Abstract
Extraction of oil from the seed of Blighia unijugata gave a yield of 50.82 ± 1.20% using hexane in a soxhlet extractor. The iodine and saponification values were 67.60 ± 0.80 g iodine/100 g and 239.20 ± 1.00 mg KOH/g respectively with C18:1 being the dominant fatty acid. Unsaturated methyl esters of Blighia unijugata which had been previously subjected to urea adduct complexation was used to synthesize methyl 9, 10-dihydroxyoctadecanoate via hydroxylation in the presence of cetyltrimethylammonium permanganate (CTAP). The reaction was monitored and confirmed using FTIR and GC-MS. This study has revealed that oxidation reaction of mono unsaturated bonds using CTAP could be achieved under solvent free condition.
Introduction
The replacement of petrochemicals by oleochemical feedstocks in many industrial and domestic applications as resulted in increase in demand for bio-based products and as such recognizing and increasing the benefits of using renewable materials. This has reduced the dependence on imported petroleum and promoted the sustainable agricultural initiative. Vegetable oils are one of the most versatile renewable substrates and can be converted into several products [1-3]. The use of lesser known seed oils is of great benefit in this regards, example of such lesser known underutilized seed oil is that of Blighia unijugata.
Blighia unijugata is a tree planted as shade tree in Nigeria. It is of attractive appearance especially when in fruit which are red or pinkish yellow. The wood is used for buildings; it is also recognized for its sedative and analgesic properties in treatment of rheumatism [4]. Presently, the seed oil has no specific use in Nigeria as the seeds are discarded as waste.
Natural polyols can be obtained by chemical modification of the vegetable oils introducing hydroxyl groups in an unsaturated triglyceride by hydroxylation of carbon-carbon double bonds and/or by alcoholysis of the triglyceride to obtain mainly a monoglyceride [3,5]. Vegetable oil contains mixture of different unsaturated and saturated fatty acids in varying amounts; these have different applications, though, there is little or scanty report on their isolation.
Urea complexation reaction is a well-established technique for elimination of saturated and monounsaturated fatty acids [6-9]. Urea complexation has the advantage that complexed crystals are extremely stable, and filtration does not necessarily have to be carried out at the very low temperatures which solvent crystallization of fatty acids would require. This method is also favorable because complexation depends upon the configuration of the fatty acid moieties due to the presence of multiple double bonds, rather than pure physical properties such as melting point or solubility [10]. The saturated and monounsaturated fatty acids easily complex with urea and crystallize out on cooling and may subsequently be removed by filtration. The liquid or non-urea complexed fraction is enriched with unsaturated fatty acids.
The fundamentally attractive concept of green Chemistry is solvent free reactions. Solvent-free reactions are an interesting alternative approach, mainly when these conditions eliminate the use of a solid support or solvent from the reaction [11]. A dry solid-phase reaction is solvent-free, also a reaction where there is liquid presence, but not acting as a solvent (i.e. nothing is dissolved in it) is also solvent-free" [12]. The economic benefits of green Chemistry are central drivers in its advancement. Industry is adopting green Chemistry methodologies because they improve the corporate bottom line. A wide array of operating costs is decreased through the use of green Chemistry. When less waste is generated, environmental compliance casts go down and when waste is eliminated treatment and disposal become unnecessary. Decreased solvent usage and fewer processing steps lessen the material and energy costs of manufacturing and increase material efficiency.
Permanganate, an important oxidant in many organic and inorganic redox reactions, involves the Mn (VII) entity, which is renowned for its versatility. The permanganate oxidation process is eco-friendly and has gained importance in green Chemistry. Cetyltrimethylammonium permanganate (CTAP) possess a long hydrocarbon chain that can draw substrate close to MnO4- ion in a micelle-like aggregation, thereby enabling reactant molecules to efficiently interact with oxidizing ion even if a homogenizing solvent is not present. This attribute pointed our attention to the hydroxylation of olefin bonds in the unsaturated methyl esters from seed oil of Blighia unijugata with cetyltrimethylammonium permanganate. In the present study, the seed oil of Blighia unijugata was chemically characterized, the unsaturation of the methyl ester was increased using urea adduct complexation reaction and 9,10-dihydroxyoctadecanoate was synthesized from the unsaturated methyl esters using cetyltrimethylammonium permanganate.
