Abstract
We report a novel COL4A5 mutation causing rapid progression to end stage renal disease in males despite the absence of clinical and biopsy findings associated with Alport syndrome. Affected males had proteinuria, variable hematuria, early progression to end stage renal disease; and renal biopsy findings which included global and segmental glomerulosclerosis, mesangial hypercellularity and basement membrane immune complex deposition.
Exon sequencing of the COL4A5 locus identified a thymine to guanine transversion at nucleotide 665, resulting in a phenylalanine to cysteine missense mutation at codon 222. This mutation was confirmed in 4 affected males and 4 female obligate carriers, but was absent in 6 asymptomatic male family members and 198 unrelated individuals. α5(IV) collagen staining in renal biopsies from affected males was normal.
The phenylalanine at position 222 is 100% conserved among vertebrates. This is the first description of a mutation in a non-collagenous interruption associated with severe renal disease, providing evidence for the importance of this structural motif. The range of phenotypes associated with COL4A5 mutations is more diverse than previously realized. COL4A5 mutation analysis should be considered when glomerulonephritis presents in an X-linked inheritance pattern, even with a distinct presentation from Alport syndrome.
INTRODUCTION
A network formed by self-assembly of α3α4α5(IV) collagen molecules in the glomerular basement membrane (GBM) maintains the structural integrity of the filtration barrier. This function is compromised by certain mutations in the COL4A3, COL4A4 or COL4A5 genes, which encode the α3–α5(IV) collagen chains, causing hereditary nephropathies.1 Mutations in the COL4A3 or COL4A4 genes cause autosomal recessive or dominant Alport syndrome.2 Mutations in the COL4A5 gene, located at Xq22, present as X-linked Alport syndrome (XLAS; OMIM #301050), which is the most prevalent form of hereditary nephritis.3 The clinical course of XLAS is heterogeneous based upon the type of GBM changes, the age of progression to ESRD, and the presence of extra-renal manifestations.4, 5 Characteristic findings in males include microscopic hematuria in 100%; gross hematuria in 60% to 70%; proteinuria in 90%; hearing loss in 90%; and ocular abnormalities in 35%.5, 6 On renal biopsy, extensive thickening and splitting of the GBM in the absence of immune complex (IC) deposition is strongly suggestive of XLAS6. Female heterozygotes for COL4A5 mutations are less symptomatic than males, but have microscopic hematuria in 95% of cases.7
More than 400 mutations in the COL4A5 gene have been described.8 Large case series of XLAS patients have led to the classification of mutations and genotype-phenotype associations.4, 5, 9, 10 Major alterations of COL4A5 — including deletions, frame-shift mutations, donor splice site mutations and nonsense mutations — result in the absence or severe truncation of the COL4A5 protein, causing severe XLAS; these patients are characterized by juvenile onset of end-stage renal disease (ESRD) and extrarenal features such as hearing loss and ocular defects.5 In contrast, missense mutations of COL4A5 alter one amino acid residue but produce an otherwise intact COL4A5 protein, generally causing a less severe clinical presentation, with adult onset of ESRD and fewer extrarenal manifestations.5
The goal of this study was to identify the genetic alteration responsible for juvenile-onset ESRD in multiple related males of a large pedigree. We report the identification of a novel missense mutation in the COL4A5 gene strongly associated with kidney disease in this cohort. This mutation occurs within a non-collagenous interruption of the α5(IV) collagen chain, providing the first direct evidence for the functional importance of this structural motif.
RESULTS
Patients
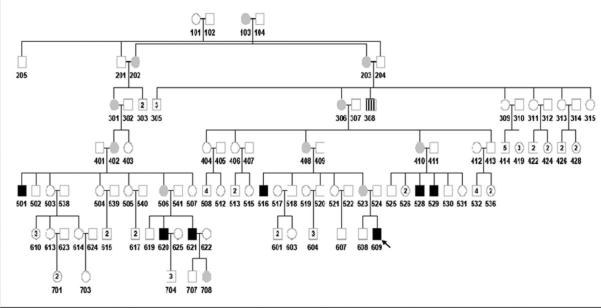
A pedigree consisting of 117 individuals across 7 generations identified 8 affected males with ESRD and 11 carrier or obligate carrier females (Figure 1). No females were identified with a history of chronic kidney disease. Medical records were available from 7 of the affected males; DNA samples were obtained on 5 affected males, 4 carrier females and 6 unaffected males. The absence of glomerular disease in unaffected males was confirmed with a dipstick urinalysis (UA) with no more than trace blood or protein.
Figure 1. Extended Pedigree.
Carriers are indicated by gray circles. Affected males are indicated by black squares. Individual 308 (square with vertical stripes) died of renal failure by history; however records to determine the precise etiology were not available. The index patient is indicated by an arrow.
