Abstract
The emergence of cancer stem cell theory has profound implications for cancer chemoprevention and therapy. Cancer stem cells give rise to the tumor bulk through continuous self-renewal and differentiation. Understanding the mechanisms that regulate self-renewal is of greatest importance for discovery of anti-cancer drugs targeting cancer stem cells. Naturally-occurring dietary compounds have received increasing attention in cancer chemoprevention. The anti-cancer effects of many dietary components have been reported for both in vitro and in vivo studies. Recently, a number of studies have found that several dietary compounds can directly or indirectly affect cancer stem cell self-renewal pathways. Herein we review the current knowledge of most common natural dietary compounds for their impact on self-renewal pathways and potential effect against cancer stem cells. Three pathways (Wnt/β-catenin, Hedgehog, and Notch) are summarized for their functions in self-renewal of cancer stem cells. The dietary compounds, including curcumin, sulforaphane, soy isoflavone, epigallocatechin-3-gallate, resveratrol, lycopene, piperine, and vitamin D3, are discussed for their direct or indirect effect on these self-renewal pathways. Curcumin and piperine have been demonstrated to target breast cancer stem cells. Sulforaphane has been reported to inhibit pancreatic tumor initiating cells and breast cancer stem cells. These studies provide a basis for preclinical and clinical evaluation of dietary compounds for chemoprevention of cancer stem cells. This may enable us to discover more preventive strategies for cancer management by reducing cancer resistance and recurrence and improving patient survival.
Keywords: cancer stem cells, chemoprevention, natural dietary compounds
1. Introduction
Cancer is the second leading cause of death in the United States. The first use of chemotherapeutic agents to treat cancer was in the early twentieth century, which became the basis of discovery and development of most current anti-cancer drugs (1, 2). Although a large majority of chemotherapeutic drugs can considerably shrink tumor sizes (3), they often fail to eradicate tumors. The cancer may eventually develop drug resistance and recurrence (3–7). In recent years, a great deal of research has demonstrated the existence of cancer stem cells (CSCs) or tumor-initiating cells (TICs) in several human cancers (8–14). However, most currently available therapeutic approaches, including chemotherapy and radiotherapy, lack the ability to effectively kill these CSCs (3, 15–17). Therefore, this CSC population has become a target for cancer prevention and therapy (7).
Since a large number of epidemiological studies have demonstrated an association between consumption of fruits and vegetables and the reduced risk of various cancers, naturally-occurring dietary compounds have received increasing attention for their efficacy in cancer chemoprevention (18). The anti-cancer effects of many dietary components have been reported for both in vitro and in vivo studies (19–26). This review aims to summarize the potential impact of natural dietary compounds on CSC self-renewal based on CSC theory and self-renewal signaling pathways.
2. Cancer Stem Cells
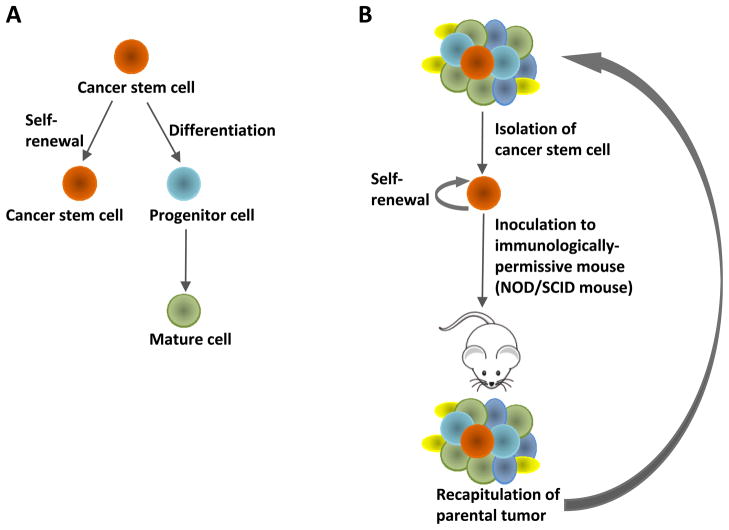
The CSC theory asserts that many types of cancer are initiated from and maintained by a minor population of tumorigenic cells that are capable of continuous self-renewal and differentiation (15, 27) (Figure 1A). This cell population undergoes unlimited proliferation and gives rise to differentiated cells, developing new tumors phenotypically recapitulating the original tumors (7) (Figure 1B). In addition, recent studies indicate that CSCs may be responsible for tumor relapse and resistance to therapy (28, 29).
Figure 1.
Cancer stem cell theory. (A) Cancer stem cells are capable of self-renewal and differentiation. (B) Isolated cancer stem cells are able to phenotypically recapitulate the parental tumor along serial passaging through multiple recipient mice.