Materials and method
Extraction and chemical analysis of the seed oil of Blighia unijugata
The dried seeds of Blighia unijugata were extracted with n-hexane for 10 hr using soxhlet extractor [13]. The oil was analyzed for iodine, saponification and free fatty acid content by method described by the Association of Official Analytical Chemist [14].
Fatty acid composition of Blighia unijugata
Fatty acid methyl esters of the oil were prepared by refluxing the sample at 70°C for 3 h in 2% sulphuric acid in methanol. The esters were extracted into ethyl acetate, washed free of acid and passed over anhydrous sodium sulphate. The ethyl acetate extracts were further concentrated using a rotary evaporator. The fatty acid composition was analyzed using an Agilent 6890 N series gas chromatography equipped with FID detector on a split injector. A fused silica capillary column (DB-225, 30 × 0.32 m i.d., J & W Scientifics, USA) was used with the injector and detector temperature maintained at 230°C and 250°C respectively. The oven temperature was programmed at 160°C for 2 min and finally increased to 230°C at 4°C/min. The carrier gas was nitrogen at a flow rate of 1.5 mL/min. The area percentages were recorded with a standard Chemstation Data System.
Urea adduct complexation reaction of Blighia unijugata methyl esters
Fatty acid methyl esters for the urea adduct complexation reaction were prepared by refluxing the oil at 70°C for 3 h in 1% KOH in methanol. The esters were extracted into ethyl acetate, washed free of acid and passed over anhydrous sodium sulphate. The ethyl acetate extracts were further concentrated using a rotary evaporator. Methyl esters (100 g) were dissolved in methanol (1000 ml) to which urea (200 g) had been added. The mixture was warmed with stirring until the whole mixture turned into a clear homogeneous solution [15]. The solution was allowed to cool to room temperature and kept refrigerated at 5°C for 8 h. Crystals were removed by filtering through a Buchner funnel to remove the urea complexes, which were washed twice with 25 ml of methanol saturated with urea. The filtrate which is rich in unsaturated methyl esters was poured into 1% hydrochloric acid (600 ml) and extracted alternatively with hexane and diethyl ether. The combined organic layers were washed with water twice (50 ml) and passed over anhydrous sodium sulphate and later concentrated using a rotary evaporator. This reaction was repeated changing the ratio of fatty acid methyl esters to urea from 1:2 to 2:1 in order to further increase the unsaturation of the methyl esters. The resulting methyl esters (BME) were then taken for the determination of the constituent fatty acids using a GC as described above.
Preparation of cetyltrimethylammonium permanganate
This was achieved by introducing a solution of 27.0 mmol of KMnO4 (4.25 g in 25 ml distilled water) into a 250 ml two-necked round bottom flask in a water bath maintained at 8°C. After about 15 min, solution of 25.0 mmol of cetyltrimethylammonium bromide (9.10 g in 50 ml dichloromethane) was added into the flask and stirred for 4 h. The organic layer was separated and the solvent recovered under reduced pressure with the crystalline cetyltrimethylammonium permanganate (purple coloured) precipitating out before the complete recovery of the solvent; it was filtered, washed with distilled water and ether and dried over P2O5 under vacuum.
Dihydroxylation of BME using cetyltrimethylammonium permanganate
The dihydroxylation was carried out in a clamped three necked round bottom flask equipped with a thermometer and a stirrer in a thermo-regulated water bath. About 2.10 g (5.2 mmol) of cetyltrimethylammonium permanganate with five drops of distilled water was introduced into the flask. About 5.0 mmol of BME was slowly added drop wise while stirring the mixture. The mixture was stirred for 1 h and extracted three times with 50 ml portion of ether; this was later washed with saturated solution of NaCl, dried over Na2SO4 and concentrated on a rotary evaporator.
