Abstract
Background
Since the discovery of the human CFTR gene in 1989 various mouse models for cystic fibrosis (CF) have been generated and used as a very suitable and popular tool to approach research on this life-threatening disease. Age related changes regarding the course of disease and susceptibility towards pulmonary infections have been discussed in numerous studies.
Methods
Here, we investigated CftrTgH(neoim)Hgu and Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J CF mice and their non-CF littermates during an acute lung infection with Pseudomonas aeruginosa for age dependent effects of their lung function and immune response.
Mice younger than three or older than six months were intratracheally infected with P. aeruginosa TBCF10839. The infection was monitored by lung function of the animals using non-invasive head-out spirometry and the time course of physiological parameters over 192 hours. Quantitative bacteriology and lung histopathology of a subgroup of animals were used as endpoint parameters.
Results
Age-dependent changes in lung function and characteristic features for CF like a shallower, faster breathing pattern were observed in both CF mouse models in uninfected state. In contrast infected CF mice did not significantly differ from their non-CF littermates in susceptibility and severity of lung infection in both mouse models and age groups. The transgenic Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J and their non-CF littermates showed a milder course of infection than the CftrTgH(neoim)Hgu CF and their congenic C57Bl/6J non-CF mice suggesting that the genetic background was more important for outcome than Cftr dysfunction.
Conclusions
Previous investigations of the same mouse lines have shown a higher airway susceptibility of older CF mice to intranasally applied P. aeruginosa. The different outcome of intranasal and intratracheal instillation of bacteria implies that infected CF epithelium is impaired during the initial colonization of upper airways, but not in the subsequent response of host defense.
Keywords: Cystic fibrosis mouse models, intranasal, intratracheal, experimental lung infection, age effect
Background
Cystic fibrosis (CF) is the most common life-shortening autosomal recessive disease within the Caucasian population and is caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene [1]. The CFTR protein functions as a cAMP-regulated chloride channel in the apical membrane of epithelial cells. The symptoms of CF are caused by an impaired function of exocrine glands in many CFTR expressing organs, predominantly within the gastrointestinal and respiratory tracts. In most cases the progressive decrease of lung function is life limiting for CF patients. In this context, the opportunistic bacterial pathogen Pseudomonas aeruginosa most commonly causes chronic microbial lung infections, leading to excessive lung tissue remodelling and destruction [2,3]. The bacteria are able to survive in the anaerobic environment of the CF lung [4] and become extremely resistant to the eradication of biofilms in the conducting airways by antibiotic treatment.
In 1989 the coding gene for the CF disease was identified on the long arm of chromosome 7 [5-7], a finding which highly advanced our understanding of CF cell biology and pathophysiology. In 1991 Tata et al. [8] and Yorifuji et al. [9] described the cloning and sequencing of the murine Cftr gene which is located in a conserved segment of chromosome 6 and shows 78% amino acid sequence homology to the human CFTR gene. Since no spontaneous mutations were known for the murine Cftr, different CF mouse models were generated by targeted mutagenesis [10]. Most of these models show massive pathological changes in the intestine, but fail to develop a lung disease comparable to human CF subjects. The reason therefore may due to the short life expectancy of mice or their ability to use alternative chloride channels in the lung epithelium [11-15]. In 1992 Dorin and her coworkers described the CftrTgH(neoim)Hgu mouse which only showed mild gastrointestinal complications, a good survival after weaning and benign respiratory symptoms [16,17]. Another well described and often used mouse model is represented by the transgenic STOCK Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J mouse, which expresses human CFTR in the gut under control of the FABP1 promoter (fatty acid binding protein1), which prevents it from acute intestinal obstruction [18].
