Abstract
A series of conformationally restrained epothilone analogs with a short bridge between the methyl groups at C6 and C8 was designed to mimic the binding pose assigned to our recently reported EpoA-microtubule binding model. A versatile synthetic route to these bridged epothilone analogs has been successfully devised and implemented. Biological evaluation of the compounds against A2780 human ovarian cancer and PC3 prostate cancer cell lines suggested that the introduction of a bridge between C6-C8 reduced potency by 25–1000 fold in comparison with natural epothilone D. Tubulin assembly measurements indicate these bridged epothilone analogs to be mildly active, but without significant microtubule stabilization capacity. Molecular mechanics and DFT energy evaluations suggest the mild activity of the bridged epo-analogs may be due to internal conformational strain.
Keywords: Epothilone, total synthesis, modeling, natural products, antitumor agents
Introduction
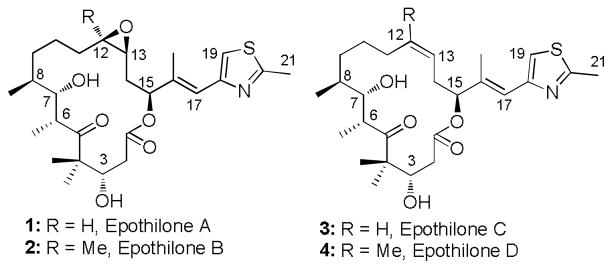
Epothilones are a family of cytotoxic polyketide natural products originally isolated from the bacterium Sorangium cellulosum.[1] Epothilone A (EpoA, 1) and epothilone B (EpoB, 2) (Figure 1), two major representatives, were recognized to be potent inhibitors against breast and colon cancer cells shortly after their initial isolation. [1] The mechanism of action of both EpoA and EpoB was established by the Merck group to be induction of tubulin polymerization in vitro resulting in the stabilization of microtubules under normally destabilizing conditions similar to the clinical anticancer drugs Taxol and docetaxel.[2] While epothilones exert their antiproliferative action in a similar way to Taxol, the two classes of compounds are distinctly different in terms of their potency and ability to inhibit the growth of multidrug-resistant cancer cell lines. [2–4] In contrast to Taxol, the epothilones are more efficacious promoters of cancer cell death with EpoB being the most active. Epothilones have also been proven to be very poor substrates for the phosphoglycoprotein 170 (P-gp) efflux pump. Thus, they retain almost full activity against P-gp-overexpressing, Taxol-resistant cell lines. Furthermore, epothilones are also active against cells with tubulin mutations which induce paclitaxel resistance.[4a] This suggests that epothilone-derived drugs might be useful for treating certain drug resistant tumors. In addition, although EpoA and EpoB were the major products isolated from the myxobacterium, numerous other related structures of the epothilone class have been identified as minor components of the fermentation of myxobacteria, including, for example, epothilone C (EpoC, 3) and D (EpoD, 4). These compounds also exhibit potent anticancer properties (Figure 1).[5]
Figure 1.
Structures of natural epothilones A-D.
These exceptional biological advantages, combined with the ease of synthesis by comparison with paclitaxel have evoked a vast research effort within academic and pharmaceutical research groups.[3] Numerous total and partial epothilone syntheses have been published since the determination of absolute stereochemistry in 1996.[6] During the development of these syntheses, a variety of methodologies have enabled the development of diverse libraries of synthetic analogs. In turn, these have contributed to mapping the extensive structure-activity relationship (SAR) profiles of epothilones and to elucidating interactions between the ligands and microtubules.[7–9] The tremendous efforts exerted to generate epothilone SAR profiles have greatly aided our understanding of the drug pharmacophore and the development of natural/unnatural analogs with improved biological activity and reduced toxicity. Significantly, these efforts have delivered at least seven compounds in advanced clinical trials, one of which recently won FDA approval for clinical use as an anti-cancer drug (Ixabepilone®).[10]
Since the discovery of the microtubule-stabilizing properties of epothilones in 1995, the details of the binding poses for the structurally diverse taxanes and epothilones have been pursued in order to facilitate the rational design of improved and perhaps structurally simplified analogs.[11–14] A variety of epothilone conformations and tubulin binding modes have been proposed by pharmacophore mapping,[11,12] solution NMR[15,16] and the superposition of epothilones on taxanes in the electron crystallographic tubulin complex.[13,14] By combining NMR spectroscopy, electron crystallography and molecular modeling, our group proposed a unique EpoA conformation and microtubule binding model that offers an alternative to the common pharmacophore model by describing the tubulin binding cavity as promiscuous.[17] According to this model, epothilone and Taxol occupy the same gross binding pocket, but the tubulin-ligand binding is mediated through different sets of hydrogen bonds and hydrophobic interactions for the two compounds. The electron crystallographic structure of epothilone was superposed with that of Taxol bound to tubulin. The overlap suggested that the thiazole moiety of epothilone A and the benzoyloxy phenyl of Taxol do not reside in the same region of the tubulin pocket. In addition, among the five oxygen-containing polar groups in epothilone, only the C7-OH falls near the similar C7-OH moiety in Taxol. In this comparison, the latter is the only common center between the two molecules.
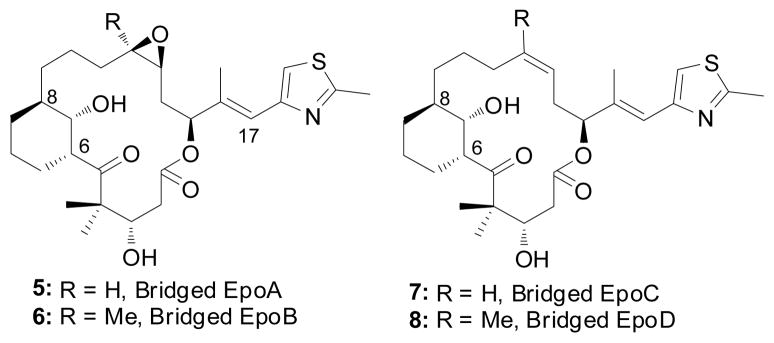
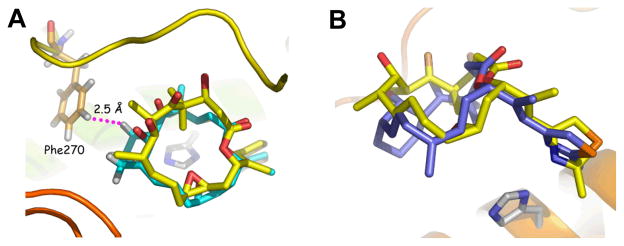
An unusual feature of the EpoA binding conformer derived by electron crystallography (EC) is the presence of a syn-pentane interaction between the methyl groups at C-6 and C-8. A conceivable test of the latter structural feature introduces a short bridge between the ring carbons to constrain the macrocycle to the EC model geometry. The cyclohexane rings depicted in 5–8 (Figure 2) illustrate one solution to the problem. A potential liability of this strategy is that the small ring, expanding the volume of the epothilone, might introduce steric congestion with the tubulin residues lining the binding pocket. To examine specific geometric details of the corresponding structures, 5 and 6 in the proposed binding form were optimized by molecular mechanics to show that both reside in a stable local minimum. Subsequent docking of the latter into the β-tubulin taxoid site suggested that the additional CH2 in the newly installed cyclohexane ring would not experience undue steric congestion with the protein (Figure 3).
Figure 2.
Structures of C6-C8 bridged epothilone analogs.
Figure 3.
Docking poses of C6-C8 bridged epothilone analogs in the electron crystallography-determined tubulin binding site: (A) Docking poses of 1 (yellow) and 5 (cyan); (B) Docking poses of 1 (yellow) and 6 (blue). The shortest H---H contact between ligand and protein is 2.5 Å, an acceptable, though minimal, van der Waals separation.
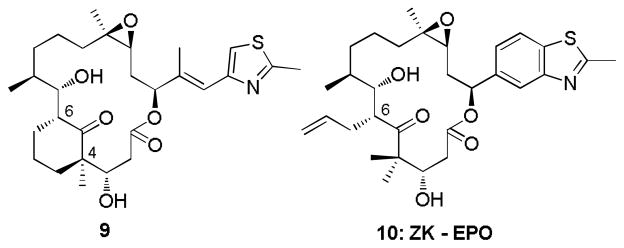
To our knowledge, modifications within the C6-C8 epothilone sector have received little previous attention (Figure 4).[18–20] In a related study, however, Martin and colleagues introduced a three carbon bridge between C4 and C6 from the pro-R methyl at C4 in the EpoB framework (9, Figure 4).[20] The compound had no effect when exposed to the MCF-7 tumor cell line. Since the EC binding conformer predicts the pro-S attachment to be the appropriate attachment direction, stereochemical inversion may be responsible for the lack of activity. Accordingly, bridged analogs 5–8 were selected as targets suitable for diagnostic tests of the EC epothilone binding model. In addition, ZK-EPO (Sagopilone, 10) in which a vinyl moiety was introduced to the C-6 methyl group and the C16-C21 side chain was displaced by a benzothiazole moiety, is currently undergoing advanced clinical development as the first fully synthetic epothilone candidate.[18]
Figure 4.
Selected epothilone analogs with modification around C6-C8.
Previously, we briefly communicated the synthesis of bridged 5, and reported the compound to be only weakly active against the A2780 ovarian cancer cell line.[19] In the present work, we describe the full synthetic details for 5–8. All four analogs were further subjected to cytotoxicity assessment against human ovarian (A2780) and prostate cancer (PC3) cell lines, tubulin assembly and microtubule cold stabilization.
Results and Discussion
Synthesis of C4-C8 Bridged Epothilones: Retrosynthetic Analysis
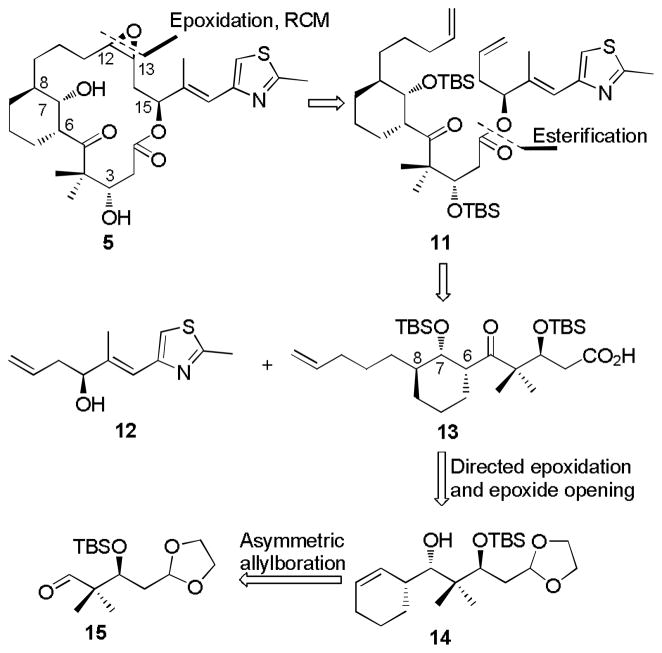
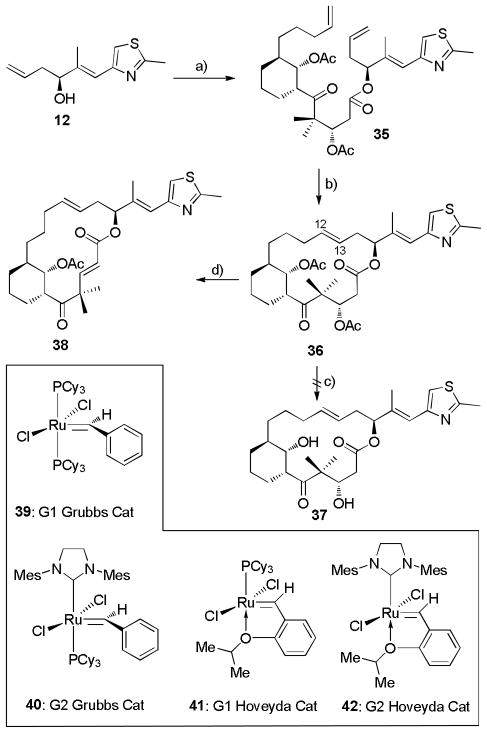
The first generation synthetic plan for the C6-C8 bridged epothilones, based on ring closure metathesis (RCM) as a key step, is summarized in Scheme 1. Compound 5 is used as an example. Although RCM is known to give both cis and trans isomers during total syntheses of natural epothilones, [21] it was applied as a key step here considering that isolation of both isomers could contribute to the structure activity profile (SAR) for the compound series. Applying a general disconnection strategy to epothilones, the bridged target 5 could be traced back to alcohol 12 and the advanced intermediate keto acid 13 after retrosynthetic epoxidation, RCM and esterification. The preparation of keto acid 13 was conceived as the key step along this route, by which the cyclohexane core structure with three adjacent chiral centers would be constructed. First, the stereochemistry at C7 and C8 in 13 was contemplated by means of sequential substrate directed epoxidation[22] and regiocontrolled epoxide opening from homoallylic alcohol 14. Moving further along the retrosynthetic path, alcohol 14 was envisioned to arise from aldehyde 15 by utilizing Brown’s asymmetric cyclohexenylboration stratetgy.[23]
Scheme 1.
