The study assessed the effect of smoking on total symptom burden, the sum of 12 common treatment-related side effects, in patients undergoing treatment for cancer using data from private medical oncology practices that were part of the National Cancer Institute's Community Clinical Oncology Program. Smokers had a higher total symptom burden than nonsmokers during cancer treatment, and this persisted at a follow-up 6 months after treatment.
Keywords: Cancer control, Smoking, Symptom management, Symptom burden
Learning Objectives
After completing this course, the reader will be able to:
Describe the influence of cigarette smoking on side effects during cancer treatment and following the end of cancer treatment.
Identify areas in your practice in which smoking status can be assessed on a regular basis and devise a plan for disseminating cessation information and free cessation aids.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Cigarette smoking has long been implicated in cancer development and survival. However, few studies have investigated the impact of smoking on symptom burden in cancer survivors during treatment and at survivorship stage. This study examines the influence of cigarette smoking on side effects among 947 cancer patients during and 6 months following treatment.
Methods.
Patients diagnosed with cancer and scheduled to receive chemotherapy and/or radiation therapy reported on current smoking status (yes, no) and total symptom burden [the sum of 12 common symptoms (fatigue, hair loss, memory, nausea, depression, sleep, pain, concentration, hot flashes, weight loss, skin problems, and dyspnea) scored on an 11-point scale ranging from 0 = “not present” to 10 = “as bad as you can imagine”] during treatment and at 6-month follow-up. The adjusted mean total symptom burden by smoking status was determined by analysis of covariance controlling for age, gender, race, education, occupation, treatment, cancer site, and Karnofsky performance score.
Results.
During treatment, smokers (S) had a significantly higher total symptom burden than nonsmokers (NS) (S = 46.3 vs. NS = 41.2; p < 0.05). At 6-month follow-up, smokers continued to report a higher total symptom burden than nonsmokers (S = 27.7 vs. NS = 21.9; p < 0.05). Participants who quit smoking before treatment levels had a total symptom burden similar to nonsmokers.
Conclusion.
Smoking was associated with an increased symptom burden during and following treatments for cancer. Targeted cessation efforts for smokers to decrease symptom burden may limit the likelihood of treatment interruptions and increase quality of life following treatment.
Introduction
Improvements in early detection and advances in treatments such as chemotherapy, radiotherapy, surgery, and hormone therapy have played significant roles in the decrease in cancer mortality rates [1–3]. Because of the combination of increasing incidence rates and decreasing mortality rates, the number of cancer patients surviving their disease is increasing. In 2007, there were almost 12 million cancer survivors living in the U.S. [4]. This number is expected to increase, as 68% of the 1.5 million Americans diagnosed with cancer in 2010 are expected to survive ≥5 years [5]. Unfortunately, the cytotoxic therapies (chemotherapy and radiotherapy) that destroy malignant cells and improve survival can also damage healthy tissues, resulting in undesired side effects and a greater symptom burden for patients [6, 7]. As the number of cancer survivors increases, it is increasingly important to identify, quantify, and reduce the symptom burden this growing population bears.
Cancer patients face a unique set of health challenges related to the side effects of their disease and its treatment. Cancer therapy is associated with a range of short-term (usually resolve within a few months of treatment completion) and long-term (persisting for years after treatment completion) side effects. One of the most common short-term side effects of cancer therapy, experienced by 60%–99% of patients, is cancer-related fatigue, characterized by overwhelming exhaustion and a decreased capacity for physical and mental work that is not relieved by rest [8–13]. Fatigue can also persist for years after treatment completion [14], with 20%–35% of cancer patients reporting persistent fatigue >5 years after treatment [14–16]. Chemotherapy-induced nausea and vomiting are among the most feared side effects [17, 18]. Although vomiting is well controlled by antiemetics, nausea remains a prevalent side effect of chemotherapy [19]. Sleep disruption is also a common side effect of cancer treatment, with rates up to three times higher in cancer patients than in the general population [20–22]. Patients who undergo adjuvant cancer therapy often report cognitive difficulties such as memory problems and difficulty concentrating; this syndrome has been termed “chemobrain” [23–25]. Short-term cognitive impairment associated with cancer treatment has been reported to occur in up to 75% of patients [26, 27]. Long-term cognitive difficulties have been observed among cancer patients [24, 25], with 20% and 45% of cancer patients reporting cognitive deficits many years after the completion of treatment [28, 29]. Whereas major depression is seen in 4%–17% of the general population, depression rates can exceed 50% in patients with cancer, depending on the site [30–32]. Depression frequently coexists with anxiety disorders (e.g., post-traumatic stress disorder, panic disorder, generalized anxiety disorder), pain, and fatigue, which can prolong recovery and result in poor outcomes [32]. Many of these short-term side effects can lead to treatment interruptions and dosage reductions, resulting in lower efficacy, and the presence of long-term side effects can significantly reduce quality of life (QOL) [33, 34].