Evidence supporting the CSC model was initially obtained from acute myeloid leukemia (AML) (30, 31). Dick et al. isolated a cell subpopulation with surface marker CD34+CD38−, which was able to recapitulate the phenotypes of the original patient neoplasms along serial passaging through multiple NOD/SCID recipient mice (8, 30, 32). Subsequent studies support that solid tumors, including breast (9, 33), pancreatic (12, 34), brain (10, 35), colon (11, 36, 37), liver (14), head/neck (38), ovarian (39, 40), and melanoma (13, 41) are also driven and sustained by CSCs (31). The first work in isolation and characterization of CSCs in solid tumors was conducted by Al-Hajj et al. (9). A breast cancer cell population expressing the surface marker, CD44+CD24−/lowLin−, was able to initiate tumors with the same heterogeneity as the primary tumor from 100 cells (9). Similarly, enzymatic activity of aldehyde dehydrogenase 1 (ALDH) was also demonstrated to be a selective marker to enrich for breast cancer stem/progenitor cells (33). These two phenotypes, ALDH-positive and CD44+CD24−/lowLin−, were identified as possessing a small overlap that has the highest tumorigenic capacity, generating tumors from as few as 20 cells (33). Recently, the CD44+CD24+ESA+ and CD133+ subpopulations were found to harbor putative pancreatic CSCs (12, 34), and an overlap was suggested to exist between these two populations (34). These cell markers have been widely used to evaluate the ability of drugs to target cancer stem/progenitor cells (42–44).
Another technique that has been developed to isolate and characterize cancer stem/progenitor cells is tumorsphere culture (45–48). This is based on the ability of stem/progenitor cells to grow in serum-free, non-adherent suspension as spherical clusters, while differentiated cells fail to survive under the same condition (45, 46). Cancer stem/progenitor cells are capable of yielding secondary spheres and differentiating along multiple lineages (45). Decreases in tumorsphere formation in primary culture in the presence of drug treatment and in subsequent passages that are cultured in the absence of drugs indicate an inhibitory effect of the drug on self-renewal capacity of cancer stem/progenitor cells (42, 45).
Cancer stem cells are able to generate the diverse cells that comprise the tumor through continuous self-renewal and differentiation (49). There is a reliable in vivo model often used to evaluate the drug efficacy against cancer stem cells (9, 49, 50). Immune-deficient mice are first implanted with human cancer cells or human primary tumors. After treatment, the dissociated tumor cells are analyzed for cancer stem cell population based on their specific cell markers, and living tumor cells are re-implanted to a second group of mice which do not receive any treatment (15). Tumorigenicity is then monitored in the recipient mice. For example, the ability of breast cancer cells from the primary NOD/SCID xenografts to re-generate tumors upon re-implantation in the mammary fat pads of secondary mice reflects the inhibitory effect of the treatment on cancer stem cells (15). Failure of tumor initiation indicates the effectiveness of the treatment against breast cancer stem cells.
3. Self-renewal Pathways of Cancer Stem Cells
CSCs produce the tumor mass through continuous self-renewal and differentiation, which may be regulated by similar signaling pathways occurring in normal stem cells (3, 27). Understanding the mechanisms that underlie the self-renewal behavior of CSCs is of greatest importance for discovery and development of anti-cancer drugs targeting CSCs. So far, several major pathways including Wnt/β-catenin, Hedgehog, and Notch have been identified to play pivotal roles in CSC self-renewal (51–53).
3.1. Wnt/β-catenin Pathway
Wnt/β-catenin pathway was demonstrated to modulate cell proliferation, migration, apoptosis, differentiation, and stem cell self-renewal (54–57). It has been shown that Wnt/β-catenin signaling is implicated in the maintenance of CSCs of leukemia (58–60), melanoma (61), breast (62, 63), colon (64), liver (65), lung (66) cancers. For example, over-expression of β-catenin in stem cell survival pathway was shown to mediate the resistance of mouse mammary stem/progenitor cells to radiation (63). Yang and his colleagues reported that Wnt/β-catenin signaling promoted expansion of the hepatic progenitor cell population when it is over-expressed in transplanted rat oval cells and when it is transiently expressed in adult mice (65). Elimination of β-catenin abrogated the chemo-resistant cell population endowed with progenitor-like features (65).
β-Catenin, the essential mediator of canonical Wnt signaling, participates in two distinct functions in the cell, depending on its cellular localization. Membrane-localized β-catenin is sequestered by the epithelial cell-cell adhesion protein E-cadherin to maintain cell-cell adhesion (67). On the other hand, cytoplasmic accumulation of β-catenin and its subsequent nuclear translocation, followed by cooperation with the transcription factors T cell factor/lymphoid enhancer factor (TCF/LEF) as a transcription activator, eventually leads to activation of Wnt target genes such as c-Jun, c-Myc, fibronectin, and cyclin D1 (27, 68–73). Binding of Wnt proteins, a family of secreted proteins, to Frizzled receptors results in the cytoplasmic accumulation of β-catenin (74). In the absence of Wnt signaling, β-catenin forms a multi-protein complex with glycogen synthase kinase 3β (GSK3β), adenomatous polyposis coli, casein kinase1α, and axin (75). When β-catenin is phosphorylated at Ser33/Ser37/Thr41 by GSK3β, it is immediately subject to ubiquitin-proteasome degradation (75, 76).
The link between Wnt/β-catenin and PI3K/Akt pathway has been established by several studies. Activated Akt (i.e., phospho-Akt Ser473) was shown to be able to phosphorylate Ser9 on GSK3β, which may decrease the activity of GSK3β, thereby stabilizing β-catenin (77–79). Furthermore, Korkaya et al. demonstrated that PI3K/Akt pathway is important in regulating the mammary stem/progenitor cells by promoting β-catenin downstream events through phosphorylation of GSK3β (15).