Both CF models were included in a study by Teichgräber et al. [19] in which they detected ceramide accumulation in the murine respiratory epithelium and hypothesized that this accumulation leads to inflammation and cell death and increases infection susceptibility towards P. aeruginosa in CF patients. A significant increase of ceramide in the lung epithelium of both CF models was found to be associated with higher bacterial numbers, an accumulation of neutrophils and alveolar macrophages and increased cell death. All effects became more prominent with increasing age and started to become visible by around week 16 of murine life. These findings are also consistent with previously published data from P. Durie and associates, who identified many pathological changes in aged CF mice [20].
In this study described here we tested the influence of the described age-dependent ceramide accumulation [21] in our well established mouse model on airway infection with P. aeruginosa [22] using young and old mice and identical mouse strains but a different, namely intratracheal infection route. In Teichgräber's study [19] the bacteria were inoculated by intranasal instillation thus targeting both the upper and lower airways. In contrast intratracheal instillation [23] bypasses the upper airways and delivers more bacteria into distal bronchi than the intranasal inoculation. Thus the two infection routes target overlapping, but not matching airway compartments. We monitored the course of infection over 192 hours via several physiological parameters supported by non invasive head-out spirometry and employed quantitative bacteriology and lung histopathology as endpoint parameters. To make a clear distinction we categorized mice in groups younger than three and older than six months. Moreover, we compared the lung function of the CF mice in the uninfected state. The differential outcome of the infection experiments in Teichgräber's and our study led to the conclusion that older CF mice are impaired in their first defence of bacterial clearance, but that otherwise the clinical course of the acute P. aeruginosa lung infection is indistinguishable between CF and non-CF mice that share the same genetic background.
Methods
Mouse strains
Infection experiments were performed with two CF mouse models (a) B6.129P2(CF/3)-CftrTgH(neoim)Hgu, (b) Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J and their respective littermates. According to the nomenclature of Teichgräber et al. the mouse lines are called (a) CftrMHH and (b) CftrKO, their non-CF littermates (a) B6 and (b) WT, respectively. In CftrTgH(neoim)Hgu mice the exon 10 of the Cftr gene had been disrupted by the insertion of the vector pMCIneoPolyA [16]. Since those mice produced low levels of Cftr [17] but showed a mixed genetic background [24], from the original CftrTgH(neoim)Hgu mutant mouse, CF strain CF/3-CftrTgH(neoim)Hgu was established at the Institute of Laboratory Animal Science of the Hannover Medical School by brother-sister mating for more than 40 generations. Next, the congenic mouse inbred strain B6.129P2(CF/3)- CftrTgH(neoim)Hgu, which is used in this study, was generated by 40 backcross generations using CF/3-CftrTgH(neoim)Hgu as donor strain and C57BL/6J as recipient strain [25]. Following the nomenclature of Teichgräber et al. [19] this strain is called CftrMHH, syngenic C57BL6/J mice are called B6 and served as controls. CftrMHH mice are regulary monitored for their congenic C57BL/6J status using 27 SNP markers and integrity of the mutant Cftr locus by intragenic microsatellite markers [24,26,27].
STOCK Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J mice were obtained from the Jackson Laboratories. These mice, in the following called CftrKO, which are of a mixed genetic background consisting of C57BL/6, FVB/N and 129, are knock-outs for the murine Cftr gene, but express human CFTR in the gut under control of the FABP1 (fatty acid binding protein1) promoter, which prevents acute intestinal obstruction 1 [12,18]. Mice were obtained homozygous and heterozygous for the Cftrtm1Unc targeted mutation (tm/tm and tm/+) as well as homozygous and hemizygous for the FABP-hCFTR transgene (tg/tg and tg/0). Tm/+ tg/0 mice were used as parents to generate wildtype control mice (called WT). Genotyping was performed using the protocols provided by the JAX lab [28].