Although cancer patients face a greater symptom burden, they also continue to engage in poor health behaviors at rates similar to those of the general population. Smoking rates at the time of diagnosis of cancer vary from 10% to >95%, depending on the cancer site [35–38]. Quit rates among newly diagnosed cancer patients also differ by cancer site, ranging from <5% for breast cancer cases to >60% for lung cancer cases [36, 37, 39]. However, data from 1999–2001 show little difference in smoking prevalence between cancer patients and the general population at all ages; 20% of cancer patients and 24% of the general population smoke [35]. Among those <40 years old, ∼44% of cancer patients report smoking whereas 27% of individuals with no reported cancer history report smoking [28, 29]. Health behaviors such as cigarette smoking during cancer treatment may have an impact on treatment outcomes for cancer patients. Patients who smoke throughout cancer treatment have a significantly lower survival rate than those who do not smoke [40–42]. Smoking during cancer treatment has also been associated with the development of second primary tumors (SPTs) [43, 44] and treatment-related complications [45, 46]. Moreover, cancer patients who smoke report lower QOL scores than cancer patients who do not smoke [47, 48]. Many QOL domains such as the physical, functional, and emotional domains are directly related to cancer-specific side effects (fatigue, pain, depression, etc.) and symptom burden.
Although smoking throughout cancer treatment is associated with a variety of adverse events that include greater mortality, more SPTs, and more treatment-related complications, there is little research on the effect of smoking on cancer treatment–related side effect severity and symptom burden. The primary aim of this study was to determine the effect of smoking on the total symptom burden, the sum of 12 common treatment-related side effects, in patients undergoing treatment for cancer. Data for this study were obtained from private medical oncology practices that were part of the National Cancer Institute's Community Clinical Oncology Program (CCOP).
Materials and Methods
This study assessed the sociodemographic characteristics, self-reported smoking status, and side effects of cancer therapy over the course of cancer treatment (chemotherapy, radiotherapy, or both). Data for this secondary analysis were part of a larger study that collected data as part of a longitudinal study to assess the information needs of cancer patients undergoing chemotherapy or radiation therapy through the University of Rochester Cancer Center CCOP research base. The recruitment methods and inclusion criteria for this study have been previously described [49–51]. The study questionnaires were completed at three time points: (a) before initiation of chemotherapy or radiation, (b) within 2 weeks after the completion of treatment (reflecting experience during treatment), and (c) 6 months after the completion of treatment. After expressing an interest at their initial oncology consultation, participants met with a research coordinator and, after giving informed consent, were briefly interviewed to collect demographic and medical information, smoking status, and symptom burden prior to beginning therapy. Follow-up surveys were then completed by participants at home and collected via postage-paid, preaddressed return envelopes within 2 weeks and again at 6 months after the termination of treatment. The study was approved by the University of Rochester Research Subjects Review Board and the internal review boards of the participating accrual sites.
Participants
Twenty geographically separate medical oncology private practice sites throughout the U.S. recruited consecutive newly diagnosed cancer patients receiving chemotherapy and/or radiation therapy. Eligibility criteria included the following: (a) having a diagnosis of breast, lung, genitourinary tract, gynecological, hematologic, gastrointestinal, or head and neck cancer; (b) being naive to chemotherapy or radiation therapy; (3) having a life expectancy of ≥10 months; (d) being ≥18 years old; and (e) being able to read and understand English. Although patients receiving treatment for palliative purposes were not excluded from this study sample, the majority were receiving chemotherapy and/or radiation with curative intent.
Smoking Status
A single question, “Are you currently a smoker (yes, no)?” was asked to assess smoking status at all three time points. For analyses that used outcome variables from time period 2, participants who responded “yes” at time points 1 and 2 were considered smokers throughout cancer treatment, participants who responded “no” at time points 1 and 2 were considered nonsmokers, and participants who responded “yes” at time point 1 and “no” at time point 2 were considered to be “nonactive smokers.” No participants responded “no” at time point 1 and “yes” at time point 2. For analyses that used outcome variables from time period 3, participants who responded “yes” at time points 2 and 3 were considered smokers throughout cancer treatment, participants who responded “no” at time points 2 and 3 were considered nonsmokers, and participants who responded “yes” at time point 2 and “no” at time point 3 were considered to be “nonactive smokers.”