Presentation and clinical course
The clinical presentation of affected males (Table 1) was notable for proteinuria, hypertension and renal dysfunction with variable hematuria. The two males with no hematuria at presentation did not develop persistent hematuria during progression to ESRD. Two of the patients had documented hypoalbuminemia and four patients had nephrotic range proteinuria. Two affected males had normal audiograms at presentation, and none reported hearing loss or hearing aid requirements at the time of consent or had documentation of hearing loss in the medical records. One patient had visual changes, determined to be secondary to pseudotumor cerebri. No patients had documented anterior lenticonus or esophageal leiomyomatosis. One patient had linear IgG deposits on a renal allograft biopsy. One of the evaluated female carriers had trace hematuria, and 2/3 (67%) manifested 100 mg/dl of proteinuria.
Table 1. Clinical Presentation and Course.
The clinical presentation and course of seven related male patients are summarized. Numbers designate the location of each patient in the extended pedigree (Figure 1). Serum creatinine in mg/dL may be converted to μmol/L by multiplying by 88.4. Blood pressures are listed as mm Hg.
| Patient | How diagnosed | BP and Physical exam. | Initial serum labs | Initial urine labs. | Treatment. | Age at diagnosis / onset of ESRD (yr) | Long term outcome |
|---|---|---|---|---|---|---|---|
| 501 | Data not available | Data not available | Data not available. | Data not available. | Data not available. | Unknown / 17 years | 2 renal transplants. Linear fluorescence for IgG noted on renal allograft biopsy. This patient died at age 26 years. |
| 516 | Proteinuria was identified during the annual primary care evaluation. | BP, 132/70. No edema. | ANA, negative. Creatinine, 0.4 mg/dl. C3, 130 mg/dl. | 2+ protein Small blood. 20–30 rbcs/hpf. | Prednisone and leukeran | 9 / 16 | A renal transplant has been functioning for the last 24 years. |
| 529 | Proteinuria was identified during the annual primary care evaluation. | Data not available. | Data not available. | Data not available. | Data not available. | 13 / 16 | The patient has had 3 renal transplants. |
| 528 | Proteinuria was identified during the annual primary care evaluation. | BP, 120/90 No edema | ANA negative. Creatinine, 1.1 mg/dl. C3, 86 mg/dl. | 4+ protein. 5–10 rbcs/hpf. | Data not available. | 9 / 13 | A renal transplant has been functioning for the last 27 years. |
| 620 | A renal evaluation was performed secondary to renal disease in his brother. | BP, 170/110. Pretibial and periorbital edema. | Albumin, 2.5 gm/dl. Creatinine, 2.2 mg/dl. C3, 95 mg/dl. C4, 14 mg/dl. | 4+ protein. Trace blood. | Ace inhibition | 17 / 22 | A renal transplant has been functioning for the last 19 years. |
| 621 | The patient was noted to be hypertensive during an evaluation for headaches. | BP, 150/100. No edema. | Albumin, 2.2 gm/dl. ANA, negative. Creatinine 4.3 mg/dl. C3, 141 mg/dl. C4, 28 mg/dl. | 4+ protein. No blood. 5.4 mg/kg/hr of protein. | Ace inhibition and imuran. | 13 / 14 | A renal transplant has been functioning for the last 21 years. |
| 609 | The patient was evaluated in the Emergency Department with nausea, vomiting and fatigue. | BP, 220/150. No edema | ANA, negative Creatinine 11.4 mg/dl C3, 130 mg/dl C4, 31 mg/dl | 4+ protein < 1 rbc/hpf. Protein: creatinine, 15.2 mg/mg. | Renal replacement therapy | 10 / 10 | The patient has been transplanted within the last year. |
Renal biopsy findings
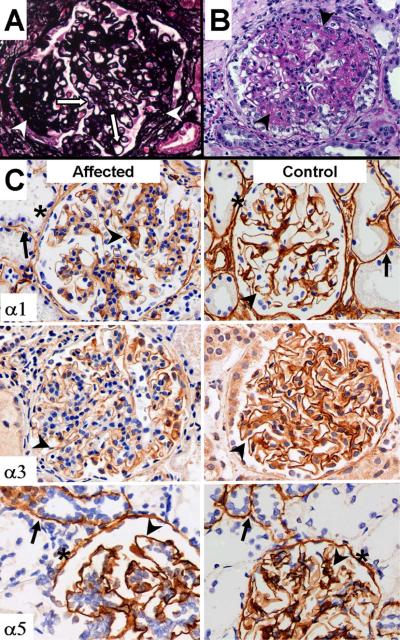
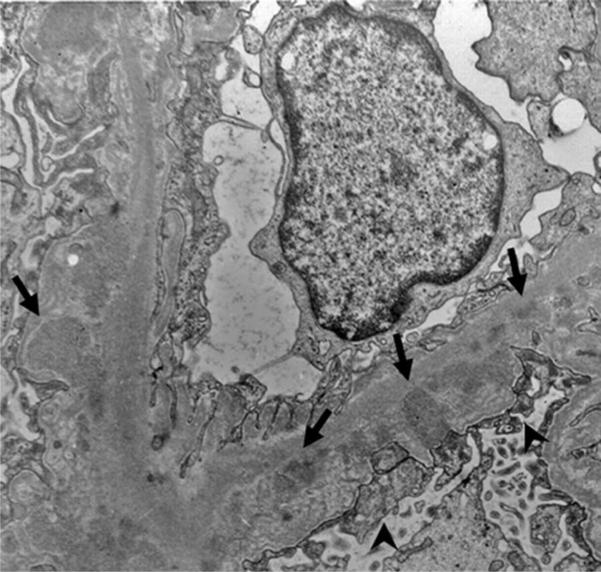