3.2. Hedgehog Pathway
Another major pathway that is involved in stem cell self-renewal is hedgehog signaling pathway (46, 51, 80, 81). For instance, Liu et al. have demonstrated that the hedgehog pathway plays a crucial role in regulating self-renewal of normal and malignant human mammary stem cells by utilizing both in vitro and mouse model systems (51). Another recent study revealed the essential role of hedgehog-Gli signaling in controlling the self-renewal behavior of human glioma CSCs and tumorigenicity (81).
In the absence of hedgehog ligands (Sonic Hedgehog, Desert Hedgehog, and Indian Hedgehog), their transmembrane receptor Patched (Ptch) associates with Smoothened (Smo) and blocks Smo function (27, 80, 82). When secreted hedgehog ligands bind to Ptch, Smo is released, triggering dissociation of transcription factors, Gli1, Gli2, and Gli3 from Fused (Fu) and suppressor of Fused (SuFu), leading to transcription of an array of genes, such as cyclin D, cyclin E, Myc, and elements of EGF pathway (27, 80, 82, 83).
Sonic hedgehog pathway is also linked to transcription factor NF-κB signaling. It was suggested that over-expression of sonic hedgehog is activated by NF-κB in pancreatic cancer and pancreatic cancer cell proliferation is accelerated by NF-κB in part through sonic hedgehog over-expression (84). Kasperczyk et al. further characterized sonic hedgehog as a novel NF-κB target gene and mapped minimal NF-κB consensus site to position +139 of sonic hedgehog promoter (85).
3.3. Notch Pathway
Notch signaling is known to control cell proliferation and apoptosis to modulate the development of many organs (86). A number of recent studies have demonstrated that Notch-activated genes and pathways can drive tumor growth through the expansion of CSCs (46, 86–91). Notch pathway is believed to be dysregulated in CSCs, ultimately leading to uncontrolled CSC self-renewal (86). For example, Notch pathway was shown to play an important role in the self-renewal function of malignant breast cancer CSCs (52, 92).
Five Notch proteins, Notch-1 to Notch-4, have been identified to express as transmembrane receptors in a variety of stem/progenitor cells (93). Binding of surface-bound ligands (Jagged1, Jagged2, Delta-like1, Delta-like3, and Delta-like4) triggers serial cleavage events at the Notch proteins by ADAM protease family and γ-secretase (93–95). Subsequently, the intracellular domain of Notch is released and translocates into the nucleus, where it acts as a transcription co-activator of recombination signal sequence-binding protein Jκ (RBP-J) to activate downstream target genes, e.g., c-Myc, cyclin D1, p21, NF-κB (95–101).
Notch1 has been reported to cross-talk with NF-κB pathway in diverse cellular situations (101–108). Specifically, Notch-1 is necessary for expression of several NF-κB subunits (102, 109) and stimulates NF-κB promoter activity (102).
4. Targeting Self-renewal Pathways of Cancer Stem Cells by Natural Dietary Compounds
The existence of CSCs has profound implications for cancer chemoprevention and therapy (3). Since CSCs are more resistant to conventional therapies in comparison with differentiated cells constituting the tumor bulk, combination of drugs that are directed against CSCs and conventional chemotherapy would have the potential to overcome tumor resistance, reduce relapse (27), and eventually improve patient survival. It was suggested that targeting CSCs could be achieved by several strategies including sensitizing them to chemotherapeutic agents, induction of differentiation, and inhibition of self-renewal signaling (7, 110). A plethora of naturally-occurring dietary compounds have been proven to be promising chemoprevention agents against various types of cancer. A number of studies have found that some dietary compounds can directly or indirectly affect CSC self-renewal pathways (110). Herein, we review the current knowledge of some natural dietary compounds with a focus upon their potential impact on CSC self-renewal pathways and CSC survival (summarized in Table 1).
Table 1.
Natural dietary compounds that potentially regulate cancer stem cell self-renewal and inhibit cancer stem cells.
| Natural Dietary Compound | Food Origin | Cancer Stem Cell | Elements of Self-renewal Pathways |
|---|---|---|---|
| Curcumin | Turmeric | Breast cancer stem cells | β-catenin, TCF-4, Frizzled-1; Notch-1 |
| Sulforaphane | Cruciferous vegetables | Pancreatic cancer stem cells, breast cancer stem cells | β-catenin, GSK3β(?), Wnt-9a |
| Soy isoflavone (especially genistein) | Soy | GSK3β, β-catenin, E-cadherin, Wnt-5a, Sfrp-2; Notch-2 | |
| Indole-3-carbinol and 3,3’-diindolylmethane | Cruciferous vegetables | β-catenin, GSK3β (?) | |
| Epigallocatechin-3-gallate | Green tea | HBP1, β-catenin, GSK3β (?) | |
| Resveratrol | Grapes, berries, plums, and peanuts | β-catenin, GSK3β; Notch-1 | |
| Lycopene | Tomatoes, watermelon, papaya, pink grapefruit | β-catenin, GSK3β (?) | |
| Piperine | Black and long pepper | Breast cancer stem cells | Wnt/β-catenin |
| Vitamin D3 | TCF-4, E-cadherin |
4.1. Curcumin
Curcumin is a well-known dietary polyphenol present in an Indian spice, turmeric, which is usually used in preparation of mustard and curry (111). Curcumin possesses anti-inflammatory and anti-oxidant activities (111, 112), and has been studied as a chemoprevention agent in several cancer models (24, 113).