Symptom Burden
A symptom inventory that assesses 12 common symptoms (fatigue, hair loss, memory loss, nausea, depression, sleep problems, pain, difficulty concentrating, hot flashes, weight loss, skin problems, and shortness of breath) was used to examine the presence and severity of treatment-related side effects. These questions were adapted from a measure created at MD Anderson Cancer Center [52] and have been used by our group in numerous studies of cancer patients [10, 11]. Content and construct validity have also been demonstrated for this instrument [52, 53]. The one-page questionnaire consists of a series of scales on which the severity of each symptom is indicated by filling in the appropriate circle on an 11-point horizontal scale ranging from 0 = not present to 10 = as bad as you can imagine. Participants were instructed to respond to the side-effect questionnaire administered 2 weeks post-treatment with answers that reflected their symptom severity at its worst at any point during treatment. Responses to the questionnaire administered 6 months post-treatment were based on symptoms during the previous 5 days. Total symptom burden was calculated by adding the severity of each of the 12 symptoms on a scale of 0 (symptom not present at all) to 10 (symptom severity as bad as you can possibly imagine). Total symptom burden scores have a possible range of 0–120 (0 = no symptoms present at all; 1–120 = symptoms present, with 120 indicating all symptoms present at the highest imaginable severity).
Statistical Analyses
For descriptive statistics, χ2 and Student's t-tests were used to analyze the difference between smokers and nonsmokers for categorical variables and continuous variables, respectively. Unadjusted between-group comparisons were made using analysis of variance and adjusted between-group comparisons were made using analysis of covariance (ANCOVA). Covariates included age, gender, racial/ethnic category, marital status, occupation, education, self-rated health status, site of diagnosis, year of diagnosis, Karnofsky performance status score, and treatment (chemotherapy, radiation, or both) reported at time point 1. In an effort to preserve statistical power, model selection was performed in a stepwise, backward manner, adding all the covariates at once. Covariates with p > .20 were removed one at a time, starting with the variables with the highest p-value. All covariates with p ≤ .20 were kept in the model. The baseline level of side-effect severity was included as a covariate in the ANCOVA model for the corresponding side effect being tested.
The presence of severe symptoms by smoking status was also investigated. Based on previous literature, each side effect was assigned a descriptor based on its 0–10 rating: 0 was assigned as not present, 1–3 was assigned as mild, 4–6 was assigned as moderate, and ≥7 was assigned as severe [52, 54]. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from covariate-adjusted logistic regression models as described above.
Results
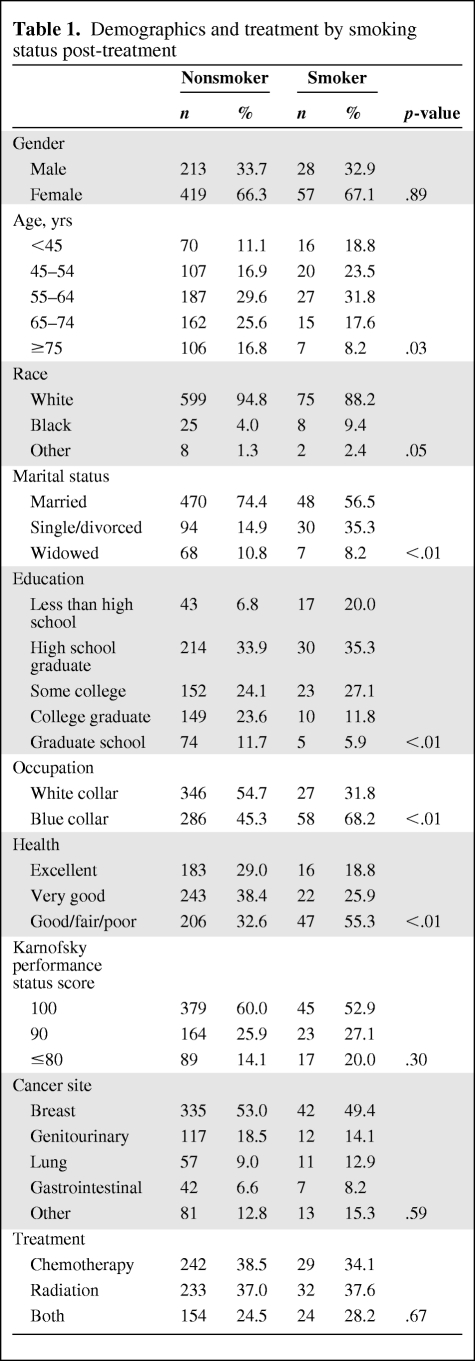
A total of 938 participants completed the survey at baseline (time point 1), 734 participants (632 nonsmokers, 85 smokers, and 17 nonactive smokers) completed the survey at the end of treatment (time point 2), and 616 participants (543 nonsmokers, 63 smokers, and 10 nonactive smokers) completed the survey at the 6-month follow-up (time point 3). Patients who completed the baseline survey but did not complete subsequent surveys either withdrew from the study or were lost to follow-up. In data not shown, there was no significant difference in smoking rates between those lost to follow-up and those who were not lost to follow-up. Table 1 displays the sociodemographic and treatment-related characteristics of smokers and nonsmokers. The distributions of gender, race, cancer site, Karnofsky performance status score, and treatment were similar for smokers and nonsmokers (p > .05). Smokers were more likely to be younger, single or divorced, less educated, employed in a manual labor job, and have a poorer self-rated health status (p < .05).