Records from 9 renal biopsies performed on 7 patients were reviewed (Table 2). Tissue from 5 biopsies was available for review. Light microscopy findings included global and segmental sclerosis and mesangial expansion (Figure 2a). No foam cells were identified. Immunofluorescence was consistently positive for IgM (granular) in the GBM, with weak (1+) intensity. No biopsy was positive for IgA. Electron microscopy revealed scattered electron dense deposits in the GBM (Figure 3). Podocyte foot process effacement was present in areas with and without basement membrane dense deposits (Figure 3). Three of the NCH biopsies had small subepithelial lucent areas suggestive for resolved deposits. Only one patient had splitting of the GBM. However, the splitting was confined to areas of dense deposits or lucent areas, indicating that it was most likely a secondary change. No patient had diffuse thinning of the GBM
Table 2. Renal Biopsy Findings.
The renal biopsy findings of six related male patients are summarized. In certain cases, serial biopsies were available. Patients are designated according to their location in the family pedigree (Figure 1). The presence of a finding is indicated by a “+” symbol in the appropriate box.
| Patient | 501 | 516 | 516 | 528 | 529@ | 529 | 620 | 621 | 708 |
| Age at biopsy (years) | ? | 9 | 14 | 10 | 13 | 16 | 17 | 13 | 9 |
| Biopsy reviewed at NCH | + | + | + | + | + | ||||
| Light microscopy | |||||||||
| mesangial expansion | + | + | + | + | + | + | + | ||
| FSGS | + | + | + | + | + | + | |||
| global sclerosis (affected/total) | + 1/15 | + 2/8 | + | + all | + 9/21 | + 3/6 | + 16/19 | ||
| interstitial fibrosis | + | + | + | ||||||
| inflammation | + | + | |||||||
| Immunofluorescence | |||||||||
| IgA | |||||||||
| IgM | + gbm | + gbm | + | + gbm mes | + gbm mes | + gbm | |||
| IgG | + gbm | + | |||||||
| C3 | + gbm | + mes | + | + gbm mes | + gbm | ||||
| Electron Microscopy | |||||||||
| electron dense deposits | + s-ep i-me s-en | + s-en i-me mes | + | + s-en s-ep mes i-me | + s-en s-ep mes | ||||
| GBM splitting | +* | +* | |||||||
| lucent areas | + s-ep | + | + s-ep | + s-ep | |||||
Shaded cells indicate when data was not available;
specific details of light microscopy not available, but findings reported as “normal”; NCH, Nationwide Children's Hospital; FSGS, focal segmental glomerulosclerosis; gbm, glomerular basement membrane; mes, mesangium; s-ep, subepithelial; s-en, subendothelial; i-me, intramenbranous;
The splitting was confined to areas of deposits or lucent areas
Figure 2. Light Microscopy and Immunohistochemistry Findings in the Renal Biopsy.
Jones silver staining (left panel) demonstrated segmental sclerosis (arrows). No splitting, spikes or lucencies were evident in the GBM (arrowheads) PAS staining (right panel) demonstrates mesangioproliferative glomerulonephritis with increased mesangial matrix and mesangial cellularity (arrowheads). Magnification 400×. (B) Staining for α1(IV), α3(IV) and α5(IV) collagen was found in the GBM (arrowheads), although α3(IV) staining was slightly diminished in affecteds. Positive staining was present in the tubular basement membranes (arrows); this staining was diffuse for α1(IV) collagen and focal for α3 (not shown) and α5 chains of type IV collagen. α1(IV) and α5(IV) collagen was also expressed in the basement membranes of Bowman's capsule (asterisks).
Figure 3. Electron Microscopy Findings in the Renal Biopsy.
Electron microscopy results are presented with electron dense immune complex (arrows) deposition. Effacement of the podocyte foot processes is present (arrowheads).