Retrosynthetic Analysis of C6-C8 Bridged EpoA 5 by Olefin Metathesis.
Model Study
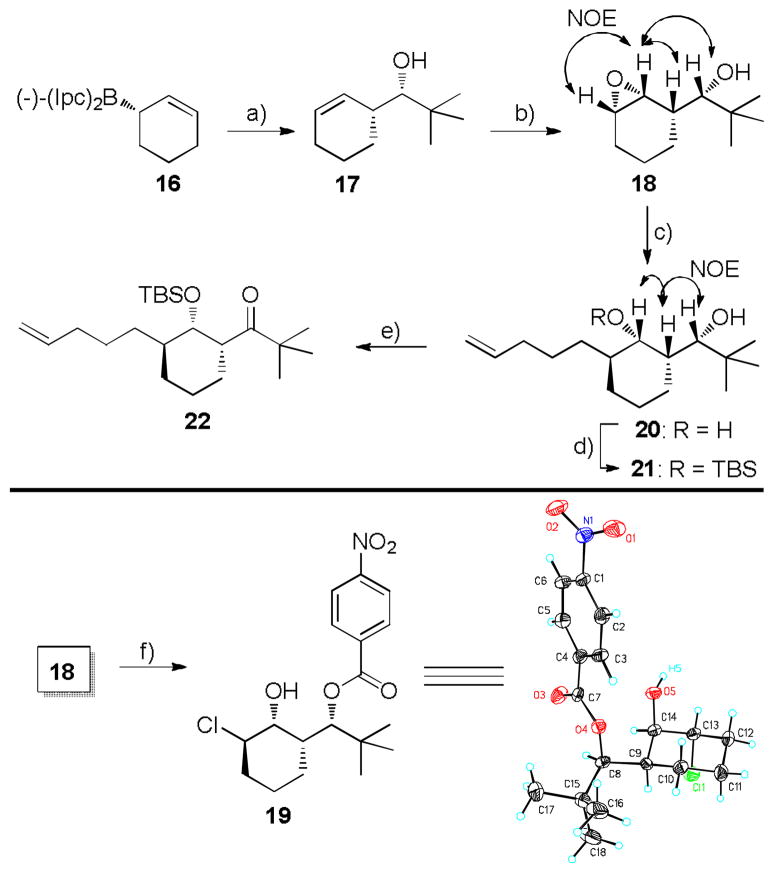
To test the feasibility of the substrate-directed epoxidation and subsequent regio-controlled epoxide opening strategy, a simplified model system was studied as shown in Scheme 2. The model study started from (−)-B-2-cyclohexen-1-yldiisopinocampheylborane 16, prepared by treating cyclohexa-1,3-diene with diisopinocampheylborane derived from (+)-α-pinene at −25 °Cin tetrahydrofuran (THF) as described by Brown.[23] The freshly prepared solution of borane 16 in THF was cooled to −100 °C, and treated with pivalaldehyde. After oxidation with H2O2 in the presence of NaHCO3, homoallylic alcohol 17 was obtained in 70% yield (dr > 95% by 1H NMR).[24]
Scheme 2.
Model Study. a) pivaldehyde, Et2O/THF, −100 °C, 70%, dr > 95%. b) t-BuOOH, VO(acac)2 (cat.), CH2Cl2, 88%; or mCPBA, CH2Cl2, 84%. c) CH2=CH(CH2)3MgBr, CuCN (cat.), Et2O, −60 → 0 °C, 89%. d) TBSOTf, 2,6-lutidine, CH2Cl2, −78°C, 85%. e) (COCl)2, Et3N, DMSO, CH2Cl2, −78 °C → RT, quant. f) 4-nitrobenzoyl chloride, pyridine, DAMP, THF, RT, 16%.
The highly stereoselective epoxidation of 17 was first achieved by a homoallylic alcohol-directed vanadium-catalyzed epoxidation strategy to afford hydroxy epoxide 18 in 88% yield as a single isomer. Further study indicated that mCPBA-based epoxidation also delivered the desired epoxide 30 in 84% yield. The relative configuration was confirmed by NOE. Considering the difference between the optical rotation value of 17 and that reported previously ([α]D25= − 3.4, c 1.0, CHCl3, Lit[23b]+ 6.83 l 0.5, neat), the p-nitro-benzoyl derivative 19 was prepared. X-ray crystallography of 19 unambiguously confirmed the absolute configuration of alcohol 17 and hydroxy epoxide 18 (Scheme 2). The site of epoxide ring opening by chloride anion further supports the original plan for regioselective nucleophilic opening of hydroxy epoxide.
With successful stereoselective epoxidation, the next key step in Scheme 2 is epoxide opening with an alkyl nucleophile in a regioselective manner. Fortunately, this transformation was successfully performed by treatment of 18 with freshly prepared 4-pentenylmaganesium bromide in the presence of CuCN (10 mol%). The desired diol 20 was obtained exclusively in 89% yield. We concur with Flippin and co-workers[25] that the regioselectivity of this metal catalyzed epoxide opening is not only controlled by the Fürst-Plattner rule favoring diaxial orientation, but also is most likely reinforced by a chelation process.
Following the remarkable success of the two key steps in the model study, we turned our attention to probe the regioselective protection of the two secondary hydroxy groups in 20 and the subsequent oxidation of the sterically hindered secondary alcohol in the model system. Selective silylation of the sterically less hindered OH group in 20 was achieved by slow addition of tert-butyldimethylsilyl triflate (TBSOTf) to a solution of 20 in CH2Cl2 at −78 °C in the presence of 2,6-lutidine to provide the mono-silyl ether 21 in 85% yield. Surprisingly, no silylation of the sterically hindered hydroxyl group was detected even when 1.5 equiv of TBSOTf was added. At this stage, a NOESY analysis for silyl ether 21 supported the previously described regioselectivity of the oxirane opening and selective TBS protection (Scheme 2). Swern oxidation of the sterically hindered alcohol afforded the desired olefinic ketone 22 in quantitative yield.
Construction of Building Blocks
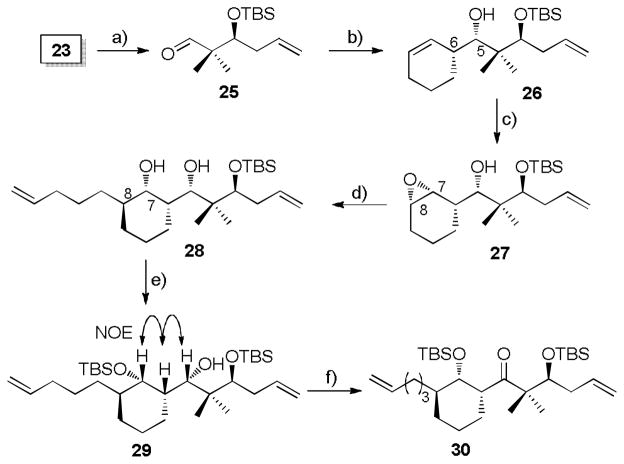
Encouraged by the results of the model studies, we proceeded to construct carboxylic acid 13. In pursuit of this advanced intermediate, the previously reported silyl ether 23[19] was subjected to ozonolysis, followed by acid catalyzed acetal protection with ethylene glycol and selective desilylation of the primary TBS silyl ether to afford primary alcohol 24 in 56% yield over three steps (Scheme 3). Exposure of 24 to Swern oxidation producedthe desired aldehyde 15 in quantitative yield.
Scheme 3.
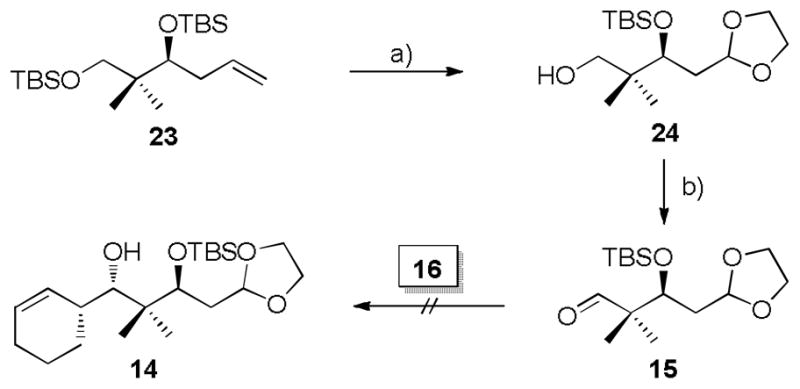
a) i) O3, CH2Cl2, −78 °C, then PPh3, RT; ii) ethylene glycol, PTSA (cat.), benzene, reflux; iii) HF/Pyridine, THF, 0 °C → RT, 53% (3 steps). b) (COCl)2, Et3N, DMSO, CH2Cl2, −78°C → RT, quant.
At this point, we were in a position to probe the feasibility of establishing the C5-C6 bond by Brown’s protocol.[23] Unfortunately, when aldehyde 15 was subjected to the standard Brown conditions, no workable amounts of product 14 could be separated (Scheme 3), while over 90% of aldehyde 15 was recovered before the oxidative quench. Attempts to facilitate the reaction by increasing temperature and reaction time did not lead to satisfactory results. We presume the allylboration is disfavored not only by steric hindrance from the α-quaternary carbon of the aldehyde, but also by the coordination between borane and the acetal oxygen atoms, which in turn interrupts the interaction between borane and aldehyde.
To address this problem, we turned our attention to an alternative aldehyde 25 (Scheme 4) in which a terminal olefin replaces the acetal in 15 and, thereby, avoids the potential coordination described above. Selective desilylation and subsequent Swern oxidation converted silyl ether 23 into the desired aldehyde 25. Upon treatment of the modified aldehyde 25 with freshly prepared borane 16, the desired homoallylic alcohol 26 was obtained in excellent yield and selectivity (96%, dr > 20:1 by 1H NMR) as shown in Scheme 4. Surprisingly, both the C-C bond formation and oxidative cleavage of the B-O bond were unexpectedly sluggish, taking about three weeks. Stereochemistry at C5 and C6 was assigned on the basis of the model study (Scheme 2).
Scheme 4.
a) i) HF/Pyridine, THF, 0 °C → RT, 88%; ii) (COCl)2, Et3N, DMSO, CH2Cl2, −78 °C α RT, 94%. b) 16, THF, −78 °C, then H2O2, NaHCO3, 40 °C, 92%, dr > 20:1. c) t-BuOOH, VO(acac)2 (cat.), CH2Cl2, 93%, dr > 20:1 d) CH2=CH(CH2)3MgBr (8–9 equiv.), CuCN (cat.), Et2O, −55 → 0 °C, 90%. e) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 85%. f) (COCl)2, Et3N, DMSO, CH2Cl2, −78 °C → RT, 99%.