Table 1.
Demographics and treatment by smoking status post-treatment
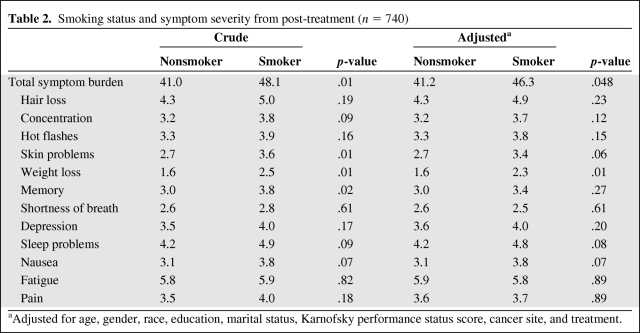
Table 2 displays the crude and adjusted means for total symptom burden along with the symptom severity for each of the 12 side effects by smoking status post-treatment. The total symptom burden and side-effect severity at this point reflect the participants' symptom severity at its worst at any point during treatment. After adjustment, the mean total symptom burden during treatment was significantly greater for smokers than for nonsmokers (46.3 versus 41.2; p = .048). Smokers reported a significantly higher severity of weight loss (2.3 versus 1.6; p = .01) as well as a trend toward a higher severity of skin problems (3.4 versus 2.7; p = .06), sleep problems (4.8 versus 4.2; p = .08), and nausea (3.8 versus 3.1; p = .07) than nonsmokers during treatment.
Table 2.
Smoking status and symptom severity from post-treatment (n = 740)
aAdjusted for age, gender, race, education, marital status, Karnofsky performance status score, cancer site, and treatment.
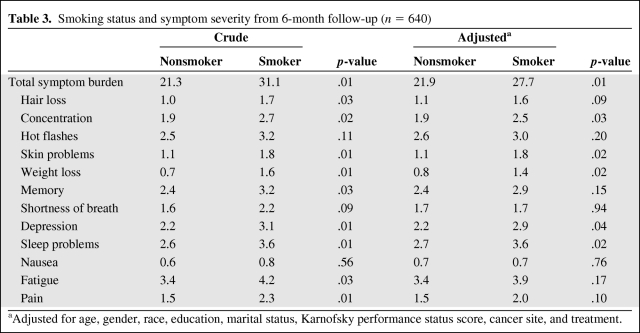
Table 3 displays the crude and adjusted means for total symptom burden along with the symptom severity for each of the 12 side effects by smoking status at the 6-month follow up. The total symptom burden and side-effect severity at this point reflect symptom severity during the previous 5 days. After adjustment, the mean total symptom burden at the 6-month follow-up was significantly greater for smokers than for nonsmokers (27.7 versus 21.9; p = .01). Smokers reported a significantly higher severity of weight loss (1.4 versus 0.8; p = .02), skin problems (1.8 versus 1.1; p = .02), sleep problems (3.6 versus 2.7; p = .02), depression (2.9 versus 2.2; p = .04), and concentration problems (2.5 versus 1.9; p = .03) than nonsmokers at the 6-month follow-up.
Table 3.
Smoking status and symptom severity from 6-month follow-up (n = 640)
aAdjusted for age, gender, race, education, marital status, Karnofsky performance status score, cancer site, and treatment.