Jaiswal et al. suggested that curcumin induced caspase-3-mediated cleavage of β-catenin, leading to inactivation of Wnt/β-catenin signaling in HCT116 intestinal cancer cells (114). The work of Park et al. strengthened the point that curcumin decreased β-catenin/TCF transcription activity in all tested cancer cell lines, including gastric, colon, and intestinal cancer cells, which was attributed to the reduced amount of nuclear β-catenin and TCF-4 proteins (111). Moreover, analysis of gene transcription profile revealed that the expression of Wnt receptor Frizzled-1 was potently suppressed by curcumin (115). Curcumin was also shown to be able to attenuate response of β-catenin to Wnt-3a in colon cancer cells through down-regulation of p300, a positive regulator of Wnt/β-catenin signaling (116). In addition, Wang and his colleagues demonstrated that curcumin down-regulated Notch-1 mRNA level in pancreatic cancer cells, indicating a transcriptional inactivation of Notch-1 by curcumin (117). Curcumin-induced inactivation of NF-κB DNA-binding activity was potentially mediated by Notch-1 signaling pathway (117).
Very recently, Kakarala et al. demonstrated that curcumin was able to target breast stem/progenitor cells, as evidenced by suppressed mammosphere formation along serial passage and by a decrease in the percent of ALDH-positive cells (118). On the contrary, curcumin had little impact on differentiated cells (118). By utilizing a TCF-LEF reporter assay system in MCF7 cells, these authors confirmed that the effect of curcumin on breast cancer stem/progenitor cells was mediated through its potent inhibitory effect on Wnt/β-catenin signaling (118).
4.2. Sulforaphane
An extensive amount of studies have substantiated the chemoprevention property of high consumption of cruciferous vegetables (e.g., broccoli and broccoli sprouts), which has been mostly attributed to the activity of isothiocyanates that are enzymatically hydrolyzed from glucosinolates contained in these vegetables (119, 120). In particular, sulforaphane, which is converted from a major glucosinolate in broccoli/broccoli sprouts (121), has been demonstrated to be not only effective in preventing chemically induced cancers in animal models (121–124), but also in inhibiting the growth of established tumors (125, 126).
In a very recent report, Kallifatidis et al. suggested that sulforaphane could abrogate the resistance of pancreatic TICs to TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) by interfering with TRAIL-activated NF-κB signaling (127). Hence, they concluded that combination of sulforaphane with TRAIL would be a promising strategy for targeting pancreatic TICs (127). The down-regulation of NF-κB function by sulforaphane treatment has been reported in prostate and colon cancer cells as well (128–130). In addition, expression of Wnt-9a was shown to be significantly suppressed in ApcMin/+ mouse adenomas treated with sulforaphane (131).
Sulforaphane was previously shown to induce down-regulation of β-catenin in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells (132). On the other hand, several studies have reported the activity of sulforaphane to down-regulate Akt pathway in ovarian, prostate, and colorectal cancers (133–135). Very recently, PI3K/Akt pathway was demonstrated to play an important role in regulating breast stem/progenitor cells by promoting β-catenin down-stream events through phosphorylation of GSK3β (15).
In our studies, we have shown that sulforaphane is effective in targeting breast cancer stem/progenitor cells in vitro and in vivo (42). Sulforaphane inhibits breast CSCs at concentrations (0.5 – 5 μM) approximately 10-fold lower of that exhibiting anti-proliferative effect on cancer cell culture. Our studies have demonstrated that sulforaphane can inhibit breast CSCs in vivo. The data showed that recipient NOD/SCID mice inoculated with tumor cells derived from sulforaphane-treated primary xenografts failed to develop tumor re-growth up to 33 days, whereas control tumor cells quickly gave rise to large tumors. We also observed a down-regulation of Wnt/β-catenin self-renewal pathway in sulforaphane-treated breast cancer cells.
4.3. Soy Isoflavone
High consumption of soy-rich food has shown an inverse correlation with the incidence of breast cancer (136). Increased plasma concentration of genistein (one of the most active soy isoflavones) due to soy food intake was associated with reduced risk of breast cancer in recent studies (137, 138). Soy isoflavones, especially genistein, exhibit potent anti-proliferative effect on various cancers (139).
Soy isoflavones were found to inhibit the phosphorylation of Akt and FOXO3a, enhance the expression of GSK3β, leading to increased phosphorylation of β-catenin in prostate cancer cells (140, 141). Genistein was reported to attenuate β-catenin-mediated expression of Wnt downstream target genes in mammary epithelial cells by up-regulating E-cadherin (142). Using gene microarray technique, a study revealed that dietary exposure to genistein down-regulated Wnt signaling through inhibiting Wnt-5a expression and enhancing Sfrp-2 (secreted frizzled-related protein-2, an extracellular Wnt receptor antagonist) expression and reduced Notch-2 expression in rat mammary epithelial cells in vivo (143). Moreover, Wang et al. have found that genistein inhibited Notch-1 signaling, thereby down-regulating NF-κB activity, eventually leading to cell growth inhibition and apoptosis in pancreatic cancer cells (144, 145). The inactivation of NF-κB by genistein in several cancers (146–148) provides a basis for further investigation in the impact on hedgehog pathway. Based on all these data, future studies on the effect of soy isoflavone, particularly genistein, on CSCs is warranted.