With this chemistry in hand, the next phase involved a crucial stereoselective epoxidation and subsequent regioselective oxirane ring opening. Following the successful strategy achieved in the model study, alcohol 26 underwent chemoselective epoxidation by vanadium-catalysis to provide hydroxy epoxide 27 in 93% yield (dr > 20:1 by 1H NMR), followed by copper-catalyzed epoxide opening with Grignard reagent to furnish diol 28 in 90% yield as the sole diastereomer (Scheme 4). It is worth noting that an excess of Grignard reagent (8–9 equiv) was required to minimize formation of the bromohydrin side product.[26] Selective silylation of the sterically less hindered OH group in 28 furnished silyl ether 29 (85% yield). The relative stereochemistry of compound 29 was confirmed on the basis of NOESY experiments. In practice, the conversion from 26 to 29 could be completed in 93% yield over three steps without purification of the intermediates 27 and 28. To complete Scheme 4, Swern oxidation converted the secondary alcohol into ketone 30 in quantitative yield. The stereochemical assignment for ketone 30 was confirmed subsequently by comparison with its analog 50 (Scheme 9), the stereochemistry of which was determined by X-ray crystallography of a derivative.[19]
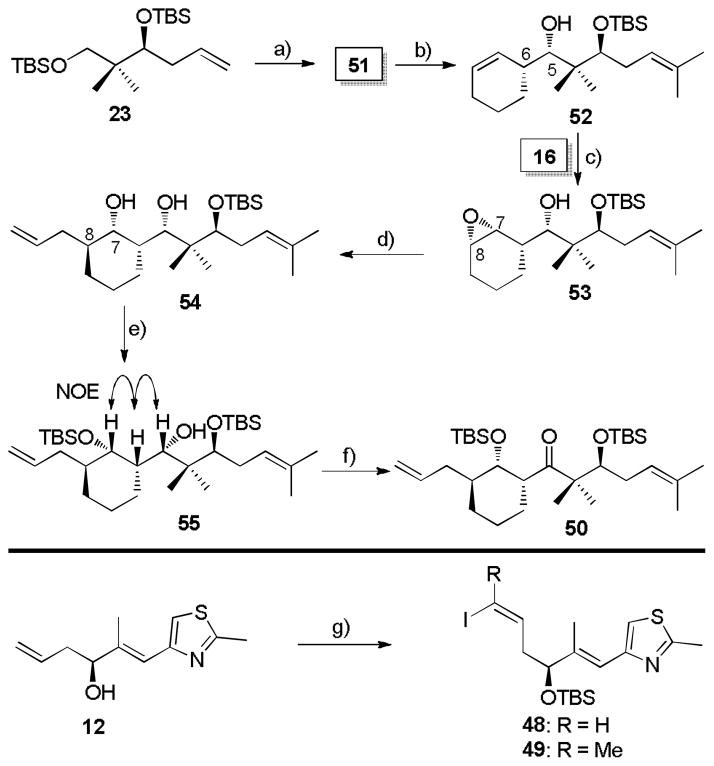
Scheme 9.
a) See Lit. [19]. b) 16, THF, −78 °C, then H2O2, NaHCO3, 40 °C, 96%, dr > 20:1. c) t-BuOOH, VO(acac)2 (cat.), CH2Cl2, 93%, dr > 20:1 d) AllylMgBr (8.0 equiv.), CuCN (cat.), Et2O, −55 → 0 °C, 85%. e) TBSOTf, 2,6-lutidine, CH2Cl2, −78 °C, 97%. f) (COCl)2, Et3N, DMSO, CH2Cl2, −78 °C → RT, 85%. g) See Lit. [39c, 42].
It is clear that aldehyde 25 has obvious advantages over aldehyde 15 in the context of cyclohexenylboration. However, application of 25 raised a second challenging problem, namely differentiation between the two terminal olefins with high structural similarity in 30. As will be shown, the terminal olefin homoallyic to OTBS could be selectively converted to a carboxylic acid by taking advantage of the masked homoallyic alcohol. Thus, we turned our attention to the hydroxy directed epoxidation to give a carboxylic acid precursor. To pursue this strategy, desilylation of 30 with trifluoroacetic acid afforded diol 31 (78% yield). Subsequently, vanadium catalyzed chemoselective epoxidation of 31, as expected, led to β-hydroxy epoxide 32 in 89% total yield as a mixture of two diastereomeric epoxides (ca. 10:1 by 1H NMR). The stereochemistry of the epoxide has been tentatively assigned in accord with the proposed model by Mihelich and coworkers.[27] In view of the subsequent cleavage of the epoxide, the diastereomeric epoxides were subjected to the next step without separation.
At this stage, we initially attempted to reinstall the TBS silyl ether onto 32 in order to pursue the original synthetic plan (Scheme 1). However, all attempts with classical conditions failed to give satisfactory results.[28] Fortunately, diacetyl epoxide 33 was cleanly obtained in 93% yield by treatment of alcohol 32 with acetic anhydride and 4-dimethylaminopyridine (DMAP). At this point, it appeared timely to transfer the primary epoxide to the carboxylic acid. The conversion was accomplished with a three step sequence. The epoxide first underwent tetrabutylammonium bisulfate catalyzed hydrolysis,[29] followed by NaIO4 cleavage of the resulting diol to furnish an aldehyde intermediate. Purification of the aldehyde by silica gel flash column chromatography was unmanageable due to the instability of this intermediate. Thus, Pinnick oxidation of the crude aldehyde with NaClO2 in the presence of 2-methyl-2-butene and NaH2PO4 in t-BuOH/H2O provided the desired carboxylic acid 34 in 45% yield over the three-step procedure (Scheme 5).
Scheme 5.
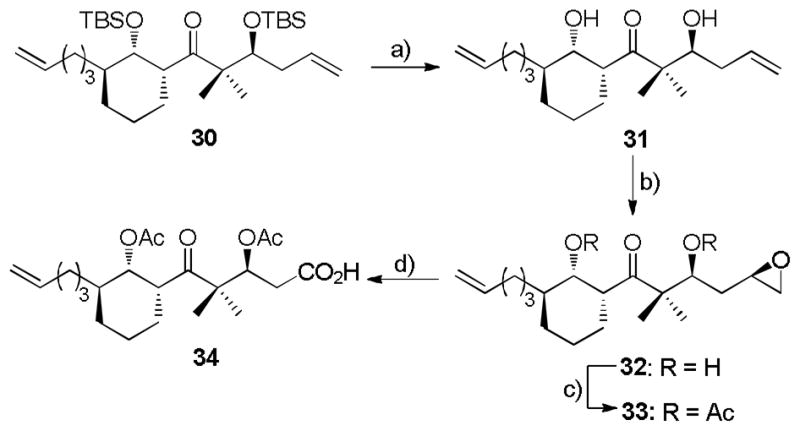
a) TFA, CH2Cl2, −20 → 0 °C, 78%. b) t-BuOOH, VO(acac)2 (cat.), CH2Cl2, 0 °C → RT, 89%, dr = 10:1 c) Ac2O, DMAP, CH2Cl2, 0 °C → RT, 93%. d) i) n-Bu4NHSO4 (cat.), CH3CN/H2O, 50 °C; ii) NaIO4, THF, RT, iii) NaClO4, NaH2PO4, 2-methyl-2-butene, t-BuOH/H2O, 45% (3 steps).
Assembly of Building Blocks and Synthesis of 12,13-trans-6,8-Bridged Epothilone via Olefin Metathesis
With the key building block 34 in hand, our attention was directed to the feasibility of the olefin metathesis strategy. Therefore, the coupling between alcohol 12 and carboxylic acid 34 was performed under the influence of 1-ethyl-3-((dimethylamino)propyl)carbodiimide hydrochloride (EDCI) and DMAP to furnish the proposed metathesis precursor 35 in 58% yield as depicted in Scheme 6. In this coupling reaction, serious β-elimination from the β-acetate of the carboxylic acid was responsible for the modest yield. However, β-elimination was completely suppressed by a modified Yonemitsu-Yamaguchi protocol,[30] giving keto ester 35 in 86% yield. Exposure of 35 to metathesis catalysts 39–42 under highly dilute conditions resulted in clean formation of a single trans-macrocyclic olefin 36 (JH12-H13 = 14.4 Hz). Grubbs catalysts 39 and 40 as well as the Hoveyda catalyst 41 gave the trans-product in high yields, while the reaction was unresponsive to Hoveyda catalyst 42 (Entry 5, Scheme 6). It is widely known that the E/Z selectivity of the ring closure metathesis depends on many factors including substrate, solvent, temperature and concentration.[31] In this specific case, attempts to modify the geometric outcome of the reaction by choosing solvents and temperatures as recorded in Table 1 were unsuccessful.
Scheme 6.
a) EDCI, DMAP, CH2Cl2, RT, 58%; or 2,4,6-trichlorobenzoylchloride, DMAP, Et3N, toluene, −78 → 0 °C, 86%. b) RCM, see text. c) DBU, CH2Cl2, 0 °C → RT, 96%.
Table 1.
Ring closure metathesis of 35 gives trans-olefin 36 exclusively.
| Entry | Cat. (10 mol%) | Conditions | Yields | E:Z [a] |
|---|---|---|---|---|
| 1 | 39 | CH2Cl2, RT, 12h | 84% | >20:1 |
| 2 | 39 | Toluene, 80 °C, 12h | 76% | >20:1 |
| 3 | 40 | CH2Cl2, RT, 12h | Quant | >20:1 |
| 4 | 40 | Toluene, 80 °C, 12h | quant | >20:1 |
| 5 | 41 | CH2Cl2, RT, 12h | < 5% | ND |
| 6 | 41 | Toluene, 80 °C, 12h | 95% | >20:1 |
The E/Z ratio was judged from crude 1H NMR.
The disheartening geometric results from olefin metathesis temporarily directed our attention to the 12,13-trans-6,8-bridged epothilone C (37, Scheme 6). Considering previous SAR studies suggesting that the non-natural epothilone analog 12,13-trans-epothilone C is only slightly less active then the natural epothilone C (3),[32] the C6-C8 bridged analog 37 was regarded as potentially providing valuable structural information for the project. With this in mind, we turned to the deacylation of 36 (Scheme 6). Surprisingly, none of the reaction conditions applied was able to accomplish deprotection to produce the dihydroxyl lactone 37. In all cases, either unreacted acetate was recovered or decomposition took place. One of the major side reactions arising from attempted deacylation was β-elimination leading to lactone 38, which could be alternatively prepared from 36 in 96% yield by treatment with 8-diazabicyclo[5.4.0]undec-7-ene (DBU). Such a process has been documented to suggest that the approximate 180° torsion angle around C2-C3 might be responsible for the elimination.[3, 33]
We presume the unsuccessful deacylation could also arise from the competition between hydrolysis and elimination of the β-acetate. In this specific case, β-elimination might be much faster than hydrolysis of the acetate. To facilitate deacylation, we envisioned introducing a substituent to the acetyl moiety which could increase the acetyl hydrolysis rate, while not significantly altering its leaving group character. With this scenario in mind, the chloroacetyl group was introduced, recognizing that it might be 350–700 fold more quickly hydrolyzed than acetyl depending on the nature of the intermediates.[34] As shown in Scheme 7, the required chloroacetate was prepared from epoxy alcohol 32 in quantitative yield. Subsequent exposure to NaIO4/H5IO6 mediated cleavage of the terminal epoxide to generate an aldehyde which was subsequently subjected to Pinnick oxidation to furnish the carboxylic acid 43 in 72% overall yield. Esterification of keto acid 43 by alcohol 12 was achieved in modest yield with the modified Yonemitsu-Yamaguchi protocol. [35] Without complication, the following olefin metathesis led cleanly to trans-product 45 (JH12-H13 = 14.8 Hz). For example, a 77% yield of 45 was achieved with Hoveyda second generation metathesis catalyst 42. After screening various conditions for the crucial deacylation step, we discovered that it could be successfully performed by careful treatment of 45 with ammonium hydroxide in methanol (1/10, v/v) followed by treatment with ammonia in methanol to afford the 12,13-trans-6,8-bridged EpoC analog 37 in 57% yield (Scheme 7).
Scheme 7.