COL4A5 sequencing
Sequencing of the coding region for COL4A5 identified a thymine to guanine (T>G) transversion at nucleotide 665 (T665G) in exon 12 of the index patient, resulting in a single phenylalanine to cysteine (Phe→Cys) amino acid substitution at codon 222 (p.F222C). Exon 12 was subsequently amplified by polymerase chain reaction (PCR) from genomic DNA of additional family members and sequenced (Figure S1). We confirmed the presence of the mutation in another 4 affected males; in addition, one mutant allele was present in 4 carrier females and absent in 6 asymptomatic males (Table 3), as well as 198 unrelated individuals. These findings suggest that the p.F222C mutation is causative of the X-linked glomerulopathy in this family.
Table 3. COL4A5 Genotyping and Screening UA Results.
Subjects are designated according to their location in the extended pedigree (Figure 1) and grouped according to their clinical phenotype, a unaffected (UN), carrier (C), or affected (A). When available, the results of dipstick urinalysis for blood and protein are listed (Neg: negative). Results of COL4A5 nucleotide 665 genotyping are listed as wild type (WT) or the T665G transversion. Shaded cells indicate when data was not available.
| Subject | Phenotype | Blood | Protein | Genotype |
|---|---|---|---|---|
| 409 | UN | Neg | Trace | |
| 413 | UN | Neg | Trace | WT |
| 502 | UN | Neg | Trace | WT |
| 541 | UN | Neg | Trace | |
| 518 | UN | Neg | Trace | WT |
| 520 | UN | Neg | Trace | WT |
| 525 | UN | Neg | Trace | WT |
| 619 | UN | WT | ||
| 408 | C | Neg | 2+ | |
| 410 | C | Trace | 2+ | WT / T665G |
| 506 | C | Neg | Trace | WT / T665G |
| 523 | C | WT / T665G | ||
| 708 | C | WT / T665G | ||
| 516 | A | T665G | ||
| 528 | A | T665G | ||
| 620 | A | T665G | ||
| 621 | A | T665G | ||
| 609 | A | T665G |
Renal survival
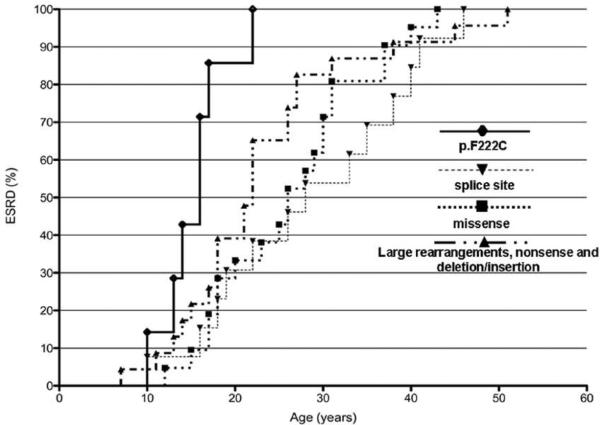
All affected males developed ESRD. The age of 50% renal survival was 16 years in males with the p.F222C mutation compared to 28 years, 26 years and 22 years in historical controls with splice site mutations, missense mutations and large rearrangements, nonsense and deletion/insertion mutations, respectively (Figure 4). (9, 10)
Figure 4. Renal Survival Curves.
Patients with the p.F222C mutation (n = 7) progressed to ESRD at a more rapid rate than historical controls with splice site mutations (n = 13), missense mutations (n = 21) and large rearrangements, nonsense and deletion/insertion mutations (n = 23) reported by Martin et al30 and Gross et al9. Differences between patients with the p.F222C mutation and patients with other types of mutations were highly significant (P < 0 .001).
Expression of collagen IV chains
IHC was performed on available diagnostic renal biopsy tissue from 4 affected males, as well as positive and negative controls. Results were consistent, with all 4 biopsies demonstrating positive (but slightly lighter than positive control) staining for α1(IV) collagen and α3(IV) collagen, and positive staining for α5(IV) collagen. Representative results are presented in Figure 2B.
DISCUSSION
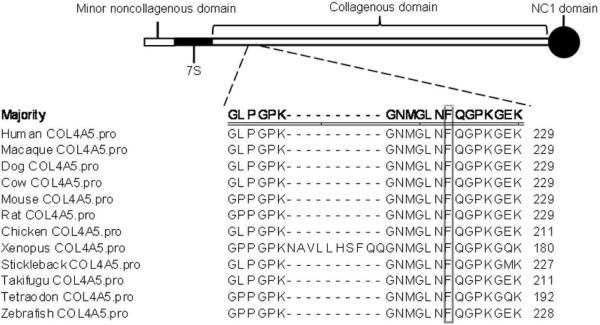
We report a series of related male patients whose presentation and clinical course included proteinuria with variable hematuria; mesangial hypercellularity with IC deposition on renal biopsy; and rapid progression to ESRD. We identified a novel T>G transversion at nucleotide 665 in COL4A5, which segregated in affected males and female carriers but was absent in unaffected males and 198 unrelated individuals, indicating that it is likely causative. The amino acid residue altered by this mutation, Phe-222, occurs within one of the 22 breaks that interrupt the Gly-X-Y repeats comprising the collagenous domain of the α5(IV) collagen chain.4 The amino acid sequence of this interruption, GLNFQG, has been phylogenetically conserved from fish to mammals suggesting an important structural and functional role for this interruption (Figure 5).