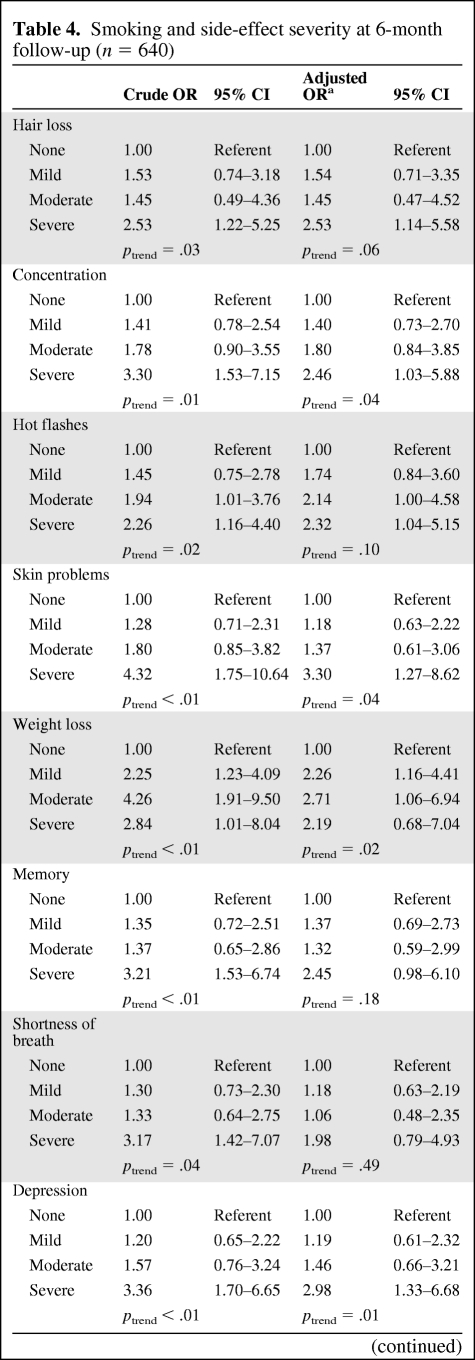
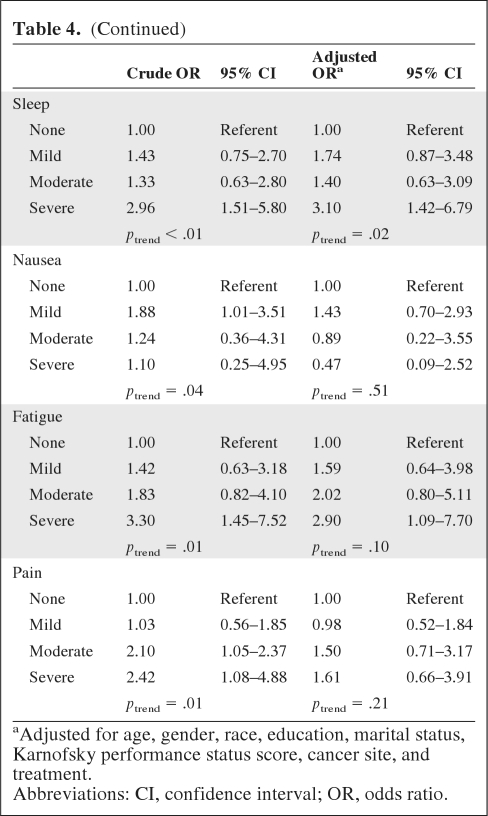
Table 4 shows the crude and adjusted ORs and corresponding 95% CIs for severe symptoms (≥7) by smoking status at the 6-month follow-up. After adjusting for confounders, smokers were significantly more likely to report severe levels of concentration problems (OR, 2.46; 95% CI, 1.03–5.88), skin problems (OR, 3.30; 95% CI, 1.27–8.62), hair loss (OR, 2.53; 95% CI, 1.14–5.58), depression (OR, 2.98; 95% CI, 1.33–6.68), sleep problems (OR, 3.10; 95% CI, 1.42–6.79), and fatigue (OR, 2.90; 95% CI, 1.09–7.70). Additionally, a significant trend was detected among smokers for higher levels of concentration problems, skin problems, weight loss, depression, and sleep problems.
Table 4.
Smoking and side-effect severity at 6-month follow-up (n = 640)
Table 4a.
(Continued)
aAdjusted for age, gender, race, education, marital status, Karnofsky performance status score, cancer site, and treatment.
Abbreviations: CI, confidence interval; OR, odds ratio.
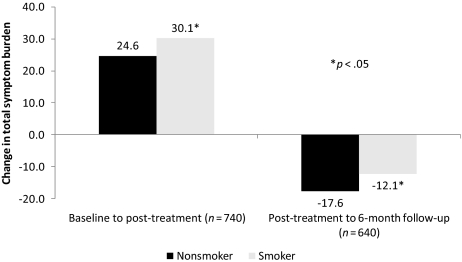
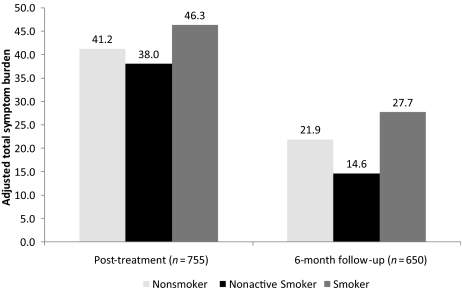
Figure 1 shows the adjusted change in total symptom burden from baseline to post-treatment and from post-treatment to 6-month follow up by smoking status. Smokers had a significantly greater increase in total symptom burden from baseline to post-treatment (+30.1 versus +24.6; p = .03) and a significantly lower decrease in total symptom burden from post-treatment to 6-month follow-up than nonsmokers (−12.1 versus −17.6; p = .02). Figure 2 shows the adjusted total symptom burden post-treatment and at the 6-month follow up for smokers, nonsmokers, and nonactive smokers (participants who smoked at baseline but stopped smoking during treatment). Nonactive smokers (NAS) and nonsmokers had statistically similar adjusted total symptom burdens post-treatment (38.0 versus 41.2, respectively; p = .55) and at the 6-month follow-up (14.6 versus 21.9, respectively; p = .16). The adjusted total symptom burden was significantly lower for nonactive smokers than for smokers at the 6-month follow up (14.6 versus 27.7; p = .02).