Isolation of methyl 9, 10-dihydroxyoctadecanoate
The final product was separated on a 1 g scale by silica gel column chromatography using a glass column 20 cm × 2 cm OD packed with 30 g activated silica gel (60-120 mesh). Hydrocarbons and other non-polar compounds were eluted with petroleum ether (boiling point, 60-80°C). The methyl 9, 10-dihydroxyoctadecanoate were eluted using a mixture of petroleum ether - diethyl ether (40:60 v/v). The fractions were screened by TLC for the identification of the compounds isolated. The eluted spots were identified using iodine vapors.
Trimethylsilylation derivatisation and GC-MS analysis of methyl 9, 10-dihydroxyoctadecanoate
The isolated methyl 9,10-dihydroxyoctadecanoate was derivatised and identified by GC-MS analysis using Agilent (Palo Alto, USA) 6890 N gas chromatography equipped with an HP-1 MS capillary column connected to an Agilent 5973 mass spectrometer operating in the EI mode (70 ev; m/z 50-550; source temperature 230°C and quadruple temperature 150°C). Methyl 9,10-dihydroxyoctadecanoate was silylated using N, O-Bis(trimethylsilyl)trifluoroacetamide; about 13 μl/mg of N, O-Bis(trimethylsilyl)trifluoroacetamide was added to methyl 9,10-dihydroxyoctadecanoate, kept at 75°C for 60 min and thoroughly shaken. The final product was extracted in ethyl acetate and concentrated using a rotary evaporator. Structural assignments were made based on interpretation of mass spectrometric fragmentation and confirmation by comparison of retention time as well as fragmentation pattern of authentic compounds and the spectral data obtained from the Wiley and NIST libraries.
Fourier Transform Infrared (FTIR)
The FTIR spectra of the methyl esters and hydroxylated methyl esters were recorded using a Perkin Elmer FTIR system spectrum BX LR64912C. The samples were spread over NaCl cells, and their spectra were recorded in the range of 4000-400 cm-1.
Results and discussion
Chemical characterization and fatty acid composition of the seed oil of Blighia unijugata
The result of the chemical characterization of Blighia unijugata is presented in Table 1. The oil yield from the seed of Blighia unijugata was found to be 50.82 ± 1.20%. The iodine value and saponification were found to be 67.60 ± 0.80 g iodine/100 g and 239.20 ± 1.00 mg KOH/g respectively. These values are different from what was initially reported [16] which might be due to the presence of impurities or solvent contamination in the previously analyzed samples. The fatty acid composition is shown in Table 2. The dominant fatty acid was found to be C18:1 (48.1 ± 0.50%) while the least present was found to be C14:0 (0.2 ± 0.10%). C16:0 was 34.5 ± 0.20% while C18:0 was 14.1 ± 0.10% with a total unsaturation of 50.4 ± 0.20%. These data are available with the online version of this paper. Additional file 1 describes the characterization and fatty acid composition of the seed oil of Blighia unijugata.
Table 1.
Characterization of the oil of B.unijugata
| Parameter | B. unijugata |
|---|---|
| Oil yield (%) | 50.82 ± 1.20 |
| FFA (%) | 7.00 ± 0.1 |
| Iodine value (g iodine/100 g) | 67.60 ± 0.80 |
| Saponification value (mg KOH/g) | 239.20 ± 1.00 |
Values are mean ± standard deviation of triplicate determinations.
Table 2.
Fatty acid composition (wt %) of B.unijugata
| Fatty acids | B. unijugata |
|---|---|
| 14:0 | 0.2 ± 0.10 |
| 16:0 | 34.5 ± 0.20 |
| 18:0 | 14.1 ± 0.10 |
| 18:1 | 48.1 ± 0.50 |
| 18:2 | 1.8 ± 0.30 |
| 18:3 | 0.3 ± 0.20 |
| 20:0 | 0.5 ± 0.10 |
| 20:1 | 0.2 ± 0.00 |
| 24:0 | 0.3 ± 0.10 |
| Unsaturated | 50.4 ± 0.20 |
| Saturated | 49.6 ± 0.10 |
Values are mean ± standard deviation of triplicate determinations.