Figure 5. Phylogenetic Conservation of the Phe 222 Residue.
Type 4 collagens consist of a minor noncollagenous domain, a minor collagenous (7S) domain, a major collagenous domain containing 15–20 noncollagenous interruptions and a carboxyl terminal noncollagenous (NC1) domain32, 33. The area of the noncollagenous interruption affected by the p.F222C mutation is represented by the dashed lines. The alignment of the predicted COL4A5 protein sequences from twelve species reveals the complete conservation of Phe222 residue in vertebrates.
Breaks in Gly-X-Y repeats introduce bends in the collagen triple helix, create flexible sites, and may form binding sites.11, 12 G4G interrupts (Gly-X1-X2-X3-X4-Gly) and G1G (Gly-X1-Gly) interrupts are the most common in all collagen IV chains.13 Peptide studies show that G4G interrupts have an ordered structure, forming a pseudo-triple helix in which hydrophobic interactions among residues at the X3 position—like Phe-222—replace glycine packing in the triple helix.14, 15 Substitution of Phe-222 is predicted to disrupt these hydrophobic interactions, altering the normal structure of this G4G interruption. Development of severe kidney disease in patients with the p.F222C mutation highlights the functional importance of Phe-222. Notably, this is the first collagen IV missense mutation causing severe kidney disease that occurs within a Gly-X-Y interruption.
Although COL4A5 mutations typically cause XLAS, the affected males in this study have clinical and pathological features inconsistent with the classic presentation of XLAS. Microscopic hematuria occurred in only 3/5 (60%) of the affected males and 1/3 (33%) of the carrier females in our series, compared to 100% of males and 95% of females for XLAS.5, 6 Since the initial urinalysis in affected males without hematuria was performed late in the clinical course, it remains possible that hematuria is more prominent in an earlier phase of the disease. Male patients without hematuria concurrently had nephrotic range proteinuria and elevated serum creatinine. The median renal survival age was 15 years, compared to 25 years for XLAS.5 Despite the severity of the nephritis, there were no documented extrarenal complications typically associated with severe XLAS. However, such extrarenal manifestations cannot be conclusively ruled out without formal audiologic and ophthalmologic evaluation. Mesangial hypercellularity and segmental sclerosis may be identified on renal biopsies of XLAS patients, but are accompanied by more characteristic findings.16 Although the basement membranes were thickened, the absence of alternate thickening and thinning of the GBM, known as “basket weaving” and splitting of the GBM (with the exception of one limited area in a single case that was suggestive of a secondary change) is unusual for XLAS.16
Basement membrane electron dense deposits and positive immunofluorescence are generally thought to be inconsistent with XLAS, though exceptions have been reported in AS patients.17, 18 In addition, IC deposition has been reported in Alport syndrome patients with a coexisting glomerulopathy, i.e., dual glomerulopathy.19, 20 The mechanism of IC deposition in patients with COL4A5 mutations is not known. We were unable to detect the presence of circulating anti-GBM antibodies in serum from the index patient by indirect immunofluorescence (D.-B.B., W.L., data not shown); however, material from additional affected individuals was unavailable, and it remains conceivable that serum factors (such as autoantibodies) could account for the glomerular deposition of immune complexes. Alternatively, structural changes in the collagen IV network of the GBM may lead to IC trapping independent of antigen recognition, which in turn contributes to glomerular damage. The presence of IC raises the question of whether antibody trapping leads to complement activation and the potential role of complement in glomerular damage in affected patients. There was no evidence of hypocomplementemia in all examined individuals at presentation (Table 1), arguing against complement activation. Immunofluorescence demonstrated positive C3 in 5/6 biopsies examined, with GBM localization in 3/5 cases (Table 2). The presence of C3 deposition in the GBM has been reported in XLAS, particularly in segmental lesions with progression of disease.21 Whether this represents non-specific trapping of C3 along with antibodies such as IgG and IgM or complement activation is unclear at this time.