Figure 1.
Adjusted change in total symptom burden throughout cancer treatment.
Figure 2.
Adjusted total symptom burden by smoking status.
Discussion
These results show that smoking is associated with a higher mean total symptom burden during treatment and a greater increase in total symptom burden from prior to the initiation of cancer treatment to the highest severity at any point during treatment. These results are consistent with other studies that found that smoking during treatment leads to a lower QOL and marked decreases in physical, social, and emotional functioning [47, 48]. A higher symptom burden can lead to interruptions in treatment, reductions in dosages, and delays in therapy. Treatment interruptions and dosage reduction can, in turn, compromise treatment efficacy, resulting in lower survival rates [33, 34].
The difference in the mean total symptom burden between smokers and nonsmokers persisted 6 months after treatment, with smokers having a significantly greater symptom burden. Smokers reported significantly higher levels of concentration problems, skin problems, sleep problems, weight loss, and depression. Additionally, the decrease in the symptom burden from the end of treatment to the 6-month follow-up was significantly less for smokers than for nonsmokers. Not only did smokers have a higher mean symptom burden, but they also had higher rates of “severe” side effects. Smokers were significantly more likely to report severe fatigue, hair loss, concentration problems, hot flashes, skin problems, sleep problems, and depression at 6 months after treatment.
The responsible biological mechanisms that might account for the associations found between smoking and cancer treatment–related adverse outcomes (shorter survival, worse QOL, more SPTs, and more treatment complications) have yet to be elucidated. It is quite possible that the same mechanism that leads to these treatment-related adverse outcomes also increases symptom burden. Certain side effects, such as fatigue, depression, and insomnia, may be precipitated by the induction of cellular damage, which in turn induces inflammation, alters hormone levels, and disrupts circadian rhythm. Natural killer (NK) cells are large granular lymphocytes that control the spread of damaged cells. Smokers have lower circulating levels of NK cells than nonsmokers, and research has shown that a lower NK cell level may result in accelerated tumor progression, which may also exacerbate the side effects of cancer treatment [55–57]. Cigarette smoking also increases carboxyhemoglobin concentrations in the blood, leading to tissue hypoxia [40]. Tissue hypoxia can reduce the efficacy of radiotherapy in cancer patients and may also increase symptom burden [58]. Although several biological mechanisms are plausible, no firm conclusions about the role of these mechanisms can yet be drawn.
In addition to finding that smokers reported a higher symptom burden than nonsmokers during and after treatment, we also found that nonactive smokers (participants who smoked at baseline but stopped smoking during treatment) had symptom burden levels similar to those of nonsmokers. Nonactive smokers also had a significantly lower symptom burden than smokers at the 6-month follow-up. Although these findings must be interpreted cautiously because of the small sample size, they nonetheless highlight the potential importance of targeted smoking cessation as a means to decrease side-effect severity in cancer patients during and after treatment.
Research shows that patient interest in smoking cessation is greatly increased following a cancer diagnosis [38, 39]. At diagnosis, a window of opportunity is open to provide cessation services and it remains open well into survivorship because of the high rate of relapse [59]. Unfortunately, the majority of smoking cessation research has focused on the primary prevention of cancer, and only a limited number of clinical trials have evaluated smoking cessation targeted for cancer patients [59–61]. The lack of smoking cessation clinical trials may be a result of the reluctance of cancer patients to participate in trials unrelated to their treatment or the fact that many cancer patients report quitting after a diagnosis is made [62]. Regardless, smoking cessation services for cancer patients are lacking and do not meet the needs of the growing population of cancer patients [63, 64]. Additional research is needed in the area of smoking cessation specifically tailored for cancer patients.
Strengths
This study has a number of strengths, including a large sample of the general cancer population recruited from diverse community clinical oncology practices across the U.S. The questionnaire used to assess symptom burden, adapted from the MD Anderson Cancer Center, has been used in numerous studies on cancer patients and has demonstrated reliability and validity [52]. This study was also able to control for a large number of potential confounding variables including age, gender, race, self-rated health status, Karnofsky performance status score, treatment, and other sociodemographic variables, limiting the likelihood of residual confounding.
Limitations
Several methodological issues should be considered when interpreting the results of this study. First, this was a secondary data analysis of a dataset originally designed to assess the information needs of patients throughout cancer treatment. Because of this, only current smoking status was assessed, and other smoking variables such as smoking duration and amount smoked were not collected. Therefore, it is not possible to investigate any potential dose–response relationship between smoking and symptom burden. Additionally, the smoking question was self-reported without subsequent biochemical verification such as expired carbon monoxide or serum cotinine. Because of the longitudinal nature of the study, there was some loss to follow-up, although analyses indicated that smokers were no more likely than nonsmokers to be lost to follow-up. Also, we were unable to control for the potential confounding effects of alcohol and drug use because information was not collected on those variables. Lastly, the number of quitters was small, perhaps because of loss to follow-up, limiting the interpretability of the results related to quitting.