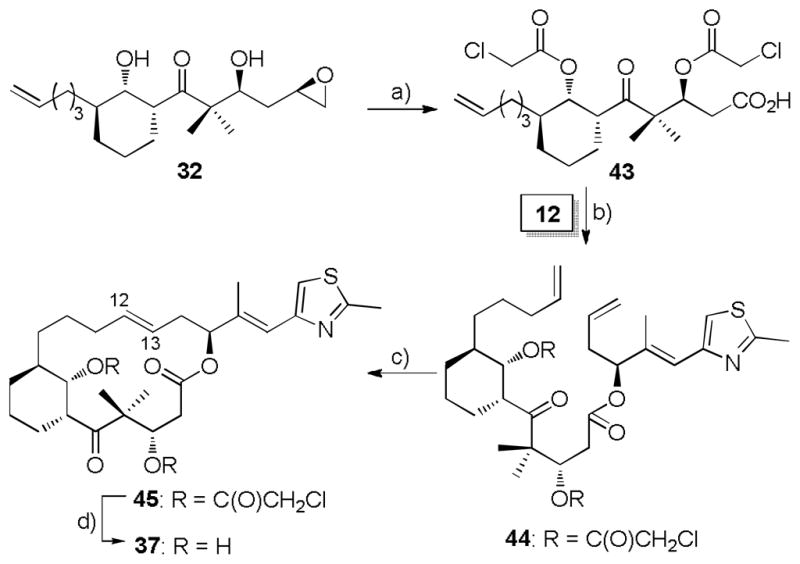
a) i) (ClCO2CO)2, DMAP, pyridine, CH2Cl2, 0 °C, quant.; ii) NaIO4/H5IO6, THF/H2 O; iii) NaClO4, NaH2PO4, 2-methyl-2-butene, t-BuOH/H2O, 72% (2 steps). b) 2,4,6-trichlorobenzoylchloride, DMAP, Et3N, toluene, −78 → −35 °C, 49%. c) RCM, cat. 42, CH2Cl2, RT, 77%. d) NH4OH/MeOH, 0 °C then NH3/MeOH, 0 °C, 57%.
To bring the synthesis to a close, we initially attempted to isomerize the C12-C13 trans double regioselectively to the desired cis geometry. Unfortunately, following attempts such as photoirradiated isomerization,[18] iodine-catalyzed free radical isomerization[36] and Vedejs isomerization,[37] no observable amounts of the isomerized product could be separated (assuming isomerization occurred). Either unreacted trans-olefin was recovered or decomposition took place.
Second Generation Synthesis via Suzuki Coupling
As discussed above, some surprising limitations surfaced in the ring forming olefin metathesis reaction. Although there is still much opportunity to optimize reaction conditions and employ alternative methods such as the molybdenum-based Schrock catalyst,[38] the epothilone literature teaches that the stereochemical outcome of the RCM process is highly substrate dependent.[21a,39] With such an ambiguous precedent, it was imperative to select a reliable method for accessing the desired Z-stereochemistry. An important alternative to introduce the Z-double bond at C12-C13 is Danishefsky’s B-alkyl Suzuki coupling strategy.[40] Given the widespread application of this strategy in epothilone synthesis, we elected to explore its utility for our targets.
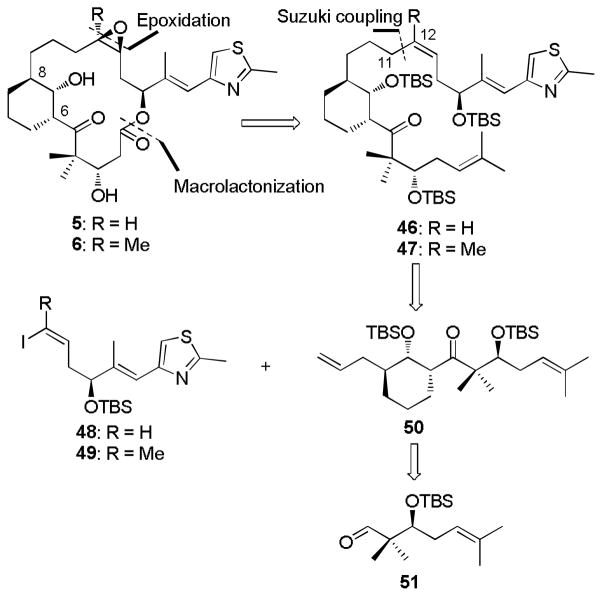
The retrosynthesis for bridged epothilones 5 and 6 via the second generation Suzuki coupling strategy is summarized in Scheme 8.[41] The key disconnection for this route is at the C11-C12 bond, leading to vinyl iodides 48/49 and olefin 50 as the Suzuki coupling partners. The keto diene 50 derives from aldehyde 51 following a sequence similar to that for the synthesis of dienyl ketone 30 in Scheme 4. In contrast to keto diene 30, a gem dimethyl moiety was introduced at the right-side terminal olefin of 50 in order to easily differentiate the two olefins.
Scheme 8.
Retrosynthesis of C6-C8 Bridged EpoA/B by Suzuki Coupling.
Construction of Building Partners for Suzuki Coupling
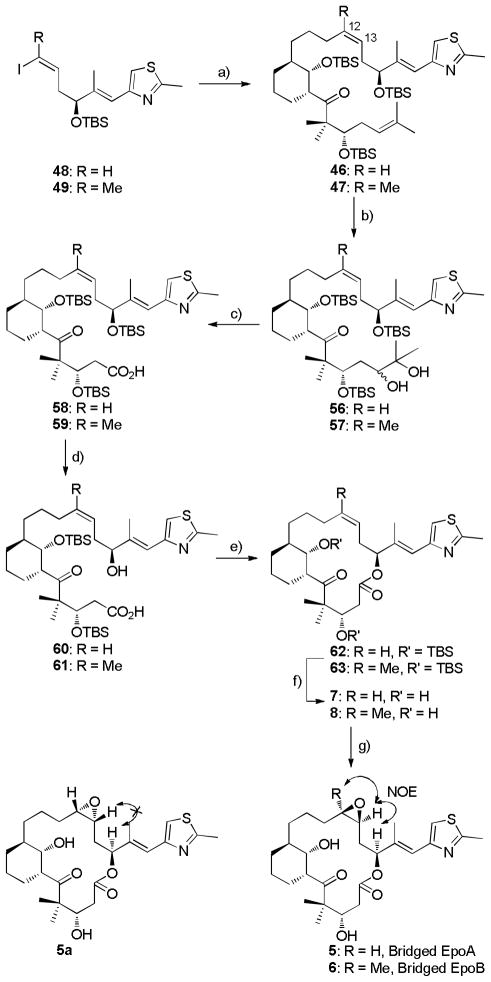
To pursue this modified route, the preparation of Suzuki coupling precursor 50 is illustrated in Scheme 9.[19] The modified aldehyde 51 was firstly obtained by a four-step sequence from 23 in 58% yield, followed by allylboration of aldehyde 51 with freshly prepared (−)-B-2-cyclohexen-1-yldiisopinocampheylborane 16 to give homoallylic alcohol 52 in 96% yield (dr > 20:1 by 1H NMR). Then, using the previously validated vanadium-catalysis strategy, alcohol 52 was converted to epoxy alcohol 53 in 93% yield (dr > 20:1 by 1H NMR). Reaction of epoxide 53 with allylmagnesium bromide in a copper catalyzed fashion furnished epoxide-opened product 54 (85% yield) along with a trace of the C7-alkylated isomer and bromohydrin. The sterically less hindered hydroxyl group from 54 was selectively converted to TBS silyl ether 55 in 97% yield. At this point, a NOESY analysis was executed to confirm the relative stereochemistry (Scheme 9). Finally, the sterically hindered secondary alcohol in diene 55 was transformed to dienyl ketone 50 by Swern oxidation in 85% yield. The absolute configuration of dienyl ketone 50 has been verified by X-ray crystal structure analysis of a derivative.[19] Preparation of the other Suzuki coupling partners, vinyl iodides 48/49, was accomplished from alcohol 12 in a four-step sequence (Scheme 9).[39c, 42]
Completion of the Synthesis of Bridged Epothilones A-D
With the requisite coupling precursors in hand, the final steps in the synthesis of bridged epothilones 5/6 were carried out as depicted in Scheme 10. After regioselective hydroboration with 9-BBN, olefin 50 was coupled with vinyl iodides 48/49 in accordance with an approach reported by Danishefsky to furnish cis-olefins 46/47 (JH12-H13 = 10.8 Hz, 46) in 92% and 57% yields respectively.[40a] The following crucial regioselective dihydroxylation of trienes 56/57 was performed under Sharpless asymmetric dihydroxylation conditions[43] to selectively convert the gem-dimethyl olefin to diols 56/57 as a mixture of diastereomers (56: 42% yield, 86% BRSM, ca. 5:1 ratio by 1H NMR; 57: 42% yield, 87% BRSM, ca. 4:1 ratio by 1H NMR ). The stereochemistry of the hydroxyl group was undefined. Without separation, the resulting mixture of diols was subjected to NaIO4 mediated glycol cleavage and subsequent Pinnick oxidation to furnish the corresponding carboxylic acids 58 and 59 in 72% and 58% yields after two steps (Scheme 10).
Scheme 10.
a) 9-BBN, Cs2CO3, AsPh3, PdCl2(dppf), DMF/H2 O, RT, 46: 92%, 47: 57%. b) (DHQD)2PHAL, K2CO3, K3Fe(CN)6, CH3SO2NH2, K2OsO4 2H2O, t-BuOH/H2O, 0 °C → RT, 56: 42% (86%, BRSM), dr = 5:1; 57: 42% (87%, BRSM), dr = 4:1. c) i) NaIO4, THF/H2 O, 0 °C; ii) NaClO4, NaH2PO4, 2-methyl-2-butene, t-BuOH/H2O, 58: 78% (2 steps), 59: 58% (2 steps). d) TBAF, THF, 0 °C → RT, 60: 95%, 61: 90%. e) 2,4,6-trichlorobenzoylchloride, Et3N, DMAP, toluene, RT, 62: 51%, 63: 60%. f) TFA, CH2Cl2, −20 → 0 °C, 7: 88%, 8: 91%. g) DMDO, CH2Cl2, −50 → −30 °C, 5: 84%, dr = 2:1, 6: 52%, dr > 20:1.
As shown in scheme 10, final steps in the synthesis of the target compounds 5/6 involved conversion of keto acids 58/59 to dihydroxy lactones 60/61 by employing a procedure utilized by Nicolaou in the total synthesis of epothilones A and B.[44] Selective desilylation with tetra-n-butylammonium fluoride (TBAF), followed by Yamaguchi lactonization (62 in 51% yield, and 63 in 60% yield) and global desilylation in the presence of a freshly prepared trifluoroacetic acid (TFA) solution in CH2Cl2 (v/v, 1/4) gave C6-C8 bridged epothilone C (7, 88% yield) and epothilone D (8, 91% yield). Finally, we were pleased to obtain the C6-C8 bridged epothilone A as a mixture of 5 and its cis-epoxide diastereomer 5a (84% total yield, ca. 2:1 ratio, 1H NMR) by treatment with 3,3-dimethyldioxirane (DMDO) as described by Danishefsky.[40a] Fortunately, these two diastereomers were separable by preparative thin-layer chromatography. In a similar fashion, the C6-C8 bridged epothilone D analog 8 was converted to the bridged epothilone B analog 6 in 52% yield (dr > 20:1 by 1H NMR). The stereochemistry of the epoxide of these bridged epothilones was determined by 1D and 2D NOE analysis (Scheme 10).
Bioactivity: Microtubule Assembly and Cytotoxicity
The C6-C8 bridged epothilone analogs 5a, 5–8, 36–38 were exposed to A2780 ovarian cancer and PC3 prostate cancer cell lines to evaluate their antiproliferative properties (Table 2). In general, these C6-C8 bridged compounds are significantly less potent than the corresponding open chain analogs. Against the A2780 cell line, compound 38 exhibited the highest potency with an IC50 = 1.1 μM, but it is still 27-fold less potent than EpoD. C6-C8 Bridged Epo B 6 exhibited the highest potency against the PC3 prostate cancer cell line with a 206-fold potency loss by comparison with the activity of EpoD.
Table 2.
