larger studies including prospective PK or PD analyses.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
Provision of study material or patients: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
Collection and/or assembly of data: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
Data analysis and interpretation: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
Manuscript writing: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
Final approval of manuscript: Josep Tabernero, Rodrigo Dienstmann, Irene Braña, Jordi Rodon
References
- 1.Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Dancey JE, Dobbin KK, Groshen S, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969–1973. doi: 10.1126/science.287.5460.1969. [DOI] [PubMed] [Google Scholar]
- 4.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: A model for targeted therapy. Clin Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 5.Solomon BM, Jatoi A. Rash from EGFR inhibitors: Opportunities and challenges for palliation. Curr Oncol Rep. 2008;10:304–308. doi: 10.1007/s11912-008-0048-1. [DOI] [PubMed] [Google Scholar]
- 6.Laux I, Jain A, Singh S, et al. Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. Br J Cancer. 2006;94:85–92. doi: 10.1038/sj.bjc.6602875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 8.Ding K, Pater J, Whitehead M, et al. Validation of treatment induced specific adverse effect as a predictor of treatment benefit: A case study of NCIC CTG BR21. Contemp Clin Trials. 2008;29:527–536. doi: 10.1016/j.cct.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.O'Byrne KJ, Bondarenko I, Barrios C, et al. Molecular and clinical predictors of outcome for cetuximab in non-small cell lung cancer (NSCLC): Data from the FLEX study. J Clin Oncol. 2009;27(15 suppl) Abstract 8007. [Google Scholar]
- 10.Manzano J, Rivera F, Galan M, et al. A phase II, open label study to evaluate the relationship between skin rash and survival in patients with unresectable and/or metastatic pancreatic cancer treated with erlotinib combined with gemcitabine. J Clin Oncol. 2010;28(15 suppl) Abstract 4094. [Google Scholar]
- 11.Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 12.Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 14.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 16.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 17.Lenz HJ, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914–4921. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 19.Douillard J, Cassidy J, Jassem J, et al. Randomized, open-label, phase III study of panitumumab with FOLFOX4 versus FOLFOX4 alone as first-line treatment for metastatic colorectal cancer: Efficacy by skin toxicity. J Clin Oncol. 2010;28(15 suppl) doi: 10.1200/JCO.2009.27.4860. Abstract 3528. [DOI] [PubMed] [Google Scholar]
- 20.Price TJ, Sobrero AF, Wilson G, et al. Randomized, open-label, phase III study of panitumumab with FOLFIRI versus FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer: Efficacy by skin toxicity. J Clin Oncol. 2010;28(15 suppl) doi: 10.1200/JCO.2009.27.6055. Abstract 3529. [DOI] [PubMed] [Google Scholar]
- 21.Gordon AN, Finkler N, Edwards RP, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: Results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–792. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu JF, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–145. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–1127. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 25.Mita A, de Jonge MJ, Verweij J, et al. Erlotinib (Tarceva) “dosing-to-rash”: Characterization of skin toxicity from a pilot phase II intra-patient dose-escalation study of E in previously treated patients with advanced non-small cell lung cancer (NSCLC): P3–115. J Thorac Oncol. 2007;2:S728. [Google Scholar]
- 26.Tejpar S, Peeters M, Humblet Y, et al. Relationship of efficacy with KRAS status (wild type versus mutant) in patients with irinotecan-refractory metastatic colorectal cancer, treated with irinotecan and escalating doses of cetuximab: The EVEREST experience (preliminary data) J Clin Oncol. 2008;26(suppl) Abstract 4001. [Google Scholar]
- 27.Buerger H, Gebhardt F, Schmidt H, et al. Length and loss of heterozygosity of an intron 1 polymorphic sequence of EGFR is related to cytogenetic alterations and epithelial growth factor receptor expression. Cancer Res. 2000;60:854–857. [PubMed] [Google Scholar]
- 28.Gebhardt F, Zänker KS, Brandt B, et al. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274:13176–13180. doi: 10.1074/jbc.274.19.13176. [DOI] [PubMed] [Google Scholar]
- 29.Amador ML, Oppenheimer D, Perea S, et al. An epidermal growth factor receptor intron 1 polymorphism mediates response to epidermal growth factor receptor inhibitors. Cancer Res. 2004;64:9139–9143. doi: 10.1158/0008-5472.CAN-04-1036. [DOI] [PubMed] [Google Scholar]