Summary and Clinical Implications
These findings show that smokers had a higher total symptom burden than nonsmokers during cancer treatment, which persisted at 6 months after treatment. Smoking at 6 months after treatment was also associated with higher odds of having severe levels of a number of side effects, including fatigue, concentration problems, depression, and others. Participants who quit smoking had symptom burden scores significantly lower than smokers, indicating the importance of smoking cessation among cancer patients. However, cessation research involving participants diagnosed with cancer and targeted cessation programs remain limited. Further research is needed to confirm these results and provide more robust data in terms of a potential dose–response relationship. Studies are also needed to test the efficacy of smoking cessation programs specifically tailored for cancer patients.
Clinically, these data suggest that patients who continue to smoke throughout cancer treatment are more likely to report a greater symptom burden. In turn, this higher symptom burden can interfere with the ability of patients to complete prescribed treatments (i.e., without dose reductions, delays, or early cessation). Clinicians should consider recommending that patients quit smoking prior to the beginning of treatment, and continue to target patients who have not quit after treatment initiation. Fortunately, the point of cancer diagnosis and the beginning of cancer treatment represent a teachable moment in health behavior change, rendering patients more receptive to cessation efforts. Clinicians can make use of the multiple resources and services available, such as state-sponsored quitlines that often offer free or discounted nicotine replacement therapy to help patients quit smoking [65]. Although clinician time with patients is limited, oncology nurses, social workers, and other health professionals may also be used to make these referrals and help patients with quit attempts and cessation efforts.
Acknowledgment
Grant Support was from the National Institutes of Health, 1R25-CA102618–01A1 and 5KL2 RR024136–05.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Luke J. Peppone, Karen M. Mustian, Gary R. Morrow, Scott McIntosh
Administrative support: Scott McIntosh
Provision of study material or patients: Gary R. Morrow
Collection and/or assembly of data: Gary R. Morrow
Data analysis and interpretation: Luke J. Peppone, Karen M. Mustian, Gary R. Morrow, Deborah J. Ossip, Scott McIntosh
Manuscript writing: Luke J. Peppone, Karen M. Mustian, Gary R. Morrow, Ann M. Dozier, Deborah J. Ossip, Michelle C. Janelsins, Lisa K. Sprod, Scott McIntosh
Final approval of manuscript: Luke J. Peppone, Karen M. Mustian, Gary R. Morrow, Ann M. Dozier, Deborah J. Ossip, Michelle C. Janelsins, Lisa K. Sprod, Scott McIntosh
References
- 1.Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97(12 suppl):3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse S, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute. Bethesda, MD, based on November 2009 SEER data submission, posted to the SEER Web site 2010. [accessed March 31, 2011]. Available at http://seer.cancer.gov/csr/1975_2007/
- 5.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Gullo S. Chemotherapy: What to do about special side effects. RN. 1977;40:30–32. [PubMed] [Google Scholar]
- 7.Redd WH, Jacobsen PB, Andrykowski MA. Behavioral side effects of adjuvant chemotherapy. Recent Results Cancer Res. 1989;115:272–278. doi: 10.1007/978-3-642-83337-3_38. [DOI] [PubMed] [Google Scholar]
- 8.Mock V. Evidence-based treatment for cancer-related fatigue. J Natl Cancer Inst Monogr. 2004:112–118. doi: 10.1093/jncimonographs/lgh025. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. The Oncologist. 2007;12(suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 10.Hickok JT, Morrow GR, Roscoe JA, et al. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Hickok JT, Roscoe JA, Morrow GR, et al. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 12.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: The scale of the problem. The Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence DP, Kupelnick B, Miller K, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 16.Servaes P, Gielissen MF, Verhagen S, et al. The course of severe fatigue in disease-free breast cancer patients: A longitudinal study. Psychooncology. 2007;16:787–795. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 17.Grunberg SM, Boutin N, Ireland A, et al. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer. 1996;4:435–439. doi: 10.1007/BF01880641. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger DS, Armstrong DK, Barbour S, et al. Antiemesis. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:572–595. doi: 10.6004/jnccn.2009.0039. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JR, MacLean AW, Brundage MD, et al. Sleep disturbance in cancer patients. Soc Sci Med May. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 21.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savard J, Villa J, Ivers H, et al. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 23.Schagen SB, Hamburger HL, Muller MJ, et al. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol. 2001;51:159–165. doi: 10.1023/a:1010635229762. [DOI] [PubMed] [Google Scholar]