Urea adduct complexation reaction of Blighia unijugata methyl esters
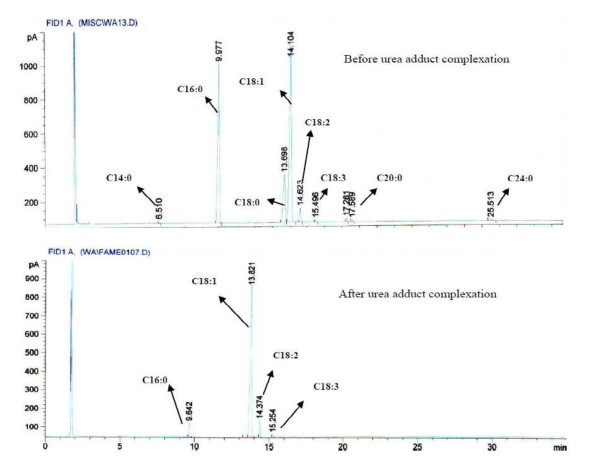
The urea adduct complexation reaction was carried out so as to increase the unsaturation of Blighia unijugata methyl esters. This increase in unsaturation allows the introduction of different functional groups in order to improve on the possible applications of the seed oil of Blighia unijugata. The effect of the complexation reaction on the methyl esters of Blighia unijugata is shown in Figure 1. After the complexation reaction C14:0, C18:0, C20:0 and C24:0 fatty acids were totally removed while the amounts of C16:0 saturated fatty acid was reduced. C16:0 was reduced from 34.5 ± 0.20% to 4.73 ± 0.10%. The unsaturation of the methyl esters was increased from 50.4 ± 0.20% to 94.56 ± 0.20% with a yield of 49.20 ± 0.20%. The amounts of C18:1 which was found as the dominant fatty acid in the oil was increased from 48.1 ± 0.50% to 88.75 ± 0.40%. This increase in C18:1 and the fair percentage yield of 49.20 ± 0.20% were the major indicators that led to the application of the methyl esters of Blighia unijugata in the synthesis of methyl 9, 10-dihydroxyoctadecanoate.
Figure 1.
Fatty acid composition before and after urea adduct complexation reaction.
Dihydroxylation of BME using cetyltrimethylammonium permanganate
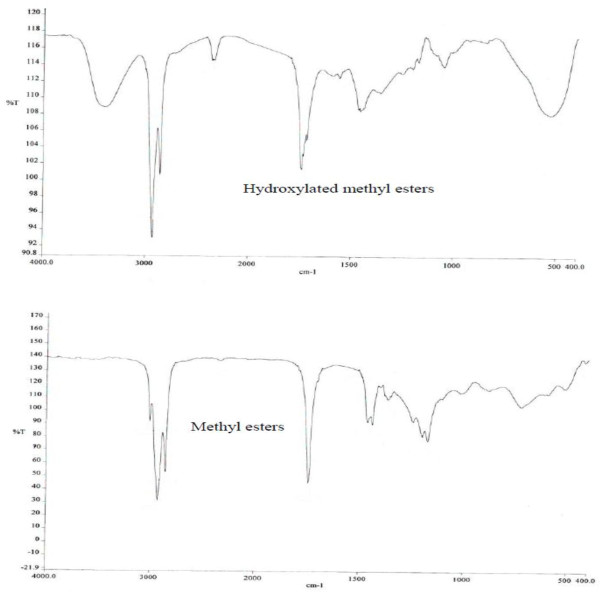
The synthesis of methyl 9, 10-dihydroxyoctadecanoate was achieved via dihydroxylation of the unsaturated methyl esters (BME) after urea adduct complexation. The reaction was monitored using TLC while the formation was confirmed using IR and GC-MS. The IR result if shown in Figure 2. The spectrum of the oil showed a peak at 3006 cm-1 which was assigned to the vibrational frequency of the C-H stretching of C = C-H present in the oil. The C = O stretching frequency of ester functional groups was found at 1743 cm-1. The peak at 2927 cm-1 and 2853 cm-1 may be accounted for to be due to the C-H stretching of -CH3 and -CH2 respectively. The values 1458 cm-1 and 1169 cm-1, could be attributed to the C-H bending frequency of saturated alkane and C-O stretching frequency of ester respectively. The spectrum of the hydroxylated methyl esters has a peak at 3404 which accounts for the presence of OH functional group. The peak at 3006 cm-1 which accounted for the presence of unsaturation in the methyl esters totally disappeared after hydroxylation suggesting the introduction of the OH functional into the methyl esters at the unsaturated carbon atoms (Figure 3).