Cytotoxicity of epothilone B, D and C6-C8 bridged epothilone analogs (IC50, μM)
| Cmpd | EpoD | 5a | 5 | 6 | 7 | 8 | 36 | 37 | 38 |
|---|---|---|---|---|---|---|---|---|---|
| A2780 | 0.04 | 24.3 | 8.5 | 3.6 | 19.0 | 5.1 | 5.6 | 9.6 | 1.1 |
| PC3 | 0.016 ± 0.001 | 10.3 ± 1.6 | 11.6 ± 0.3 | 3.3 ± 0.2 | 16.3 ± 1.8 | 6.7 ± 0.2 | 7.6 ± 0.2 | 10.7 ± 0.6 | 9.8 ± 0.8 |
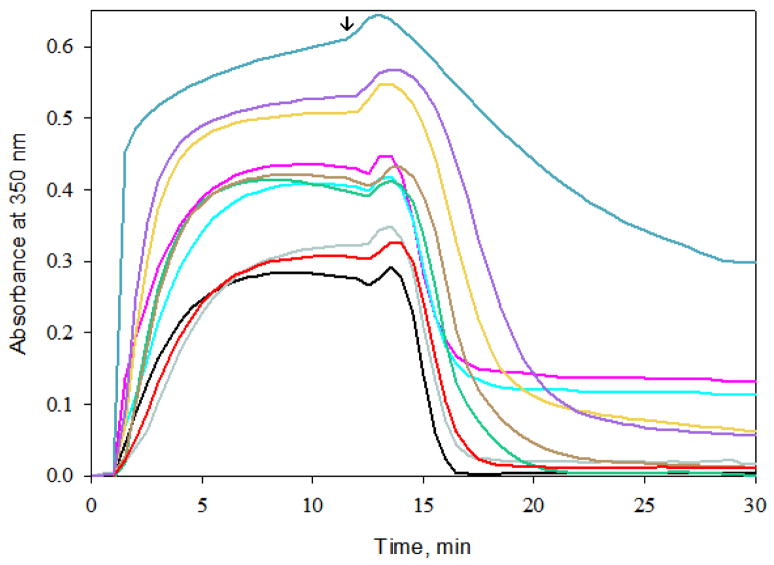
The ability of C6-C8 bridged epothilones to promote tubulin assembly and stabilize the microtubules against cold induced disassembly was also studied using 50 μM of the compounds in 4% (v/v) DMSO. The results were compared to that of 10 μM PTX under identical conditions (Figure 5). Compounds 37 and 5a displayed the least effect on tubulin assembly. Compounds 6 and 8 were the most active, however, less so than PTX. None of the C6-C8 bridged epothilones showed significant ability to stabilize the microtubules against cold induced disassembly.
Figure 5.
Tubulin assembly and microtubule cold stabilizing activity of bridged epothilones and PTX (
 ), 5a (
), 5a (
 ), 5 (
), 5 (
 ), 6 (
), 6 (
 ), 7 (
), 7 (
 ), 8 (
), 8 (
 ), 36 (
), 36 (
 ), 37 (
), 37 (
 ), 38 (
), 38 (
 ). Tubulin (10 μM) was incubated with 50 μM of a bridged epothilone or 10 μM PTX in the presence of 1 mM GTP and 4% DMSO in PME. Assembly was monitored in terms of apparent absorption at 350 nm. The arrow indicates temperature drop from 37 °C to 4 °C. For reference, a tubulin sample in the absence of promoter (–) was also included.
). Tubulin (10 μM) was incubated with 50 μM of a bridged epothilone or 10 μM PTX in the presence of 1 mM GTP and 4% DMSO in PME. Assembly was monitored in terms of apparent absorption at 350 nm. The arrow indicates temperature drop from 37 °C to 4 °C. For reference, a tubulin sample in the absence of promoter (–) was also included.
Molecular Mechanics and DFT Energy Evaluations
Obviously, these biological outcomes are not consistent with our having installed a C6-C8 bridge that constrains 5–8 to an efficacious bioactive epothilone pose. There are several possible explanations for this: 1) the C6-C8 bridge has retained the target conformation of the epothilone ring, but altered the binding mode to prevent effective coordination with the protein; 2) incorporation of the C6-C8 bridge has raised the energy of the bound target conformations of 5–8 making them inaccessible to microtubules; 3) the molecules have been constrained as expected (Figure 3), but the target conformation is not the de facto bound form as proposed. Concerning 1), there are precedents for loss of activity resulting from internal bridge-building in taxanes. In two cases, NMR evidence supported the conclusion that the ligand conformations appear to be retained, but the additional tether interferes sterically with the tubulin binding site, lifts the molecule higher in the pocket and thereby reduces ligand binding.[45] In one of these reports involving cyclic constraints in the C-13 side chain, the differences in tubulin binding and MCF7 cell cytotoxicity (5–10 fold and 37–120 fold, respectively) arose from expansion of ring size from 5-members to 6.[50a] Thus, in spite of our initial assessment that a 6-membered ring in 5–8 would cause little steric congestion (Figure 3), the degree of crowding may have been underestimated. With respect to 2), we have examined the conformational landscapes of 1 and 5 to determine the energy differences between the respective bound conformations (Figure 3A) and their corresponding global minima (GM). Thus, individual 25,000 step conformational searches for the two structures were performed with the MMFF/GBSA/H2O protocol in Schrodinger’s Macromodel.[46] The GM was then re-optimized with the OPLS-2005, MM3 and AMBER molecular mechanics methods.[46] The EpoA EC structure (1, Figure 3A) and that of 5 based on it were likewise optimized with the same four methods to put the structures (LM or local minimum forms) on the same energy scales. A comparison of the EC-related structures and the optimized variants demonstrated minimal average root mean square deviations (RMSD) of 0.098 to 0.35 Å for all heavy atoms. Superposition of the EC-based structures and the optimized OPLS-2005 conformers (average heavy-atom RMSD values of 0.26 and 0.14 Å, respectively) are provided in the Supporting Information to demonstrate essentially a perfect match between the EC bound and force-field optimized conformers. The energy differences between GM and LM for epoA and 5 for each method (ΔΔE((GM-LM)epoA – (GM-LM)5) were computed to show that epoA falls lower by 3.0 (OPLS-2005), 2.4 (AMBER), 1.1 (MMFF) and 0.8 (MM3) kcal/mol (see SI for details). Thus, in the molecular mechanics regime, the bridged structure 5 is predicted to require an additional 1.8 kcal/mol on average relative to epoA to achieve the bound form on tubulin in the context of the EC structure. In order to avoid issues surrounding the variable parameterization of the force field protocols, we calculated the energies of the OPLS-2005 optimized GM and LM forms for EpoA (1) and 5 with the density functional protocol B3LYP/6-31G*. The ΔΔE = 5.5 kcal/mol reinforces the force field trend and suggests that the weaker binding for compound 5 may be a result of increased internal conformational strain energy relative to EpoA. The possibility remains that 3) is the underlying cause of the bio-data presented here. However, until a definitive structure of the tubulin-epothilone complex is determined, the current EC[17] and NMR[47] structures remain the front-line contenders for the bound conformation of epothilones to β-tubulin.
Conclusion
A series of conformationally restrained epothilone analogs with a short bridge between methyl groups at C6 and C8 was designed to mimic the binding pose derived for our recently reported EpoA-microtubule binding model. A versatile synthetic route to these bridged epothilone analogs has been successfully devised and implemented. The key stereochemistry within the bridged C6-C8 sector was controlled by asymmetric allyboration followed by hydroxy-directed epoxidation and regiocontrolled opening of the resultant epoxide. The cis-C12-C13 double was constructed via Suzuki coupling while the ring closure metathesis exclusively gave trans selectivity.
The C6-C8 bridged epothilones were evaluated for their biological activity against the A2780 human ovarian cancer and PC3 prostate cancer cell lines. The cytotoxicity data implies that these epothilone analogs are considerably less potent than the natural epothilones. The tubulin assembly and microtubule cold stabilization assay reveal that compounds 6 and 8 inhibit tubulin assembly weakly, while none of the bridged epothilone analogs show significant microtubule stabilization against cold induced disassembly. Possible causes for the poor activity of the bridged epothilones include steric congestion between tubulin and the C6-C8 bridge, torsional strain in the flexible portion of the epothilone ring raising the energy requirement for binding and a mismatch with the empirical binding pose. Insights into the first two explanations are provided.
Experimental Section
General
Unless otherwise noted, commercial reagents and solvents were used as received unless otherwise noted. Flash column chromatography was performed by employing either Sorbent Technologies 200–400 mesh or Waterman 230–400 mesh silica gel 60. Analytical thin-layer chromatography (TLC) was performed on pre-coated silica gel 60 F254 (0.25mm thick) from EM Science. TLC plates were visualized by exposure to ultraviolet light (UV) and/or exposure to phosphomolybdic acid or potassium permanganate TLC stains followed by brief heating on a hot plate. Preparative TLC separation was performed on Analtech preparative plates pre-coated with silica gel 60 UV254 (0.5, 1.0 or 1.5 mm thick). Melting points (mp), determined on a MEL-TEMP Melting Point Apparatus from Laboratory Devices, are uncorrected. Optical rotations were measured on a Perkin Elmer Model 341 digital polarimeter with a sodium lamp at room temperature. Infrared (IR) spectra were recorded on a Nicolet 370 with a diamond probe or an ASI ReactIR 1000 FI-IR Spectrophotometer with a silicone probe (wavenumbers (cm−1)). Where noted “neat”, the sample was loaded as a thin film. Proton nuclear magnetic resonance (1H NMR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra were determined on an INOVA400 (1H NMR: 400 MHz, and 13C NMR: 100 MHz) or INOVA600 (1H NMR: 600 MHz, and 13C NMR: 150 MHz) instrument. Chemical shifts for 1H NMR are reported in parts per million (δ scale) with deuterated chloroform (CDCl3) as the internal standard (7.26 ppm) and coupling constants are in hertz (Hz). The following abbreviations are used for spin multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, bs = broad singlet. Chemical shifts for 13C NMR are reported in parts per million (δ scale) relative to the central line of the triplet at 77.23 ppm for deuterated chloroform (CDCl3). High resolution mass spectra (HRMS) were obtained on a JEOL JMS-SX102/SX102A/E or Thermo Finnigan LTQ-FTMS instrument.
Experimental details, characterization data for all new compounds, NMR spectra of key intermediates, X-ray crystal structure data for 19 and results for energy calculations for 1 and 5 are available in the Supporting Information.
Molecular Modeling and Docking
The 3-D structure of bridged epothilones 5 and 6 were constructed based on the electron crystallographic (EC) pose of EpoA bound to a β-tubulin.[17] The resulting structure of 5 and 6 was then fully optimized with the MMFF/GBSA/H2O force field to provide the nearest local minimum. The latter was flexibly Glide-docked[48] into the electron crystallographic structure of EpoA-tubulin. [17] The best docking pose was chosen on the basis of the Glide scoring function together with visualizationto ensure a reasonable binding mode and match with the EC complex. Conformational analysis for 1 and 5 and energy evaluations are described in the Supporting Information.