The positive staining for α5(IV) and α3(IV) collagen in the renal biopsies of the affected patients indicates that the p.F222C mutation does not interfere with the assembly of α3α4α5(IV) collagen. This is not surprising, because the specific assembly of α3α4α5(IV) molecules and networks is driven by molecular recognition sites within the non-collagenous (NC1) domains at the carboxyl end of each collagen IV chain22, 23. The most frequently detected missense mutations map to conserved glycine residues within the Gly-X-Y repeats comprising the collagenous domain of α5(IV) collagen.5, 24 Mutations of these glycine residues introduces an abnormal break in the collagen triple-helix, altering the structure and reducing the stability of α3α4α5(IV) molecules. As a result, the affected patients develop comparatively milder forms of XLAS.5 More severe forms of XLAS, with juvenile onset of ESRD, are caused by COL4A5 mutations such as large deletions, nonsense mutation, or splice site donor mutations that yield a truncated α5(IV) chain lacking the NC1 domain.5 Because these mutations prevent the assembly of α3α4α5(IV) heterotrimers, they cause the absence of α3, α4 and α5(IV) collagen from the GBM, resulting in a more severe phenotype.25 In our series of patients, severe kidney disease is not due to the impaired deposition of α3α4α5(IV) collagen in the GBM, but may reflect a specific functional impairment of this network caused by the p.F222C mutation.
Although the precise mechanism whereby the p.F222C mutation results in kidney disease remains to be determined, some contributing factors may be inferred. As discussed, the pseudo-triple helical structure of G4G interruptions is stabilized by interactions among hydrophobic residues at the third position, like Phe-222. Although cysteine is hydrophobic, it is not a conservative substitution for phenylalanine because of its smaller size and propensity to form disulfide bonds. The collagenous triple helix of α3α4α5(IV) molecules is characterized by numerous intra- and inter-molecular disulfide bridges.26 The collagenous domains of α3(IV) and α4(IV) contain 7 and 15 cysteine residues, respectively, whereas that of α5(IV) chain (residues 42–1456) contains only 3 cysteine residues at positions 451, 481 and 484. Therefore, introduction of a new cysteine at position 222 in the α5(IV) chain can have a detrimental effect on the structure and function of the α3α4α5(IV) collagen network by interfering with the formation of the normal disulfide bonds and/or by introducing a new disulfide bridge at an undesirable position. Cysteine substitutions in other collagenopathies appear to dramatically alter the phenotype. Defects of COL1A1 or COL1A2 typically involve substitution of a glycine in the triple repeat, triple helix domain, resulting in osteogenesis imperfecta.27 Interestingly, when cysteine is substituted for arginine in the triple repeat, the phenotype changes to that of Ehlers-Danlos with a high propensity for arterial rupture and minimal bone involvement.28 Functional studies indicate the cysteine causes abnormal formation of disulfide bridges, which can result in either premature degradation of the protein or delayed secretion and formation of abnormal collagen fibrils in the extracellular matrix (depending on the location of the mutation). We speculate a similar phenomenon may be occurring with this COL4A5 mutation, with secretion of an intact but abnormal protein.
Due to random X chromosome inactivation, females carriers with XLAS have variable expression of the mutant α5(IV) collagen chain – even within a given glomerulus - and as many as 25% develop severe renal disease29 Of the 5 carrier females enrolled in our study, all denied a history of abnormal renal function upon interview, and renal function tests were not available. Yet 2 out of the 3 (67%) carrier females evaluated by urine dipstick did manifest 2+ proteinuria, suggestive of GBM abnormalities. Whether GBM abnormalities do occur and result in subclinical disease could not be further assessed due to the absence of kidney biopsy tissue from carrier females.
In summary, we have described a set of patients with X linked glomerulopathy without characteristic findings of XLAS by clinical history or renal biopsy findings who progressed to ESRD at an early age. To our knowledge, this is the first report to associate IC deposition with a specific mutation in the COL4A5 gene. Our findings emphasize that the detection of IC deposition should not exclude the diagnosis of XLAS, nor should it automatically implicate the p.F222C mutation described in this study. Additionally, this is the first COL4A5 mutation associated with rapid progression to ESRD, in patients otherwise lacking the characteristic clinical and biopsy findings associated with XLAS. Finally, this is the first COL4A5 missense mutation that occurs within a non-collagenous interrupt of α5(IV) collagen. Testing for COL4A5 mutations should be a consideration when glomerulopathy presents with an X-linked inheritance pattern, regardless of the consistency of the presentation with XLAS. Future studies should identify the structural and functional differences between mutant and wild-type COL4A5 and determine if the p.F222C mutation is also present in unrelated patients with similar biopsy findings.
METHODS
Patient selection
This study was approved by the Nationwide Children's Hospital Institutional Review Board. Participants were enrolled with informed consent. A pedigree was constructed based on interviews and historical records. Dipstick UA was performed by presumed unaffected male as well as female family members. Controls comprised race matched individuals of North European ancestry from CEPH families (120 individuals) obtained from the Coriell Cell Repository (Camden, NJ, USA), and from central Ohio (78 individuals).
DNA isolation and COL4A5 sequencing
DNA isolated from peripheral blood of the index patient (#609) was submitted for exon sequencing of COL4A5 (Athena Diagnostics). For sequencing of COLA45 exon 12, DNA was isolated from saliva (Oragene). PCR Primers flanking exon 12 of COL4A5 were: 5'-TATCTTTTATTTGGTGTGGA-3' and 5'-TCAAGAAGCAAGAGAAAGAA-3'. Conditions were: 10 min at 94°C; then 36 cycles of 94°C for 30 sec, 59.3°C for 30 sec, 72°C for 45 sec; then 72°C for 10 min, then 4°C. PCR reactions were purified and sequenced bi-directionally.