Figure 2.
FTIR of the methyl esters and hydroxylated unsaturated methyl esters.
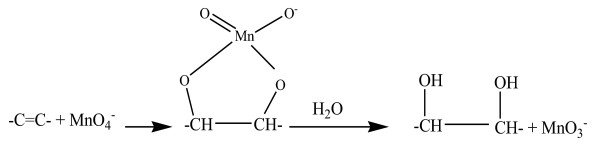
Figure 3.
Reaction scheme for the production of methyl 9, 10-dihydroxyoctadecanoate.
Isolation and GC-MS analysis of methyl 9, 10-dihydroxyoctadecanoate
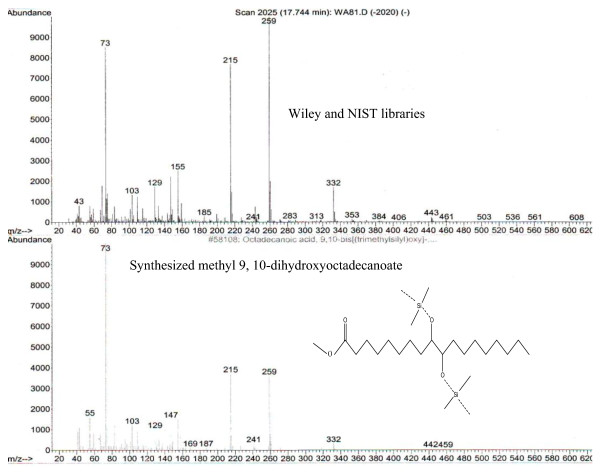
Pure methyl 9, 10-dihydroxyoctadecanoate was isolated on a 1 g scale with a yield of 85.10 ± 0.70%. The result of the GC-MS is presented in Figure 4. The fragmentation pattern of the synthesized methyl 9, 10-dihydroxyoctadecanoate compared favorably with those of Wiley and NIST library.
Figure 4.
GC-MS analysis of methyl 9, 10-dihydroxyoctadecanoate.
Conclusion
Oil was extracted from the seed of Blighia unijugata using hexane in a soxhlet extractor. The iodine value was 67.60 ± 0.80 g iodine/100 g while the saponification value was 239.20 ± 1.00 mg KOH/g. The dominant fatty acid was found to be C18:1. The unsaturation of the methyl esters of Blighia unijugata was increased using the urea adduct complexation reaction. Methyl 9, 10-dihydroxyoctadecanoate was synthesized from the methyl esters via hydroxylation using cetyltrimethylammonium permanganate. The reaction was monitored and confirmed using FTIR and GC-MS. This study has revealed that oxidation reaction using CTAP do not require solvent medium and can be achieved under complete solvent free condition.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AA: Contributed and participated in the design of the study. He carried out the synthesis of the methyl 9, 10-dihydroxyoctadecanoate and drafted the manuscript.
RO: Contributed in the design of the study.
BR: Contributed and participated in the design of the study and co-supervised it.
RP: Contributed in the design of the study and coordination. He also provided a laboratory space for the research work.
MN: Participated in the GC and GC-MS analysis.
All authors have read and approved the final manuscript.
Supplementary Material
Characterization and fatty acid composition of Blighia unijugata seed oil. Additional file 1 describes the characterization and fatty acid composition of the seed oil of Blighia unijugata.
Contributor Information
Adewale Adewuyi, Email: walexy62@yahoo.com.
Rotimi A Oderinde, Email: ra_oderinde@yahoo.co.uk.