4.4. Epigallocatechin-3-Gallate (EGCG)
Green tea is one of the most widely consumed beverages in the world. Epidemiological studies suggest an association between green tea consumption and cancer prevention effects (149). The various polyphenolic catechins contained in green tea are thought to largely account for its chemoprevention activity against certain types of cancer. In particular, several studies indicate that epigallocatechin-3-gallate (EGCG), the most abundant catechin in green tea, is a potent chemoprevention agent (150). EGCG has been shown to inhibit NF-κB activity, MAPK pathway, activator protein-1 (AP-1) activity, and EGFR-mediated downstream signaling pathways, etc. (151).
EGCG was demonstrated to block Wnt signaling by stabilizing mRNA of HBP1, a suppressor of Wnt signaling, thereby reducing breast cancer cell tumorigenic proliferation as well as invasiveness (110, 152). The nuclear import of β-catenin was decreased in adenomas isolated from EGCG-treated ApcMin/+ mice, a widely used transgenic model recapitulating human colon cancer that bears an Adenomatous Polyposis Coli (APC) gene mutation (153, 154). In addition, several studies revealed that EGCG suppressed Akt activation in both colon cancer cell lines and in vivo mouse models (151, 153–155). In our previous study, EGCG was shown to inhibit the chaperoning function of heat shock protein 90 (Hsp90) by impairing the interaction between Hsp90 with its co-chaperones in pancreatic cancer cells, thereby down-regulating Hsp90 client proteins including Akt (156). Additionally, EGCG has been found to negatively regulate NF-κB activity and inhibit the ATP- or IL-1β induced activation of NF-κB (141, 157–160). It is still unknown whether this could have impact on sonic hedgehog expression and hedgehog signaling pathway. Taken together, these studies support the further evaluation of EGCG in CSCs.
4.5. Resveratrol
During the last decade, resveratrol, a polyphenol derived from a wide variety of plants such as grapes, berries, plums, and peanuts (161), has been shown to possess chemopreventive and chemotherapeutic potential against human cancers (162). Resveratrol exhibited inhibitory effect on the proliferation of various human cancer cells and on the carcinogenesis in animal models (162, 163).
Low concentrations of resveratrol were shown to significantly decrease the nuclear localization of β-catenin in colon cancer cells (164). The inhibitory effects of resveratrol on Waldenstrom’s macroglobulinemia cells were suggested to be mediated through the down-regulation of Akt and Wnt signaling pathways (141, 165). Cecchinato and his colleagues reported that resveratrol inhibited the PI3K/Akt pathway, thereby activating GSK3β in acute lymphoblastic leukemia cells (166). Furthermore, these authors showed for the first time that escalating doses of resveratrol led to a progressive decrease in Notch-1 protein level, as well as the mRNA levels of its downstream effectors (166). Therefore, the potential impact of resveratrol against CSCs may be warranted for future exploration.
4.6. Lycopene
Lycopene, one of the most extensively studied carotenoids in tomatoes, possesses potent anti-oxidant activity due to its extended conjugated hydrocarbon chain (167). Lycopene has been shown to induce apoptosis and inhibit cell cycle progression in various cancer cells (168–174), and the efficacy of lycopene against xenograft tumors was reported in a number of in vivo studies (172, 175–177).
In colon cancer cells, lycopene suppressed Akt activation and non-phosphorylated β-catenin protein level, and augmented the phosphorylated form of β-catenin, which were associated with reduced protein expression of cyclin D1 (178). Hence, lycopene may inhibit Wnt/β-catenin signaling via the connection along Akt/GSK3β/β-catenin. Further studies on CSCs in response to lycopene would perhaps be promising.
4.7. Piperine
Piperine, a dietary polyphenol isolated from black and long peppers, has been reported to reduce cancer incidence in chemical rodent models of lung cancer (118, 179–183). Although the chemoprevention effect of piperine in breast cancer as a single agent has not been explored, Kakarala et al. demonstrated that piperine was able to target breast CSCs and inhibit Wnt/β-catenin signaling pathway (118). In addition, piperine was shown to suppress the nuclear import and activation of NF-κB (180, 184), the effect of which on sonic hedgehog signaling is not yet clear.
4.8. Vitamin D3
Vitamin D3 has been shown to reduce the incidence of human breast, prostate, and colon cancers (185–187), and induce apoptosis and cell cycle arrest of various cancer cells (188). In 2001, Palmer et al. demonstrated that vitamin D3 promoted the differentiation of colon carcinoma cells by the induction of E-cadherin expression and the inhibition of β-catenin signaling (189). Ligand-activated vitamin D receptor competed with TCF-4 for β-catenin binding, thereby reducing levels of c-Myc, peroxisome proliferator-activated receptor, TCF-1, and CD44 (189). These findings would trigger further investigations of vitamin D3 in terms of chemoprevention of CSCs.