Probability of ESRD curve analysis
Graphs for the age of ESRD in the affected males with the p.F222C mutation and historical controls9, 30 were generated with GraphPad Prism v5.0b (GraphPad Software) by the Kaplan-Meier method. Differences between curves were analyzed for significance with the Log-rank (Mantel-Cox) Test.31
Immunohistochemistry (IHC) analyses
Since frozen sections were not available, expression of collagen IV chains was analyzed by IHC using 3 μm-thick sections of formalin-fixed, paraffin-embedded renal needle biopsies. IHC was automated on the BondMax IHC system (Leica Microsystems) For antigen retrieval, sections were treated with proteinase K for 10 minutes at 37 °C. Mab-1 and Mab-5 (Alpco Diagnostics) were used for staining α1(IV) and α5(IV) collagen, respectively. Mab 5D6, used for detection of α3(IV) collagen, is a murine IgG2a monoclonal antibody raised against recombinant human α3NC1 monomers (Molecular Recognition Shared Facility, Vanderbilt University). The specificity of mAb 5D6 was verified by indirect ELISA using recombinant NC1 monomers and by immunofluorescence staining of normal human kidney (D.-B.B., unpublished observations). Staining with mAb 5D6 or Mab5 was performed after treatment with acid urea (Alpco Diagnostics). Positive control sections were obtained from a normal autopsy kidney. Negative controls were obtained by substituting the primary antibody with a Universal Negative Control Serum (cat # NC49BL, Biocare Medical).
Evaluation of phylogenetic sequence conservation
Protein sequence alignments to evaluate phylogenetic sequence conservation were performed with MegAlign (DNASTAR, Inc.), using the Clustal W method.
Supplementary Material
ACKNOWLEDGMENTS
The expert help of Dr. Yan Heping, Dr. Raymond Mernaugh and Dr. Xu-ping Wang in the production of anti-α3NC1 monoclonal antibody 5D6 is gratefully acknowledged.
SOURCES OF RESEARCH SUPPORT K.M. and A.S. received support from the Research Institute at Nationwide Children's Hospital. Research funding was assisted with a research donation to the Division of Nephrology, Nationwide Children's Hospital from the Elks Foundation of Logan County, OH. D.-B.B. is supported by R01 grant DK080799 from the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT The authors have nothing to disclose.
REFERENCES
- 1.Hudson BG, Tryggvason K, Sundaramoorthy M, et al. Mechanisms of Disease: Alport's syndrome, Goodpasture's Syndrome, and Type IV Collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 2.Mochizuki T, Lemmink HH, Mariyama M, et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nature genetics. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- 3.Barker DF, Hostikka SL, Zhou J, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Hertz JM, Leinonen A, et al. Complete amino acid sequence of the human alpha 5 (IV) collagen chain and identification of a single-base mutation in exon 23 converting glycine 521 in the collagenous domain to cysteine in an Alport syndrome patient. J Biol Chem. 1992;267:12475–12481. [PubMed] [Google Scholar]
- 5.Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history in 195 families and genotype- phenotype correlations in males. J Am Soc Nephrol. 2000;11:649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 6.Pirson Y. Making the diagnosis of Alport's syndrome. Kidney international. 1999;56:760–775. doi: 10.1046/j.1523-1755.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history and genotype phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14:2603–2610. doi: 10.1097/01.asn.0000090034.71205.74. [DOI] [PubMed] [Google Scholar]
- 8.Gubler MC. Inherited diseases of the glomerular basement membrane. Nature clinical practice. 2008;4:24–37. doi: 10.1038/ncpneph0671. [DOI] [PubMed] [Google Scholar]
- 9.Gross O, Netzer KO, Lambrecht R, et al. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant. 2002;17:1218–1227. doi: 10.1093/ndt/17.7.1218. [DOI] [PubMed] [Google Scholar]
- 10.Bekheirnia MR, Reed B, Gregory MC, et al. Genotype-Phenotype Correlation in X-Linked Alport Syndrome. J Am Soc Nephrol. doi: 10.1681/ASN.2009070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann H, Voss T, Kuhn K, et al. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol. 1984;172:325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- 12.Miles AJ, Knutson JR, Skubitz AP, et al. A peptide model of basement membrane collagen alpha 1 (IV) 531–543 binds the alpha 3 beta 1 integrin. J Biol Chem. 1995;270:29047–29050. doi: 10.1074/jbc.270.49.29047. [DOI] [PubMed] [Google Scholar]