BVSK Rao, Email: raobvsk@gmail.com.
RBN Prasad, Email: Rbnprasad@iict.res.in.
M Nalla, Email: madhunalla@iict.res.in.
References
- Schwab AW, Bagby MO, Freeman B. Preparation and properties of diesel fuels from vegetable oils. Fuel. 1987;66:1372–1378. doi: 10.1016/0016-2361(87)90184-0. [DOI] [Google Scholar]
- Gervasio GC Hui YH. Fatty Acids and Derivatives from Coconut Oil Bailey's Industrial Oil & Fat Products 199655John Wiley & Sons, NY; 33–83.22147648 [Google Scholar]
- Khot SN, Lascala JJ, Can E, Morye SS, Williams GI, Palmese GR, Kusefoglu SH, Wool RP. Development and application of triglyceride-based polymers and composites. J. Appl Polym Sci. 2001;82:703–723. doi: 10.1002/app.1897. [DOI] [Google Scholar]
- Burkill HM. The useful plants of West Tropical Africa. 2. Vol. 5. Royal botanical garden, Kew, Richmond; 2000. pp. 11–13. [Google Scholar]
- Hu YH, Gao Y, Wang DN, Hu CP, Zhu S, Vanoverloop L, Randall D. Rigid polyurethane foam prepared from a rape seed oil based polyol. J Appl Polym Sci. 2002;84:591–597. doi: 10.1002/app.10311. [DOI] [Google Scholar]
- Strocchi A, Bonaga G. Correlation between urea inclution compounds and conformational structure of unsaturated C18 fatty acid methyl esters. Chem Phys Lipids. 1975;15:87–94. doi: 10.1016/0009-3084(75)90033-X. [DOI] [Google Scholar]
- Lucy SH, Jer HL. Fractionation of urea-pretreated squid visceral oil ethyl esters. J Am Oil Chem Soc. 2001;78:473–476. doi: 10.1007/s11746-001-0288-x. [DOI] [Google Scholar]
- Tor C, Yi H. Polyunsaturated fatty acid concentrates from borage and linseed oil fatty acid. J Am Oil Chem Soc. 2001;78:485–488. doi: 10.1007/s11746-001-0290-3. [DOI] [Google Scholar]
- Gamez MN, Noriega RJA, Medina JLA, Ortega GJ, Monroy RJ, Toro VFJ, Garcia HS, Angulo O. Concentration of EPA and DHA from fish oil by hydrolysis and urea complexation. Food Res Int. 2003;36:721–727. doi: 10.1016/S0963-9969(03)00052-8. [DOI] [Google Scholar]
- Udaya N, Wanasundara X, Shahidi F. Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation: Optimization of reaction conditions. Food Chem. 1999;65:41–49. doi: 10.1016/S0308-8146(98)00153-8. [DOI] [Google Scholar]
- Kidwai M, Poonam M. Neat reaction technology: A green tool. Ind J Chem. 2005;45:2330–2336. [Google Scholar]
- Welton T. Ionic liquids in Green Chemistry. Green Chem. 2011;13:225–225. doi: 10.1039/c0gc90047h. [DOI] [Google Scholar]
- Adewuyi A, Oderinde RA, Ajayi IA. The Metal Composition, Proximate Properties and the Effect of Refining on the Physico-Chemical Characterization of Baphia nitida and Gliricidia sepium Seed and Seed Oil. J Food Technol. 2009;7:43–49. [Google Scholar]
- AOAC. Official Methods of Analysis. 14. Vol. 67. Association of Official Analytical Chemists, Arlington, VA; 1994. [Google Scholar]
- Christie WW. Lipid analysis. 2. Pergamoon press, Oxford; 1982. pp. 107–130. [Google Scholar]
- Oderinde RA, Ajayi IA, Adewuyi A. Evaluation of the mineral nutrients, characterization and some possible uses of Blighia unijugata bak seed and oil. Seed Science and Biotechnology. 2008;2:79–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization and fatty acid composition of Blighia unijugata seed oil. Additional file 1 describes the characterization and fatty acid composition of the seed oil of Blighia unijugata.