5. Conclusions and Future Perspectives
Naturally-occurring dietary compounds are advantageous in several aspects as chemoprevention agents: (1) they are present in commonly consumed food, which is readily available to most people in daily life; (2) they usually have very low or no toxicity, in contrast to most chemotherapy drugs; (3) many of these compounds have shown potential as an adjunct to chemotherapy drugs in some clinical trials. Although the reports were very limited for dietary compounds to inhibit CSCs, many of them have been shown to be involved in modulation of CSC self-renewal pathways. Three dietary components, sulforaphane, curcumin, and piperine, have been shown to inhibit Wnt/β-catenin signaling and breast CSCs at relatively low concentrations (42, 43, 190). For instance, our data showed that sulforaphane inhibited breast CSCs at concentrations of 0.5 to 5 μM (42). The inhibitory effect on the self-renewal pathway may contribute to the preferential inhibition of CSCs. Further studies are needed to investigate the underlying mechanisms. For other dietary compounds of interest, it would be very promising to study their efficacy and effective concentrations against CSCs. Given that these diet-based compounds are usually multi-targeted, they may mediate other cellular events, e.g., induction of CSC differentiation and sensitization of CSCs to chemotherapeutic agents, in addition to their potential impact on self-renewal signaling.
Investigating the efficacy of the dietary compounds against CSCs will provide rationale for preclinical and clinical evaluation of these compounds or potentially their native food extracts for chemoprevention of CSCs. These studies will eventually enable us to discover more effective strategies for cancer treatment to reduce cancer resistance and recurrence and to improve patient survival.
Acknowledgments
Grant support: This work was supported by the National Institutes of Health (RO1 CA120023 and R21 CA143474); University of Michigan Cancer Center Research Grant (Munn); and University of Michigan Cancer Center Core Grant to DS.
References
- 1.Gilman A. The initial clinical trial of nitrogen mustard. Am J Surg. 1963;105:574–8. doi: 10.1016/0002-9610(63)90232-0. [DOI] [PubMed] [Google Scholar]
- 2.Kohn KW. Beyond DNA cross-linking: history and prospects of DNA-targeted cancer treatment--fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1996;56:5533–46. [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–40. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 5.Stockler M, Wilcken NR, Ghersi D, Simes RJ. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26:151–68. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 6.Lippman ME. High-dose chemotherapy plus autologous bone marrow transplantation for metastatic breast cancer. N Engl J Med. 2000;342:1119–20. doi: 10.1056/NEJM200004133421508. [DOI] [PubMed] [Google Scholar]
- 7.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 11.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 13.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–66. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–6. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer. 2003;107:1001–11. doi: 10.1002/ijc.11490. [DOI] [PubMed] [Google Scholar]
- 19.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132:3623–31. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 21.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–36. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Li X, Sarkar FH. Gene expression profiles of I3C-and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–9. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 23.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–40. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys. 2003;410:177–85. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 26.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–54. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 30.Bednar F, Simeone DM. Pancreatic cancer stem cells and relevance to cancer treatments. J Cell Biochem. 2009;107:40–5. doi: 10.1002/jcb.22093. [DOI] [PubMed] [Google Scholar]
- 31.Ischenko I, Seeliger H, Schaffer M, Jauch KW, Bruns CJ. Cancer stem cells: how can we target them? Curr Med Chem. 2008;15:3171–84. doi: 10.2174/092986708786848541. [DOI] [PubMed] [Google Scholar]
- 32.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 33.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 38.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 40.Fong MY, Kakar SS. The role of cancer stem cells and the side population in epithelial ovarian cancer. Histol Histopathol. 25:113–20. doi: 10.14670/HH-25.113. [DOI] [PubMed] [Google Scholar]
- 41.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakarala M, Brenner DE, Korkaya H, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 122:777–85. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: current opinion and future challenges. Pathobiology. 2008;75:75–84. doi: 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall GP, 2nd, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol. 2007;8:141–5. doi: 10.2174/138920107780906559. [DOI] [PubMed] [Google Scholar]
- 48.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 50.Dick JE. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100:3547–9. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–30. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- 54.Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–23. doi: 10.1159/000098207. [DOI] [PubMed] [Google Scholar]
- 55.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 56.Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–24. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- 57.Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–82. doi: 10.1016/s1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 58.Kawaguchi-Ihara N, Murohashi I, Nara N, Tohda S. Promotion of the self-renewal capacity of human acute leukemia cells by Wnt3A. Anticancer Res. 2008;28:2701–4. [PubMed] [Google Scholar]
- 59.Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138:338–48. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- 60.Ysebaert L, Chicanne G, Demur C, et al. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20:1211–6. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]
- 61.Chien AJ, Moore EC, Lonsdorf AS, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–8. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A. 2003;100:15853–8. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulenburg A, Cech P, Herbacek I, et al. CD44-positive colorectal adenoma cells express the potential stem cell markers musashi antigen (msi1) and ephrin B2 receptor (EphB2) J Pathol. 2007;213:152–60. doi: 10.1002/path.2220. [DOI] [PubMed] [Google Scholar]
- 65.Yang W, Yan HX, Chen L, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287–95. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 66.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 67.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 69.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 70.Mann B, Gelos M, Siedow A, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–6. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112 ( Pt 8):1237–45. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 74.Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20:581–9. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 77.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–32. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 78.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 79.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 80.Cohen MM., Jr The hedgehog signaling network. Am J Med Genet A. 2003;123A:5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- 81.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis MT, Veltmaat JM. Next stop, the twilight zone: hedgehog network regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:165–81. doi: 10.1023/B:JOMG.0000037160.24731.35. [DOI] [PubMed] [Google Scholar]