trans-12,13-Macrolactone 36. Procedure A
To a solution of diene 35 (7.5 mg, 0.0125 mmol, 1.0 equiv) in CH2Cl2 (12.5 mL, 0.001 M) was added Grubbs catalyst I (1.1 mg, 0.00125 mol, 10 mol %), and the reaction mixture was allowed to stir at 25 °C for 12 h. After the completion of the reaction as established by TLC, the solvent was removed under reduced pressure and the crude product was purified by preparative thin-layer chromatography (Hexanes/ethyl acetate, 3/1) to afford the trans-lactone 36 (6.0 mg, 84%) as a white foam. Procedure B: To a solution of diene 35 (15 mg, 0.0249 mmol, 1.0 equiv) in toluene (25 mL) was added with Grubbs catalyst I (2.1 mg, 0.00249 mol, 10 mol %), and the reaction mixture was allowed to stir at 80 °C for 12 h. After the reaction is complete, the mixture was worked up according to the procedure described in procedure A to furnish 36 (10.9 mg, 76%). Procedure C: Diene 35 (13 mg, 0.0216 mmol, 1.0 equiv) was converted to 36 (12.2 mg, 100%) in accordance with the procedure described in procedure A except for the use of Grubbs catalyst II (1.8 mg, 0.0022 mol, 10 mol %). Procedure D: Diene 35 (13 mg, 0.0216 mmol, 1.0 equiv) was converted to 36 (10.1 mg, 100%) in accordance with the procedure described in procedure B except for the use of Grubbs catalyst II (1.8 mg, 0.0022 mol, 10 mol %). Procedure E: Diene 35 (12 mg, 0.02 mmol, 1.0 equiv) was converted to 36 (10.8 mg, 95%) in accordance with the procedure described in procedure A except for the use of Hoveyda-Grubbs catalyst II (0.6 mg, 0.002 mol, 5 mol %). The crude reaction mixtures in procedures A, B, C, D and E were determined to be >20:1 ratio of diastereomeric trans-olefin by 1H NMR spectroscopy. Rf = 0.37 (hexanes/ethyl acetate, 2/1); [α]22 D −47.8 (c 1.0, CHCl3); IR (thin film) νmax 2926, 2862, 1731, 1707, 1504, 1443, 1371, 1239, 1180, 1029, 972, 916, 731 cm−1; 1H NMR (600 MHz, CDCl3) δ ppm 6.97 (s, 1H, SCH=C), 6.50 (s, 1H, CH=CCH3), 5.87 (dd, J = 7.2, 5.2 Hz, 1H, CH2CHOAc), 5.57 (ddd, J =14.4, 7.2, 7.2 Hz, 1H, CH=CH), 5.47 (ddd, J =14.4, 7.2, 7.2 Hz,, 1H, CH=CH), 5.31 (dd, J = 9.4, 1.9 Hz, 1H, CHOC(O)CH2), 5.07 (s, 1H, CHCHOAc), 3.29-3.23 (m, 1H, CHC(O)), 2.69 (s, 3H, N=C(S)CH3), 2.71-2.68 (m, 1H, CH2CO2), 2.62-2.56 (m, 2H), 2.41 (dd, J = 15.3, 4.9 Hz, 1H), 2.21-2.15 (m, 1H), 2.10 (s, 3H, ArCH=CCH3), 2.05 (s, 3H, CH3CO2), 2.03 (s, 3H, CH3CO2), 1.98-1.89 (m, 2H), 1.86-1.76 (m, 1H), 1.72-1.67 (m, 2H), 1.57-1.493 (m, 3H), 1.40-1.35 (m, 2H), 1.33-1.21 (m, 2H), 1.14 (s, 3H, C(CH3)2), 1.07 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 211.62, 170.71, 170.27, 169.21, 164.79, 152.88, 137.68, 132.67, 126.91, 119.64, 116.73, 79.62, 71.33, 70.48, 53.62, 41.94, 37.83, 37.71, 36.50, 31.30, 28.82, 26.94, 24.99, 24.09, 21.42, 21.24, 20.00, 19.89, 19.50, 18.87, 15.39; HRMS calcd for C31H44NO7S 574.28385 [M + H]+, found 574.28292.
trans-2,3-keto lactone 38
A mixture of macrolactone 36 (21 mg, 0.0366 mmol, 1.0 equiv) in anhydrous CH2Cl2 (2 mL) was treated with 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) (55.7 mg, 0.366 mmol, 10.0 equiv) at room temperature. After being stirred for 3 h, no more 36 was detected from TLC. The solvent was removed under reduced pressure without further workup. The resultant residue was purified by preparative thin-layer chromatography (Hexanes/ethyl acetate, 4/1) to furnish product 38 (18.4 mg, 96%) as a colorless oil: Rf = 0.51 (hexanes/ethyl acetate, 2/1); [α]22D +17.4 (c 1.68, CHCl3); IR (thin film) νmax 2929, 2861, 1713, 1645, 1503, 1444, 1379, 1362, 1294, 1242, 1177, 1048, 1017, 992, 970, 913, 879, 731 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.40 (d, J = 16.0 Hz, 1H, CH=CHC(O)), 6.96 (s, 1H, SCH=C), 6.61 (s, 1H, CH=CCH3), 6.07 (d, J = 16.0 Hz, 1H, CH=CHC(O)), 5.56 (dd, J = 10.3, 2.2 Hz, 1H, CHOC(O)CH2), 5.53-5.37 (m, 2H, CH2CH=CH), 4.86 (s, 1H, CHCHOAc), 3.01-2.98 (m, 1H, CHC(O)), 2.71 (s, 3H, N=C(S)CH3), 2.52-2.49 (m, 1H), 2.44-2.39 (m, 1H), 2.19-2.14 (m, 1H), 2.11 (s, 3H, ArCH=CCH3), 2.00 (s, 3H, CH3CO2), 2.04-1.95 (m, 1H), 1.93-1.85 (m, 1H), 1.69-1.62 (m, 2H), 1.58-1.52 (m 1H), 1.50-1.37 (m, 3H), 1.26-1.12 ( m, 10H); 13C NMR (100 MHz, CDCl3) δ 210.45, 170.59, 165.38, 164.88, 152.72, 152.39, 138.22, 132.55, 127.20, 121.98, 112.00, 116.39, 77.77, 71.34, 51.91, 43.51, 39.24, 36.58, 33.09, 28.54, 26.72, 23.64, 23.29, 23.00, 22.79, 21.38, 19.46, 15.61; HRMS calcd for C29H40NO5S 514.26272 [M + H]+, found 574.26186.
trans-12,13-Hydroxy Lactone 37
A solution of chlorolactone 45 (60 mg, 0.093 mmol) in methanol (10 mL) at 0 °C was treated with ammonium hydroxide (0.5 mL), and stirred at that temperature until the reaction was complete (ca. 12 h). The solvent was removed under reduced pressure to give white foam. Next, the white foam was dissolved in methanol (10 mL), and treated with amino methanol (1mL, 7N in methanol) at 0 °C. After being stirred for 48 h, 1H NMR suggested the reaction was complete. The solvent was removed under reduced pressure and the residue was purified by preparative thin-layer chromatography (Hexanes/ethyl acetate, 15/4) to afford hydroxy lactone 37 (26 mg, 57%) as a white foam: Rf = 0.38 (CH2Cl2/MeOH, 15:1); [α]22D −11.6 (c 0.85, CHCl3); IR (thin film) νmax 3486 (br), 2930, 2860, 1729, 1679, 1505, 1444, 1405, 1374, 1336, 1297, 1247, 1177, 1123, 1085, 1046, 984, 915, 865, 726, 676 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.96 (s, 1H, SCH=C), 6.52 (s, 1H, CH=CCH3), 5.48 (dd, J = 9.8, 3.7 Hz, 1H, CHOC(O)), 5.45-5.40 (m, 1H, CH=CH), 5.37-5.32 (m, 1H, CH=CH), 4.41 (dd, J = 10.5, 1.8 Hz, 1H, CHOHC(CH3)2), 4.16 (s, 1H, CHCHOH), 3.67 (d, J = 1.5 Hz, 1H, CHOHC(CH3)2), 3.65 (d, J = 2.5 Hz, 1H, CHCHOH), 2.81 (d, J = 10.4 Hz, 1H, CHC(O)), 2.70 (s, 3H, N=C(S)CH3), 2.50-2.42 (m, 3H), 2.24-2.19 (m, 1H), 2.17 (d, J = 17.0 Hz, 1H, CH2CO2), 2.07 (s, 3H, CH=CCH3), 1.93-1.83 (m, 3H), 1.73 (bs, 1H), 1.59-1.52 (m, 3H), 1.51-1.43 (m, 1H), 1.34-1.22 (m, 5H), 1.21-1.12 (m, 2H), 1.02 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 221.27, 173.30, 165.04, 152.42, 137.41, 134.02, 126.68, 120.60, 116.80, 79.52, 71.59, 71.53, 53.85, 43.83, 38.41, 37.97, 36.69, 31.70, 28.14, 27.45, 24.84, 24.27, 22.49, 20.64, 19.46, 16.05, 15.12; HRMS calcd for C27H40NO5S 490.26272 [M + H]+, found 490.26064.
Bridged Epothilone C (7)
A solution of Yamaguchi lactonization product 62 (89 mg, 0.124 mmol) in CH2Cl2 (0.1 mL) was treated with a freshly prepared CF3CO2H/CH2Cl2(0.73 mL, v/v, 1:4) at −20 °C. The reaction mixture was allowed to reach 0 °C in 20 min and was stirred for additional 1 h at that temperature at which time all silyl ether disappeared from TLC plate. The mixture was diluted with CH2Cl2 (5 mL) and carefully neutralized by saturated aqueous NaHCO3. After separation, the aqueous phase was further extracted with CH2Cl2 (2 × 5 mL). The combined organics were dried over MgSO4, filtered and concentrated. The resulting residue was purified by preparative thin-layer chromatography (CH2Cl2/MeOH, 20/1) to afford pure desired epothilone C analog 7 (53.4 mg, 88%) as a colorless oil: Rf = 0.36 (CH2Cl2/MeOH, 15:1); [α]22D −86.8 (c 1.0, CHCl3); IR (thin film) νmax 3478 (br), 2928, 2860, 1736, 1678, 1507, 1443, 1409, 1291, 1248, 1187, 1084, 1046, 982, 913, 731 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.97 (s, 1H, SCH=C), 6.62 (s, 1H, CH=CCH3), 5.50 (ddd, J = 10.5, 10.5, 5.0 Hz, 1H, CH=CH), 5.40 (ddd, J = 10.5, 10.5, 5.0 Hz, 1H, CH=CH), 5.22 (d, J = 8.3 Hz, 1H, CHOC(O)), 4.42 (dd, J = 11.5, 1.8 Hz, 1H, CHOHC(CH3)2), 4.20 (s, 1H, CHCHOH), 3.89 (s, 1H, OH), 3.49 (s, 1H, OH), 2.98 (d, J = 10.8 Hz, 1H, CHC(O)), 2.76-2.63 (m, 4H), 2.50 (dd, J = 14.7, 11.6 Hz, 1H, CH2CO2), 2.32 (dd, J = 14.7, 2.3 Hz, 1H, CH2CO2), 2.30-2.24 (m, 1H), 2.18 (tt, J = 10.7, 7.6 Hz, 1H), 2.08 (s, 3H, CH=CCH3), 2.04-1.85 (m, 3H), 1.84-1.74 (m, 1H), 1.66-1.43 (m, 4H), 1.36-1.29 (m, 2H), 1.28 (s, 3H, C(CH3)2), 1.26-1.16 (m, 2H), 1.06 (s, 3H, C(CH3)2)); 13C NMR (100 MHz, CDCl3) δ 220.74, 170.52, 165.35, 152.04, 139.43, 133.17, 125.23, 119.44, 115.82, 78.76, 73.05, 69.62, 54.22, 43.97, 39.79, 39.54, 31.91, 30.05, 28.83, 28.10, 25.17, 23.92, 23.58, 20.85, 19.25, 17.77, 16.26; HRMS calcd for C27H40NO5S 490.26272 [M + H]+, found 490.26144.