- 13.Thiagarajan G, Li Y, Mohs A, et al. Common interruptions in the repeating tripeptide sequence of non-fibrillar collagens: sequence analysis and structural studies on triple-helix peptide models. J Mol Biol. 2008;376:736–748. doi: 10.1016/j.jmb.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohs A, Popiel M, Li Y, et al. Conformational features of a natural break in the type IV collagen Gly-X-Y repeat. J Biol Chem. 2006;281:17197–17202. doi: 10.1074/jbc.M601763200. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Brodsky B, Baum J. NMR shows hydrophobic interactions replace glycine packing in the triple helix at a natural break in the (Gly-X-Y)n repeat. J Biol Chem. 2007;282:22699–22706. doi: 10.1074/jbc.M702910200. [DOI] [PubMed] [Google Scholar]
- 16.Chugh KS, Sakhuja V, Agarwal A, et al. Hereditary nephritis (Alport's syndrome)--clinical profile and inheritance in 28 kindreds. Nephrol Dial Transplant. 1993;8:690–695. doi: 10.1093/ndt/8.8.690. [DOI] [PubMed] [Google Scholar]
- 17.Sessa A, Cioffi A, Conte F, et al. Hereditary nephropathy with nerve deafness (Alport's syndrome). Electron microscopic studies on the renal glomerulus. Nephron. 1974;13:404–415. doi: 10.1159/000180417. [DOI] [PubMed] [Google Scholar]
- 18.Nasr SH, Markowitz GS, Goldstein CS, et al. Hereditary nephritis mimicking immune complex-mediated glomerulonephritis. Hum Pathol. 2006;37:547–554. doi: 10.1016/j.humpath.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Meroni M, Sessa A, Battini G, et al. Alport syndrome with type I membranoproliferative glomerulonephritis. Nephron. 1993;65:479–480. doi: 10.1159/000187539. [DOI] [PubMed] [Google Scholar]
- 20.Cheong HI, Cho HY, Moon KC, et al. Pattern of double glomerulopathy in children. Pediatric nephrology (Berlin, Germany) 2007;22:521–527. doi: 10.1007/s00467-006-0342-9. [DOI] [PubMed] [Google Scholar]
- 21.Heidet L, Gubler MC. The renal lesions of Alport syndrome. J Am Soc Nephrol. 2009;20:1210–1215. doi: 10.1681/ASN.2008090984. [DOI] [PubMed] [Google Scholar]
- 22.Boutaud A, Borza DB, Bondar O, et al. Type IV collagen of the glomerular basement membrane: Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem. 2000;275:30716–30724. doi: 10.1074/jbc.M004569200. [DOI] [PubMed] [Google Scholar]
- 23.Kang JS, Colon S, Hellmark T, et al. Identification of noncollagenous sites encoding specific interactions and quaternary assembly of alpha 3 alpha 4 alpha 5(IV) collagen: implications for Alport gene therapy. J Biol Chem. 2008;283:35070–35077. doi: 10.1074/jbc.M806396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi K, Yoshikawa N, Iijima K, et al. Immunohistochemical study of alpha 1–5 chains of type IV collagen in hereditary nephritis. Kidney international. 1994;46:1413–1421. doi: 10.1038/ki.1994.413. [DOI] [PubMed] [Google Scholar]
- 26.Gunwar S, Ballester F, Noelken ME, et al. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of α3, α4 and α5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J Biol Chem. 1998;273:8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 27.Lund A, Joensen F, Christensen E, et al. A novel arginine-to-cysteine substitution in the triple helical region of the alpha1(I) collagen chain in a family with an osteogenesis imperfecta/Ehlers-Danlos phenotype. Clinical genetics. 2008;73:97–101. doi: 10.1111/j.1399-0004.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 28.Malfait F, Symoens S, De Backer J, et al. Three arginine to cysteine substitutions in the pro-alpha (I)-collagen chain cause Ehlers-Danlos syndrome with a propensity to arterial rupture in early adulthood. Human mutation. 2007;28:387–395. doi: 10.1002/humu.20455. [DOI] [PubMed] [Google Scholar]
- 29.Kashtan CE. Alport syndrome and the X chromosome: implications of a diagnosis of Alport syndrome in females. Nephrol Dial Transplant. 2007;22:1499–1505. doi: 10.1093/ndt/gfm024. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Heiskari N, Zhou J, et al. High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J Am Soc Nephrol. 1998;9:2291–2301. doi: 10.1681/ASN.V9122291. [DOI] [PubMed] [Google Scholar]
- 31.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timpl R. Structure and biological activity of basement membrane proteins. European journal of biochemistry / FEBS. 1989;180:487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 33.Harvey SJ, Zheng K, Jefferson B, et al. Transfer of the alpha 5(IV) collagen chain gene to smooth muscle restores in vivo expression of the alpha 6(IV) collagen chain in a canine model of Alport syndrome. The American journal of pathology. 2003;162:873–885. doi: 10.1016/s0002-9440(10)63883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.