- 83.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 84.Nakashima H, Nakamura M, Yamaguchi H, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–9. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 85.Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580:2860–8. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 88.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol. 2008;26:2883–9. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan X, Eberhart CG. Medulloblastoma stem cells. J Clin Oncol. 2008;26:2821–7. doi: 10.1200/JCO.2007.15.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 92.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169–75. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 93.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–65. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 94.Wu JY, Rao Y. Fringe: defining borders by regulating the notch pathway. Curr Opin Neurobiol. 1999;9:537–43. doi: 10.1016/S0959-4388(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 95.Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66:1631–46. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Satoh Y, Matsumura I, Tanaka H, et al. Roles for c-Myc in self-renewal of hematopoietic stem cells. J Biol Chem. 2004;279:24986–93. doi: 10.1074/jbc.M400407200. [DOI] [PubMed] [Google Scholar]
- 98.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103:18261–6. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–34. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rangarajan A, Talora C, Okuyama R, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–36. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol. 1998;18:2077–88. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jang MS, Miao H, Carlesso N, et al. Notch-1 regulates cell death independently of differentiation in murine erythroleukemia cells through multiple apoptosis and cell cycle pathways. J Cell Physiol. 2004;199:418–33. doi: 10.1002/jcp.10467. [DOI] [PubMed] [Google Scholar]
- 103.Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–55. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Chan SL, Miele L, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004;101:9458–62. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin HM, Minter LM, Cho OH, et al. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25:129–38. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J, Shelly L, Miele L, Boykins R, Norcross MA, Guan E. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J Immunol. 2001;167:289–95. doi: 10.4049/jimmunol.167.1.289. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–84. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 2007;101:427–33. doi: 10.1111/j.1742-7843.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 109.Cheng P, Zlobin A, Volgina V, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167:4458–67. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 110.Kawasaki BT, Hurt EM, Mistree T, Farrar WL. Targeting cancer stem cells with phytochemicals. Mol Interv. 2008;8:174–84. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 111.Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579:2965–71. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 112.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–4. [PubMed] [Google Scholar]
- 113.Shao ZM, Shen ZZ, Liu CH, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–40. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 114.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–27. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 115.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–41. [PubMed] [Google Scholar]
- 116.Ryu MJ, Cho M, Song JY, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Commun. 2008;377:1304–8. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 117.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 118.Kakarala M, Brenner DE, Korkaya H, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–5. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–91. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 125.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 126.Jackson SJ, Singletary KW. Sulforaphane: a naturally occurring mammarycarcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25:219–27. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- 127.Kallifatidis G, Rausch V, Baumann B, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–63. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 128.Choi S, Lew KL, Xiao H, et al. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–62. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 129.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 130.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–70. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 131.Khor TO, Hu R, Shen G, et al. Pharmacogenomics of cancer chemopreventive isothiocyanate compound sulforaphane in the intestinal polyps of ApcMin/+ mice. Biopharm Drug Dispos. 2006;27:407–20. doi: 10.1002/bdd.522. [DOI] [PubMed] [Google Scholar]
- 132.Park SY, Kim GY, Bae SJ, Yoo YH, Choi YH. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol Rep. 2007;18:181–7. [PubMed] [Google Scholar]
- 133.Shen G, Khor TO, Hu R, et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937–44. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 134.Chaudhuri D, Orsulic S, Ashok BT. Antiproliferative activity of sulforaphane in Akt-overexpressing ovarian cancer cells. Mol Cancer Ther. 2007;6:334–45. doi: 10.1158/1535-7163.MCT-06-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–66. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- 136.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–27. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 137.Iwasaki M, Inoue M, Otani T, et al. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case-control study from the Japan Public Health Center-based prospective study group. J Clin Oncol. 2008;26:1677–83. doi: 10.1200/JCO.2007.13.9964. [DOI] [PubMed] [Google Scholar]
- 138.Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH. Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol. 2007;25:648–55. doi: 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- 139.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125:777S–83S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283:27707–16. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–7. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–9. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 143.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 144.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–93. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 145.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–6. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 146.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35:167–74. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 147.Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol. 2000;165:2719–28. doi: 10.4049/jimmunol.165.5.2719. [DOI] [PubMed] [Google Scholar]