Bridged Epothilone A (5) and (5a)
To a solution of bridged epothilone C (7) (23 mg, 0.047 mmol, 1.0 equiv) in dry CH2Cl2 (2mL) at −50 °C was added a freshly prepared dry solution of 3,3-dimethyldioxirane (1.18 mL, ca. 0.094 mmol, 0.08 M in acetone, 2.0 equiv). The resulting solution was allowed to warm to −30 °C for 2 h. A stream of argon was then bubbled through the solution to remove excess dimethyldioxirane. The crude mixture was determined to be a mixture of diastereomeric cis-epoxides (ca. 5:2 ratio by 1H NMR). Preparative thin-layer chromatography (CH2Cl2/MeOH, 20/1) to afford bridged epothilone A (7) (13.0 mg, 55%) as a white foam and the cis-epoxide diastereomer 5a (7.0 mg, 29%) as a white solid. 5: Rf = 0.34 (CH2Cl2/MeOH, 15:1); [α]22 D −26.9 (c 0.87, CHCl3); IR (thin film) νmax 3462 (br), 2930, 2864, 1731, 1679, 1509, 1444, 1413, 1390, 1293, 1258, 1181, 1154, 1085, 1046, 980, 919, 725 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.98 (s, 1H, SCH=C), 6.61 (s, 1H, CH=CCH3), 5.35 (dd, J = 9.9, 1.5 Hz, 1H, CHOC(O)), 4.38 (d, J = 10.3 Hz, 1H, CHOHC(CH3)2), 4.31 (s, 1H, CHCHOH), 4.00 (s, 1H, OH), 3.83 (bs, 1H, CHCHOH), 3.03 (ddd, J = 9.6, 3.0, 3.0 Hz, 1H, CH2CH-O(epoxide)CH ), 2.98 (d, J = 10.6 Hz, 1H, CHC(O)), 2.96-2.93 (ddd, J = 9.6, 3.0, 3.0 Hz, 1H, CH2CH-O(epoxide)CH), 2.68 (s, 3H, N=C(S)CH3), 2.52 (dd, J = 14.5, 11.3 Hz, 1H, CH2CO2), 2.30 (dd, J = 14.5, 2.5 Hz, 1H, CH2CO2), 2.24 -2.18 (m, 1H), 2.09 (s, 3H, CH=CCH3), 2.08-2.02 (m, 1H), 1.95-1.84 (m, 3H), 1.80 (ddd, J = 15.0, 9.9, 9.9 Hz, 1H), 1.68 (ddd, J = 25.4, 12.1, 5.3 Hz, 1H), 1.63-1.56 (m, 2H), 1.56-1.46 (m, 2H), 1.44-1.39 (m, 1H), 1.38-1.22 (m, 6H), 1.08 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 220.87, 170.45, 165.54, 151.68, 138.84, 120.16, 116.19, 76.96, 72.93, 68.08, 57.70, 55.75, 54.11, 43.80, 39.82, 39.65, 31.89, 30.90, 27.98, 25.34, 25.26, 24.74, 23.36, 21.04, 19.25, 17.96, 16.07; HRMS calcd for C27H40NO6S 506.25763 [M + H]+, found 506.25654. cis-Epoxide diastereomer 5a: Rf = 0.34 (CH2Cl2/MeOH, 15:1); [α]22D −58.9 (c 1.0, CHCl3); IR (thin film) νmax 3466 (br), 2926, 2860, 1737, 1679, 1556, 1509, 1447, 1413, 1390, 1324, 1297, 1254, 1189, 1150, 1085, 1042, 1004, 984, 953, 919 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.99 (s, 1H, SCH=C), 6.65 (s, 1H, CH=CCH3), 5.60 (t, J =3.9 Hz, 1H, CHOC(O)), 4.36 (dd, J = 11.1, 1.9 Hz, 1H, CHOHC(CH3)2), 4.16 (s, 1H, CHCHOH), 3.99 (s, 1H, OH), 3.80 (bs, 1H, OH), 3.24 (ddd, J = 7.4, 4.4, 4.4 Hz, 1H, CH2CH-O(epoxide)CH), 3.11 (dd, J = 12.3, 1.7 Hz, 1H, CHC(O)), 3.05-3.02 (m, 1H, CH2CH-O(epoxide)CH), 2.69 (s, 3H, N=C(S)CH3), 2.56 (dd, J = 14.7, 11.2 Hz, 1H, CH2CO2), 2.37 (dd, J = 14.6, 2.6 Hz, 1H, CH2CO2), 2.10 (s, 3H, CH=CCH3), 2.09-1.96 (m, 3H), 1.93-1.82 (m, 3H), 1.66-1.46 (m, 6H), 1.39-1.18 (m, 6H), 1.08 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 221.01, 169.96, 165.32, 151.91, 136.90, 119.81, 116.12, 75.86, 72.88, 68.25, 57.23, 54.24, 53.79. 44.18, 39.78, 39.59, 30.83, 29.01, 28.28, 25.42, 25.06, 24.51, 22.20, 21.08, 19.27, 18.82, 16.31; HRMS calcd for C27H40NO6S 506.25763 [M + H] +, found 506.25659. The direction of the epoxide was further determined by 1D and 2D NOE.
Bridged Epothilone D (8)
The desired C6-C8 bridged epothilone D (8) was prepared from bis(silyl ether) macrolactone 63 (205 mg, 0.28 mmol) by treatment with CF3CO2H according to the same procedure described above for the preparation of 7, to obtain pure lactone 10 (137 mg, 91%) as a colorless oil or white foam: Rf = 0.46 (CH2Cl2/MeOH, 15:1); [α]22D −109.9 (c 1.35, CHCl3); IR (thin film) νmax 3435 (br), 2934, 2864, 1733, 1679, 1509, 1447, 1413, 1378, 1336, 1293, 1251, 1185, 1143, 1081, 1046, 984, 938, 914, 849, 714, 683 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.95 (s, 1H, SCH=C), 6.60 (s, 1H, ArCH=CCH3), 5.13 (m, 2H, CHOC(O), CH2CH=CCH3), 4.62-4.39 (m, 1H, CHOHC(CH3)), 4.28 (s, 1H, CHCHOH), 3.87 (s, 1H, OH), 3.69 (d, J = 5.9 Hz, 1H, OH), 2.98 (dd, J = 12.4, 2.2 Hz, 1H, CHC(O)), 2.67 (s, 3H, N=C(S)CH3), 2.62 (ddd, J = 15.2, 10.1, 10.1 Hz, 1H), 2.44 (dd, J = 14.2, 11.4 Hz, 1H, CH2CO2), 2.35-2.30 (m, 1H), 2.26-2.21 (m, 2H), 2.06 (d, J = 1.1 Hz, 3H, ArCH=CCH3), 2.02-1.85 (m, 2H), 1.81-1.74 (m, 2H), 1.67 (s, 3H, CH=CCH3CH2), 1.62-1.44 (m, 5H), 1.38-1.19 (m, 6H), 1.04 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 221.06, 170.48, 165.33, 151.94, 140.02, 138.48, 121.17, 119.17, 115.61, 79.13, 73.02, 69.77, 54.28, 43.81, 40.03, 39.82, 32.89, 32.13, 29.93, 27.03, 25.26, 23.91, 23.88, 23.39, 20.89, 19.18, 17.02, 16.28; HRMS calcd for C28H42NO5S 504.27837 [M + H]+, found 504.27762.
C6-C8 bridged epothilone B (6)
To a solution of bridged desoxyepothilone B (8) (0.20 mg, 0.04 mmol, 1.0 equiv) in CH2Cl2 (0.4 mL) at −78 °C was added freshly prepared 3,3-dimethyldioxirane (1.0 mL, ca. 0.08 mmol,ca. 0.08 M in acetone, 2.0 equiv) dropwise. The resulting solution was warmed to −50 °C for 1 h, and another portion of dimethyldioxirane (0.4 mL, 0.032 mmol) was added. After stirring at −50 °C for additional 2.5 h, A stream of argon was then bubbled through the solution at −50 °C to remove excess dimethyldioxirane and solvent. The crude reaction mixture was determined to be >20:1 ratio of diastereomeric cis-epoxides by 1H NMR spectroscopy. The resulting residue was purified by preparative thin-layer chromatography (CH2Cl2/MeOH, 30/1) to afford bridged epothilone B (6) (10.8 mg, 52%) as a white foam: Rf = 0.39 (CH2Cl2/MeOH, 15:1); [α]22D −57.8 (c 1.0, CHCl3); IR (thin film) νmax 3470 (br), 2934, 2864, 1737, 1679, 1505, 1467, 1444, 1413, 1382, 1324, 1293, 1251, 1177,1154, 1073, 1042, 984, 941, 880, 760 cm−1; 1H NMR (600 MHz, CDCl3) δ 6.98 (s, 1H, SCH=C), 6.61 (s, 1H, ArCH=CCH3), 5.33 (dd, J = 8.9, 2.7 Hz, 1H, CHOC(O)), 4.43 (d, J = 10.0 Hz, 1H, CHOHC(CH3)2), 4.37 (s, 1H, CHCHOH), 4.13 (s, 1H, OH), 4.00 (s, 1H, OH), 3.01 (dd, J = 12.1, 1.8 Hz, 1H, CHC(O)), 2.79 (dd, J = 9.1, 2.8 Hz, 1H, CH2CH-O(epoxide)C), 2.68 (s, 3H, N=C(S)CH3), 2.50 (dd, J = 14.2, 10.7 Hz, 1H, CH2CO2), 2.24 (dd, J = 14.3, 2.6 Hz, 1H, CH2CO2), 2.16 (ddd, J = 15.1, 2.8, 2.8 Hz, 1H), 2.08 (s, 3H, CH=CCH3), 2.07-2.00 (m, 1H), 1.97-1.72 (m, 5H), 1.72-1.47 (m, 5H), 1.41-1.20 (m, 9H), 1.07 (s, 3H, C(CH3)2); 13C NMR (100 MHz, CDCl3) δ 221.45, 170.43, 165.59, 151.62, 138.88, 120.07, 116.10, 77.02, 72.99, 68.27, 62.51, 61.72, 53.94, 43.76, 40.21, 39.76, 33.06, 32.68, 30.92, 25.47, 25.33, 24.77, 23.50, 23.00, 21.14, 19.23, 17.70, 16.18; HRMS calcd for C28H42NO6S 520.27328 [M + H]+, found 520.27228.
Tubulin Purification
Tubulin was prepared by two cycles of temperature dependent assembly–disassembly as described by Williams and Lee. [49] Determination of protein concentration was carried out as previously described. [50]
Tubulin Polymerization Assay
Tubulin assembly was monitored by apparent absorption at 350 nm. Tubulin samples (10 μM) were incubated at 37 °C in PME buffer (100 mM PIPES, 1 mM MgSO4, and 2 mM EGTA) containing 1 mM GTP in the spectrometer until a baseline was obtained. The ligand to be tested in DMSO was added a final concentration of 50 μM (4% DMSO). After the assembly reached steady state, the temperature was dropped to 4 °C. A tubulin sample (10 μM) with 4% DMSO and no promoter was used as the reference.
Determination of Cytotoxic Activity
Cytotoxicity of bridged epothilones in PC3 cells was assessed with the SRB assay. [51] The A2780 ovarian cancer cell line assay was performed at Virginia Polytechnic Institute and State University as previously reported. [52]
Supplementary Material
Acknowledgments
The work was supported in part by the NIH Grant No. CA-69571. We thank Kenneth Hardcastle (Emory University) for determination of the X-ray crystal structure of compound 19.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/.
Contributor Information
Dr. Weiqiang Zhan, Email: weiqiang.zhan@emory.edu.
Dr. James P. Snyder, Email: jsnyder@emory.edu.
References
- 1.Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. J Antibiot. 1996;49:560. doi: 10.7164/antibiotics.49.560. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325. [PubMed] [Google Scholar]; (b) Kowalski RJ, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 3.For recent general reviews on epothilones, see: Altmann KH, Florsheimer A, Bold G, Caravatti G, Wartmann M. Chimia. 2004;58:686. doi: 10.1016/s0960-894x(00)00555-2.Altmann KH. Cur Pharm Design. 2005;11:1595. doi: 10.2174/1381612053764715.Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. Chemmedchem. 2007;2:396. doi: 10.1002/cmdc.200600206.Feyen F, Cachoux F, Gertsch J, Wartmann M, Altmann K. Acc Chem Res. 2008;41:21. doi: 10.1021/ar700157x.
- 4.(a) Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS. J Biol Chem. 1997;272:17118. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]; (b) Wolff A, Technau A, Brandner G. Int J Oncol. 1997;11:123. doi: 10.3892/ijo.11.1.123. [DOI] [PubMed] [Google Scholar]; (c) Altmann KH, Wartmann M, O’Reilly T. Biochim Biophys Acta. 2000;1470:M79. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]; (d) Wartmann M, Altmann KH. Curr Med Chem Anticancer Agents. 2002;2:123. doi: 10.2174/1568011023354489. [DOI] [PubMed] [Google Scholar]
- 5.(a) Princen K, Hatse S, Vermeire K, Aquaro S, De Clercq E, Gerlach LO, Rosenkilde M, Schwartz TW, Skerlj R, Bridger G, Schols D. J Virol. 2004;78:12996. doi: 10.1128/JVI.78.23.12996-13006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hatse S, Princen K, De Clercq E, Rosenkilde EMM, Schwartz TW, Hernandez-Abad PE, Skerlj RT, Bridger GJ, Schols D. Biochem Pharmacol. 2005;70:752. doi: 10.1016/j.bcp.2005.05.035. [DOI] [PubMed] [Google Scholar]; (c) Hu JS, Freeman CM, Stolberg VR, Chiu BC, Bridger GJ, Fricker SP, Lukacs NW, Chensue SW. Am J Pathol. 2006;169:424. doi: 10.2353/ajpath.2006.051234. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schwarz MK, Wells TN. Nat Rev Drug Discov. 2002;1:347. doi: 10.1038/nrd795. [DOI] [PubMed] [Google Scholar]
- 6.For recent reviews on the synthesis of epothilones and analogs, see: Rivkin A, Cho YS, Gabarda AE, Yoshimura F, Danishefsky SJ. J Nat Prod. 2004;67:139. doi: 10.1021/np030540k.Luduvico I, Le Hyaric M, De Almeida MV, Da Silva MVAD. Mini-Rev Org Chem. 2006;3:49.Watkins EB, Chittiboyina AG, Avery MA. Eur J Org Chem. 2006:4071.