- 30.Graziano F, Ruzzo A, Loupakis F, et al. Pharmacogenetic profiling for cetuximab plus irinotecan therapy in patients with refractory advanced colorectal cancer. J Clin Oncol. 2008;26:1427–1434. doi: 10.1200/JCO.2007.12.4602. [DOI] [PubMed] [Google Scholar]
- 31.Klinghammer K, Knödler M, Schmittel A, et al. Association of epidermal growth factor receptor polymorphism, skin toxicity, and outcome in patients with squamous cell carcinoma of the head and neck receiving cetuximab-docetaxel treatment. Clin Cancer Res. 2010;16:304–310. doi: 10.1158/1078-0432.CCR-09-1928. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Gurubhagavatula S, Zhou W, et al. Epidermal growth factor receptor polymorphisms and clinical outcomes in non-small-cell lung cancer patients treated with gefitinib. Pharmacogenomics J. 2008;8:129–138. doi: 10.1038/sj.tpj.6500444. [DOI] [PubMed] [Google Scholar]
- 33.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010;102:8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 35.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 37.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 38.An MM, Zou Z, Shen H, et al. Incidence and risk of significantly raised blood pressure in cancer patients treated with bevacizumab: An updated meta-analysis. Eur J Clin Pharmacol. 2010;66:813–821. doi: 10.1007/s00228-010-0815-4. [DOI] [PubMed] [Google Scholar]
- 39.Geiger-Gritsch S, Stollenwerk B, Miksad R, et al. Safety of bevacizumab in patients with advanced cancer: A meta-analysis of randomized controlled trials. The Oncologist. 2010;15:1179–1191. doi: 10.1634/theoncologist.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: Intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495–499. doi: 10.1038/sj.bjc.6605514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rini BI, Garcia JA, Cooney MM, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–6283. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 46.Morere JF, Des Guetz G, Mourad J, et al. Mechanism of bevacizumab-induced arterial hypertension: Relation with skin capillary rarefaction in patients treated for metastatic colorectal cancer. J Clin Oncol. 2007;25(18 suppl) Abstract 3557. [Google Scholar]
- 47.van der Veldt AA, de Boer MP, Boven E, et al. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21:439–446. doi: 10.1097/CAD.0b013e3283359c79. [DOI] [PubMed] [Google Scholar]
- 48.Steeghs N, Gelderblom H, Roodt JO, et al. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 49.Steeghs N, Rabelink TJ, op 't Roodt J, et al. Reversibility of capillary density after discontinuation of bevacizumab treatment. Ann Oncol. 2010;21:1100–1105. doi: 10.1093/annonc/mdp417. [DOI] [PubMed] [Google Scholar]
- 50.de Boer MP, van der Veldt AA, Lankheet NA, et al. Sunitinib-induced reduction in skin microvascular density is a reversible phenomenon. Ann Oncol. 2010;21:1923–1924. doi: 10.1093/annonc/mdq335. [DOI] [PubMed] [Google Scholar]
- 51.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 52.Burnette BL, Grothey A. Hypertension and survival in unresectable metastatic colorectal cancer (mCRC) patients receiving first-line bevacizumab (BEV) and FOLFOX. J Clin Oncol. 2010;28(suppl) Abstract e14066. [Google Scholar]
- 53.De Stefano A, Carlomagno C, Pepe S, et al. Bevacizumab-related arterial hypertension as a predictive marker in metastatic colorectal cancer patients. Cancer Chemother Pharmacol. 2011 Mar 16; doi: 10.1007/s00280-011-1604-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Friberg G, Kasza K, Vokes EE, et al. Early hypertension as a potential pharmacodynamic marker for survival in pancreatic cancer patients treated with bevacizumab and gemcitabine. J Clin Oncol. 2005;23(16 suppl) Abstract 3020. [Google Scholar]
- 55.Rini BI, Schiller JH, Fruehauf JP, et al. Association of diastolic blood pressure > 90 mmHg with overall survival in patients treated with axitinib (AG-013736) J Clin Oncol. 2008;26(suppl) Abstract 3543. [Google Scholar]
- 56.Bono P, Elfving H, Utriainen T, et al. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009;20:393–394. doi: 10.1093/annonc/mdn729. [DOI] [PubMed] [Google Scholar]
- 57.Nozawa M, Nagae S, Nishigaki K, et al. Association of hypertension with objective response in patients treated with sorafenib [abstract 341]. Presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium; February 28, 2009; Orlando, Florida. [Google Scholar]
- 58.Kim RD, Byrne MT, Hammel J, et al. Association of hypertension with overall outcome in patients taking sorafenib in advanced hepatocellular carcinoma. J Clin Oncol. 2010;28(suppl) Abstract e14536. [Google Scholar]
- 59.Hurwitz H, Douglas PS, Middleton JP, et al. Analysis of early hypertension and clinical outcome with bevacizumab. J Clin Oncol. 2010;28(15 suppl) doi: 10.1634/theoncologist.2012-0339. Abstract 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahlberg SE, Sandler AB, Brahmer JR, et al. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–954. doi: 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thatcher N, Tsai CM, Laskin J, et al. MO19390 (SAiL): Hypertension in patients with advanced of recurrent non-squamous non-small cell lung cancer receiving first-line bevacizumab plus chemotherapy. J Thor Oncol. 2009;4(suppl) Abstract C2.4. [Google Scholar]