- 148.Natarajan K, Manna SK, Chaturvedi MM, Aggarwal BB. Protein tyrosine kinase inhibitors block tumor necrosis factor-induced activation of nuclear factor-kappaB, degradation of IkappaBalpha, nuclear translocation of p65, and subsequent gene expression. Arch Biochem Biophys. 1998;352:59–70. doi: 10.1006/abbi.1998.0576. [DOI] [PubMed] [Google Scholar]
- 149.Landis-Piwowar KR, Huo C, Chen D, et al. A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67:4303–10. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 150.Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin Oncol. 1999;125:589–97. doi: 10.1007/s004320050321. [DOI] [PubMed] [Google Scholar]
- 151.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)- Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 152.Kim J, Zhang X, Rieger-Christ KM, et al. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 153.Bose M, Hao X, Ju J, et al. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (-)-epigallocatechin-3-gallate and fish oil. J Agric Food Chem. 2007;55:7695–700. doi: 10.1021/jf071004r. [DOI] [PubMed] [Google Scholar]
- 154.Ju J, Hong J, Zhou JN, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–31. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 155.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–19. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 156.Li Y, Zhang T, Jiang Y, Lee HF, Schwartz SJ, Sun D. (-)-Epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca-2. Mol Pharm. 2009;6:1152–9. doi: 10.1021/mp900037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–46. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 158.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (-)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–44. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 159.Guo S, Lu J, Subramanian A, Sonenshein GE. Microarray-assisted pathway analysis identifies mitogen-activated protein kinase signaling as a mediator of resistance to the green tea polyphenol epigallocatechin 3-gallate in her-2/neu-overexpressing breast cancer cells. Cancer Res. 2006;66:5322–9. doi: 10.1158/0008-5472.CAN-05-4287. [DOI] [PubMed] [Google Scholar]
- 160.Kim SJ, Jeong HJ, Lee KM, et al. Epigallocatechin-3-gallate suppresses NF-kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J Nutr Biochem. 2007;18:587–96. doi: 10.1016/j.jnutbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 161.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 162.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–840. [PubMed] [Google Scholar]
- 163.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila Pa) 2009;2:409–18. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 164.Hope C, Planutis K, Planutiene M, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52 (Suppl 1):S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roccaro AM, Leleu X, Sacco A, et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom's macroglobulinemia. Clin Cancer Res. 2008;14:1849–58. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 166.Cecchinato V, Chiaramonte R, Nizzardo M, et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007;74:1568–74. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 167.van Breemen RB, Pajkovic N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008;269:339–51. doi: 10.1016/j.canlet.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nahum A, Zeller L, Danilenko M, et al. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45:275–82. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- 169.Nahum A, Hirsch K, Danilenko M, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–36. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 170.Hantz HL, Young LF, Martin KR. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med (Maywood) 2005;230:171–9. doi: 10.1177/153537020523000303. [DOI] [PubMed] [Google Scholar]
- 171.Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed Pharmacother. 2007;61:366–9. doi: 10.1016/j.biopha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 172.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–74. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 173.Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of human breast cell line (MCF-7) Toxicol In Vitro. 2007;21:217–23. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 174.Gunasekera RS, Sewgobind K, Desai S, et al. Lycopene and lutein inhibit proliferation in rat prostate carcinoma cells. Nutr Cancer. 2007;58:171–7. doi: 10.1080/01635580701328339. [DOI] [PubMed] [Google Scholar]
- 175.Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J Nutr. 2005;135:287–90. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- 176.Nagasawa H, Mitamura T, Sakamoto S, Yamamoto K. Effects of lycopene on spontaneous mammary tumour development in SHN virgin mice. Anticancer Res. 1995;15:1173–8. [PubMed] [Google Scholar]
- 177.Sharoni Y, Giron E, Rise M, Levy J. Effects of lycopene-enriched tomato oleoresin on 7,12-dimethyl-benz[a]anthracene-induced rat mammary tumors. Cancer Detect Prev. 1997;21:118–23. [PubMed] [Google Scholar]
- 178.Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol Nutr Food Res. 2008;52:646–54. doi: 10.1002/mnfr.200700272. [DOI] [PubMed] [Google Scholar]
- 179.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–50. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 180.Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol. 2004;4:1795–803. doi: 10.1016/j.intimp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 181.Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis. 2002;19:703–8. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]
- 182.Selvendiran K, Banu SM, Sakthisekaran D. Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta. 2004;350:73–8. doi: 10.1016/j.cccn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 183.Selvendiran K, Prince Vijeya Singh J, Sakthisekaran D. In vivo effect of piperine on serum and tissue glycoprotein levels in benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pulm Pharmacol Ther. 2006;19:107–11. doi: 10.1016/j.pupt.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, Ghosh B. Piperine inhibits TNF-alpha induced adhesion of neutrophils to endothelial monolayer through suppression of NF-kappaB and IkappaB kinase activation. Eur J Pharmacol. 2007;575:177–86. doi: 10.1016/j.ejphar.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 185.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 186.Grant WB, Garland CF. Evidence supporting the role of vitamin D in reducing the risk of cancer. J Intern Med. 2002;252:178–9. doi: 10.1046/j.1365-2796.2002.01016.x. author reply 9–80. [DOI] [PubMed] [Google Scholar]
- 187.Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–38. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- 188.Danilenko M, Studzinski GP. Enhancement by other compounds ofthe anti-cancer activity of vitamin D(3) and its analogs. Exp Cell Res. 2004;298:339–58. doi: 10.1016/j.yexcr.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 189.Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Y, et al. Sulforaphane inhibits Hsp90 function by disrupting Hsp90/p50Cdc37 complex in pancreatic cancer cells through direct interaction with specific residues. 2010 Submitted. [Google Scholar]