- 24.Schagen SB, van Dam FS, Muller MJ, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 26.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 27.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 28.Mehnert A, Scherwath A, Schirmer L, et al. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns. 2007;66:108–118. doi: 10.1016/j.pec.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ‘Subjective’ complaints and ‘objective’ neuropsychological test results. Psychooncology. 2009;18:775–782. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 30.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249:751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y, Hickok JT, Morrow G. Fatigue and depression in cancer patients undergoing chemotherapy: An emotion approach. J Pain Symptom Manage. 2006;32:311–321. doi: 10.1016/j.jpainsymman.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 33.Frei E, 3rd, Canellos GP. Dose: A critical factor in cancer chemotherapy. Am J Med. 1980;69:585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- 34.Reich SD. The clinical application of drug dosing schedules in cancer therapy–Part II. Cancer Nurs. 1984;7:59–61. [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Peterson B, McBride C, et al. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 37.Dresler CM, Bailey M, Roper CR, et al. Smoking cessation and lung cancer resection. Chest. 1996;110:1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 38.Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995;75:569–576. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 39.Gritz ER, Nisenbaum R, Elashoff RE, et al. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes Control. 1991;2:105–112. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 40.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 41.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2009;74:1062–1069. doi: 10.1016/j.ijrobp.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 43.Khuri FR, Kim ES, Lee JJ, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]
- 44.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 45.Clark JR, McCluskey SA, Hall F, et al. Predictors of morbidity following free flap reconstruction for cancer of the head and neck. Head Neck. 2007;29:1090–1101. doi: 10.1002/hed.20639. [DOI] [PubMed] [Google Scholar]
- 46.Eifel PJ, Jhingran A, Bodurka DC, et al. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 47.Duffy SA, Ronis DL, Valenstein M, et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 48.Gritz ER, Carmack CL, de Moor C, et al. First year after head and neck cancer: Quality of life. J Clin Oncol. 1999;17:352–360. doi: 10.1200/JCO.1999.17.1.352. [DOI] [PubMed] [Google Scholar]
- 49.Hofman M, Morrow GR, Roscoe JA, et al. Cancer patients' expectations of experiencing treatment-related side effects: A University of Rochester Cancer Center–Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004;101:851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 50.Mustian KM, Griggs JJ, Morrow GR, et al. Exercise and side effects among 749 patients during and after treatment for cancer: A University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. 2006;14:732–741. doi: 10.1007/s00520-005-0912-6. [DOI] [PubMed] [Google Scholar]
- 51.Yates JS, Mustian KM, Morrow GR, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]
- 52.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.Armstrong TS, Cohen MZ, Eriksen L, et al. Content validity of self-report measurement instruments: An illustration from the development of the Brain Tumor Module of the M.D. Anderson Symptom Inventory. Oncol Nurs Forum. 2005;32:669–676. doi: 10.1188/05.ONF.669-676. [DOI] [PubMed] [Google Scholar]
- 54.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: How consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferson M, Edwards A, Lind A, et al. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer. 1979;23:603–609. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- 56.Peppone LJ, Mahoney MC, Cummings KM, et al. Colorectal cancer occurs earlier in those exposed to tobacco smoke: Implications for screening. J Cancer Res Clin Oncol. 2008;134:743–751. doi: 10.1007/s00432-007-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartter PI, Steinberg B, Barron DM, et al. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987;122:1264–1268. doi: 10.1001/archsurg.1987.01400230050009. [DOI] [PubMed] [Google Scholar]
- 58.Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 1986;65:1186–1188. [PubMed] [Google Scholar]
- 59.Gritz ER, Fingeret MC, Vidrine DJ, et al. Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 60.Klosky JL, Tyc VL, Garces-Webb DM, et al. Emerging issues in smoking among adolescent and adult cancer survivors: A comprehensive review. Cancer. 2007;110:2408–2419. doi: 10.1002/cncr.23061. [DOI] [PubMed] [Google Scholar]
- 61.Park ER, Puleo E, Butterfield RM, et al. A process evaluation of a telephone-based peer-delivered smoking cessation intervention for adult survivors of childhood cancer: The partnership for health study. Prev Med. 2006;42:435–442. doi: 10.1016/j.ypmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Abeloff MD. Abeloff's Clinical Oncology, Fourth Edition. Philadelphia: Churchill Livingstone/Elsevier, 2008:1–2592. [Google Scholar]
- 63.de Moor JS, Puleo E, Butterfield RM, et al. Availability of smoking prevention and cessation services for childhood cancer survivors. Cancer Causes Control. 2007;18:423–430. doi: 10.1007/s10552-006-0110-y. [DOI] [PubMed] [Google Scholar]
- 64.Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: Designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137(1 suppl):243S–248S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 65.Fiore M. U.S. Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, 2008:1–178. [Google Scholar]