- 63.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18:1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 65.Rini BI, Cohen DP, Lu D, et al. Hypertension (HTN) as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib [abstract 312]. Presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium; March 7, 2010; San Francisco, California. [Google Scholar]
- 66.Goodwin R, Ding K, Seymour L, et al. Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: NCIC Clinical Trials Group Study BR24. Ann Oncol. 2010;21:2220–2226. doi: 10.1093/annonc/mdq221. [DOI] [PubMed] [Google Scholar]
- 67.Rixe O, Dutcher J, Motzer R, et al. Diastolic blood pressure and pharmacokinetics as predictors of axitinib efficacy in metastatic renal cell cancer. J Clin Oncol. 2009;27(15 suppl) Abstract 5045. [Google Scholar]
- 68.Kim JJ, Vaziri SA, Elson P, et al. VEGF single nucleotide polymorphisms and correlation to sunitinib-induced hypertension in metastatic renal cell carcinoma patients. J Clin Oncol. 2009;27(15 suppl) Abstract 5005. [Google Scholar]
- 69.Kim JJ, Vaziri SA, Elson P, et al. Role of VEGF and VEGFR2 single nucleotide polymorphisms in predicting treatment-induced hypertension and clinical outcome in metastatic clear cell RCC patients treated with sunitinib. J Clin Oncol. 2010;28(15 suppl) doi: 10.1002/cncr.26491. Abstract 4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: Toxicity or target? Clin Cancer Res. 2009;15:5947–5949. doi: 10.1158/1078-0432.CCR-09-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 72.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: A prospective evaluation. Br J Cancer. 2008;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tamaskar I, Bukowski R, Elson P, et al. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann Oncol. 2008;19:265–268. doi: 10.1093/annonc/mdm483. [DOI] [PubMed] [Google Scholar]
- 74.Makita N, Miyakawa M, Fujita T, et al. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid. 2010;20:323–326. doi: 10.1089/thy.2009.0414. [DOI] [PubMed] [Google Scholar]
- 75.Mannavola D, Coco P, Vannucchi G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 76.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 77.Bladou F, Gravis G, Sabatier R, et al. Hypothyroidism and survival during sunitinib therapy in metastatic renal cell carcinoma (mRCC): A prospective observational analysis. J Clin Oncol. 2010;28(suppl) Abstract e15013. [Google Scholar]
- 78.Baldazzi V, Tassi R, Lapini A, et al. The impact of sunitinib-induced hypothyroidism on progression-free survival of metastatic renal cancer patients: A prospective single-center study. Urologic Oncol. 2010 Sep 28; doi: 10.1016/j.urolonc.2010.07.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 79.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: Blessing or curse? Cancer. 2011;117:534–544. doi: 10.1002/cncr.25422. [DOI] [PubMed] [Google Scholar]
- 80.Mishkin SY, Pollack R, Yalovsky MA, et al. Inhibition of local and metastatic hepatoma growth and prolongation of survival after induction of hypothyroidism. Cancer Res. 1981;41:3040–3045. [PubMed] [Google Scholar]
- 81.Theodossiou C, Skrepnik N, Robert EG, et al. Propylthiouracil-induced hypothyroidism reduces xenograft tumor growth in athymic nude mice. Cancer. 1999;86:1596–1601. [PubMed] [Google Scholar]
- 82.Nelson M, Hercbergs A, Rybicki L, et al. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:1041–1046. doi: 10.1001/archotol.132.10.1041. [DOI] [PubMed] [Google Scholar]
- 83.Hercbergs AA, Goyal LK, Suh JH, et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003;23:617–626. [PubMed] [Google Scholar]
- 84.Cristofanilli M, Yamamura Y, Kau SW, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103:1122–1128. doi: 10.1002/cncr.20881. [DOI] [PubMed] [Google Scholar]
- 85.Mukku VR. Regulation of epidermal growth factor receptor levels by thyroid hormone. J Biol Chem. 1984;259:6543–6547. [PubMed] [Google Scholar]
- 86.Bergh JJ, Lin HY, Lansing L, et al. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- 87.Garfield DH, Wolter P, Schöffski P, et al. Documentation of thyroid function in clinical studies with sunitinib: Why does it matter? J Clin Oncol. 2008;26:5131–5132. doi: 10.1200/JCO.2008.18.8680. [DOI] [PubMed] [Google Scholar]
- 88.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: Theoretical considerations and practical challenges. J Clin Oncol. 2009;27:4027–4034. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nallari AS, Karrison T, Rosner GL, et al. Fasting glucose and triglycerides as biomarkers of mTOR inhibition, evidence of a categorical response. J Clin Oncol. 2010;28(15 suppl) Abstract 3091. [Google Scholar]