- 7.Su DS, Balog A, Meng DF, Bertinato P, Danishefsky SJ, Zheng YH, Chou TC, He LF, Horwitz SB. Angew Chem, Int Ed Engl. 1997;36:2093. [Google Scholar]
- 8.Nicolaou KC, Vourloumis D, Li TH, Pastor J, Winssinger N, He Y, Ninkovic S, Sarabia F, Vallberg H, Roschangar F, King NP, Finlay MRV, Giannakakou P, VerdierPinard P, Hamel E. Angew Chem, Int Ed Engl. 1997;36:2097. [Google Scholar]
- 9.For selected reviews on SAR study of epothilones, see: Harris CR, Danishefsky SJ. J Org Chem. 1999;64:8434.Nicolaou KC, Roschangar F, Vourloumis D. Angew Chem, Int Ed. 1998;37:2015. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2.
- 10.(a) Conlin A, Fornier M, Hudis C, Kar S, Kirkpatrick P. Nat Rev Drug Discovery. 2007;6:953. [Google Scholar]; (b) Kaminskas E, Jiang X, Aziz R, Bullock J, Kasliwal R, Harapanhalli R, Pope S, Sridhara R, Leighton J, Booth B, Dagher R, Justice R, Pazdur R. Clin Cancer Res. 2008;14:4378. doi: 10.1158/1078-0432.CCR-08-0015. [DOI] [PubMed] [Google Scholar]
- 11.Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, Kuduk SD, Danishefsky SJ. Proc Natl Acad Sci USA. 1999;96:4256. doi: 10.1073/pnas.96.8.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang MM, Xia XY, Kim Y, Hwang D, Jansen JM, Botta M, Liotta DC, Snyder JP. Org Lett. 1999;1:43. doi: 10.1021/ol990521v. [DOI] [PubMed] [Google Scholar]
- 13.He L, Jagtap PG, Kingston DGI, Shen HJ, Orr GA, Horwitz SB. Biochemistry. 2000;39:3972. doi: 10.1021/bi992518p. [DOI] [PubMed] [Google Scholar]
- 14.Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. Proc Natl Acad Sci USA. 2000;97:2904. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. Proc Natl Acad Sci USA. 2000;97:2904. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RE, Chen Y, Galvin GM, Pabba PK. Org Biomol Chem. 2004;2:127. doi: 10.1039/b312213c. [DOI] [PubMed] [Google Scholar]
- 17.Nettles JH, Li HL, Cornett B, Krahn JM, Snyder JP, Downing KH. Science. 2004;305:866. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 18.(a) Klar U, Buchmann B, Schwede W, Skuballa W, Hoffmann J, Lichtner RB. Angew Chem Int Ed. 2006;45:7942. doi: 10.1002/anie.200602785. [DOI] [PubMed] [Google Scholar]; (b) Klar U, Hoffmann J, Giurescu M. Expert Opin Investig Drugs. 2008;17:1735. doi: 10.1517/13543784.17.11.1735. [DOI] [PubMed] [Google Scholar]
- 19.Zhan W, Jiang Y, Brodie PJ, Kingston DGI, Liotta DC, Snyder JP. Org Lett. 2008:1565. doi: 10.1021/ol800422q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin HJ, Pojarliev P, Kahlig H, Mulzer J. Chem Eur J. 2001;7:2261. doi: 10.1002/1521-3765(20010518)7:10<2261::aid-chem2261>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.(a) Nicolaou KC, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z, Trujillo JI. J Am Chem Soc. 1997;119:7960. [Google Scholar]; (b) Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou KC. Angew Chem, Int Ed. 1997;36:166. [Google Scholar]
- 22.For a review of substrate directed reactions, see: Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307.
- 23.(a) Brown HC, Jadhav PK, Bhat KS. J Am Chem Soc. 1985;107:2564. [Google Scholar]; (b) Brown HC, Bhat KS, Jadhav PK. J Chem Soc, Perkin Trans. 1991;1:2633. [Google Scholar]
- 24.Young D, Kitching W. Aust J Chem. 1985:1767. [Google Scholar]
- 25.(a) Flippin LA, Brown PA, Jalaliaraghi K. J Org Chem. 1989;54:3588. [Google Scholar]; (b) Chini M, Crotti P, Flippin LA, Gardelli C, Macchia F. J Org Chem. 1992;57:1713. [Google Scholar]
- 26.Carre MC, Houmounou JP, Caubere P. Tetrahedron Lett. 1985;26:3107. [Google Scholar]
- 27.Mihelich ED, Daniels K, Eickhoff DJ. J Am Chem Soc. 1981;103:7690. [Google Scholar]
- 28.For selected example for TBS protection of primary β-hydroxy epoxide, see: Felpin FX, Lebreton J. J Org Chem. 2002;67:9192. doi: 10.1021/jo020501y.Taylor RE, Jin MZ. Org Lett. 2003;5:4959. doi: 10.1021/ol0358814.Leung LMH, Boydell AJ, Gibson V, Light ME, Linclau B. Org Lett. 2005;7:5183. doi: 10.1021/ol052009h.
- 29.Fan RH, Hou XL. Org Biomol Chem. 2003;1:1565. doi: 10.1039/b301081c. [DOI] [PubMed] [Google Scholar]
- 30.a) Hikota M, Sakurai Y, Horita K, Yonemitsu O. Tetrahedron Lett. 1990;31:6367. [Google Scholar]; b) Paterson I, Chen DYK, Acena JL, Franklin AS. Org Lett. 2000;2:1513. doi: 10.1021/ol000027n. [DOI] [PubMed] [Google Scholar]
- 31.Prunet J. Angew Chem, Int Ed. 2003;42:2826. doi: 10.1002/anie.200301628. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 32.a) Nicolaou KC, Sarabia F, Ninkovic S, Yang Z. Angew Chem, Int Ed. 1997;36:525. [Google Scholar]; b) Nicolaou KC, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature. 1997;387:268. doi: 10.1038/387268a0. [DOI] [PubMed] [Google Scholar]; c) Altmann KH, Bold G, Caravatti G, Denni D, Florsheimer A, Schmidt A, Rihs G, Wartmann M. Hel Chim Acta. 2002;85:4086. [Google Scholar]
- 33.Regueiro-Ren A, Leavitt K, Kim SH, Hofle G, Kiffe M, Gougoutas JZ, DiMarco JD, Lee FYF, Fairchild CR, Long BH, Vite GD. Org Lett. 2002;4:3815. doi: 10.1021/ol026589j. [DOI] [PubMed] [Google Scholar]
- 34.Reese CB, Stewart JCM, Vanboom JH, Leeuw HPMD, Nagel J, Rooy JFMD. J Chem Soc, Perkin Trans. 1975;1:934. doi: 10.1039/p19750000934. [DOI] [PubMed] [Google Scholar]
- 35.Only trace amounts of product was detectable through the classical EDCI coupling procedure together with large amounts of β-eliminated side products.
- 36.a) Sonnet PE. Tetrahedron. 1980;36:557. [Google Scholar]; b) Smith AB, Mesaros EF, Meyer EA. J Am Chem Soc. 2006;128:5292. doi: 10.1021/ja060369+. [DOI] [PubMed] [Google Scholar]
- 37.(a) Vedejs E, Fuchs PL. J Am Chem Soc. 1973;95:822. [Google Scholar]; (b) Vedejs E, Snoble KAJ, Fuchs PL. J Org Chem. 1973;38:1178. [Google Scholar]
- 38.Schrock RR, Murdzek JS, Bazan GC, Robbins J, Dimare M, Oregan M. J Am Chem Soc. 1990;112:3875. [Google Scholar]
- 39.(a) Balog A, Meng DF, Kamenecka T, Bertinato P, Su DS, Sorensen EJ, Danishefsky SJ. Angew Chem, Int Ed Engl. 1996;35:2801. [Google Scholar]; (b) Su DS, Meng DF, Bertinato P, Balog A, Sorensen EJ, Danishefsky SJ, Zheng YH, Chou TC, He LF, Horwitz SB. Angew Chem, Int Ed Engl. 1997;36:757. [Google Scholar]; c) Schinzer D, Bauer A, Schieber J. Chem Eur J. 1999;5:2492. [Google Scholar]
- 40.Meng DF, Bertinato P, Balog A, Su DS, Kamenecka T, Sorensen EJ, Danishefsky SJ. J Am Chem Soc. 1997;119:10073.For a good review on Suzuki coupling reaction in natural product synthesis, see: Chemler SR, Trauner D, Danishefsky SJ. Angew Chem, Int Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n.
- 41.The synthesis of compound 5 has been briefly reported, see ref 19.
- 42.Sawada D, Kanai M, Shibasaki M. J Am Chem Soc. 2000;122:10521. [Google Scholar]
- 43.For Sharpless asymmetric dihydroxylation rate of substituted alkenes, see: Andersson PG, Sharpless KB. J Am Chem Soc. 1993;115:7047.For reviews on Sharpless asymmetric dihydroxylation of polyenes, see: Becker H, Soler MA, Sharpless KB. Tetrahedron. 1995;51:1345.Kolb HC, Vannieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483.
- 44.Nicolaou KC, Ninkovic S, Sarabia F, Vourloumis D, He Y, Vallberg H, Finlay MRV, Yang Z. J Am Chem Soc. 1997;119:7974. [Google Scholar]
- 45.a) Barboni L, Lambertucci C, Appendino G, Vander Velde DG, Himes RH, Bombardelli E, Wang M, Snyder JP. J Med Chem. 2001;44:1576. doi: 10.1021/jm001103v. [DOI] [PubMed] [Google Scholar]; b) Metaferia BB, Hoch J, Glass TE, Bane SL, Chatterjee SK, Snyder JP, Lakdawala A, Cornett B, Kingston DGI. Org Lett. 2000;3:2461. doi: 10.1021/ol016124d. [DOI] [PubMed] [Google Scholar]; c) Kingston DGI, Bane S, Snyder JP. Cell Cycle. 2005;4:279. [PubMed] [Google Scholar]
- 46.a) Chang G, Guida WC, Still WC. J Am Chem Soc. 1989;111:4379. [Google Scholar]; b) Schrödinger, Inc; 120 West 45th Street, 29th Floor, New York, NY 10036–4041: [accessed 08/15/11]. http://www.schrodinger.com/products/14/12/ [Google Scholar]
- 47.a) Carlomagno T, Blommers MJJ, Meiler J, Jahnke W, Schupp T, Petersen F, Schinzer D, Altmann K-H, Griesinger C. Angew Chem Int Ed. 2003;42:2511. doi: 10.1002/anie.200351276. [DOI] [PubMed] [Google Scholar]; b) Reese M, Sanchez-Pedregal VM, Kubicek K, Meiler J, Blommers JMJJ, Griesinger C, Carlomagno T. Angew Chem Int Ed. 2007;46:1864. doi: 10.1002/anie.200604505. [DOI] [PubMed] [Google Scholar]
- 48.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shaw DE, Shelley M, Perry JK, Sander LC, Shenkin PS. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 49.Williams RC, Jr, Lee JC. Methods Enzymol. 1982;85:376. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S, Ganesh T, Kingston DGI, Bane S. Anal Biochem. 2007;360:56. doi: 10.1016/j.ab.2006.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. J Natl Cancer Inst. 1990;82:1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 52.Pan E, Harinantenaina L, Brodie PJ, Callmander M, Rakotonandrasana S, Rakotobe E, Rasamison VE, TenDyke K, Shen Y, Suh EM, Kingston DGI. Bioorg Med Chem. 2011;19:422. doi: 10.1016/j.bmc.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.