Mice were maintained at the Central Animal Facility of the Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany. They were held in groups of three to five animals animals in microisolator cages (910 cm2) with filter top lids and free access to sterilised standard laboratory chow (diet No. 1324, Altromin, Lippe, Germany) and autoclaved, acidulated water at 21 ± 2°C, 55 ± 5% humidity and a 10:14 light-dark-cycle. None of the CF mice showed gastrointestinal complications which would require a special diet. All mice were regularly monitored for infection by typical pathogens according to the FELASA recommendations [29]. All procedures performed on mice were approved by the local district governments (AZ. 33.9-42502-04-08/1528) and carried out according to the ILAR guidelines for the care and use of laboratory animals [30]. Experimental groups of mice were allocated by age of either younger than three (henceforth designated as young) or elder than six months (henceforth designated as old). This selection aimed to mimic the categories of Teichgräber et al. [19] that mice older than 4 months were susceptible to an infection with P. aeruginosa whereas the younger mice were more or less resistant.
Due to breeding limitations B6 mice showed a strong predominance of males and no 192 h value of old B6 exist.
Spirometry
Non-invasive head-out spirometry with 14 parameters was performed on conscious restrained mice [22]. In brief, four mice were investigated in parallel by placing them in glass inserts with their heads protruding out through a set of membranes ensuring an airtight fit. Respiration caused air to flow through a pneumotachograph positioned above the thorax of the animals. The airflow was digitalized and analyzed with the Notocord Hem 4.2.0.241 software package (Notocord Systems SAS, Croissy Sur Seine, France).
Bacteria
Pseudomonas aeruginosa strain TBCF10839 [31] was grown in Luria broth (LB) overnight at 37°C. The overnight culture was washed twice with the same volume of sterile PBS to remove cell detritus and secreted exopolysaccharides, then the optical density of the bacterial suspension was determined and the intended number of colony forming units (CFU) was extrapolated from a standard growth curve. Inocula with 6.0 × 105 CFU in 30 μl were prepared by dilution with sterile PBS. This infection dose is approximately one tenth of the LD50 of strain TBCF10839 for C57BL/6J mice and was able to produce a clinical infection without mortality.
Infection protocol
Mice were infected intratracheally (i.t.) with 6.0 × 105 CFU of P. aeruginosa strain TBCF10839 under a light anaesthesia. For detailed description of the view-controlled intratracheal instillation see Munder et al. [23]. To characterize the course of the bacterial infection, the body condition, weight, rectal temperature and lung function of the mice were evaluated as described previously [32]. In brief the overall health of the animals was assessed by vocalisation, piloerection, posture, locomotion, breathing, curiosity, nasal secretion, grooming and dehydration. Dysfunctions in single parameters were assessed by zero, one or two points. The overall fitness of the mice was determined by adding the points resulting in the following score of the mouse body condition: unaffected (0-1); slightly affected (2-4); moderately affected (5-7); severely affected (8-10); moribund (≥ 11).
Non-invasive head-out spirometry. First, spirometric values of uninfected animals (B6, WT, CftrKO and CftrMHH with age young: < 99 days and old: > 179 days) were averaged (median) from three independent measurements preformed on consecutive days. Prior measurements assured that the mice had adapted to the procedure. Lung function measurements of infection experiments were taken daily at the five days prior to inoculation and at time points 4, 6, 8, 10, 12, 18, 24, 48, 72, 96, 120, 144, 168, 192 hours post inoculation.
Forty-eight hours after challenge subgroups of mice were euthanized. Their left lungs were taken for the determination of bacterial counts and the right lungs were stained and examined histologically.
Pathohistology of the lungs
The right lungs were fixed via the trachea (4% paraformaldehyd), embedded in paraffin and stained with haematoxilin/eosin. One section was selected that showed aspects from all three lobes of the right lung. This slide was examined in twenty fields of view at a 100 fold magnification using a Zeiss Axiophot photomicroscope. Inflammation was assessed using a semi quantitative pathohistological score [22]. Shortly, lung histological changes were scored on a scale from one to three points. Points were given separately for lung parenchyma, airways and lung vessels. The total score classified airway inflammation into the categories: almost not visible (0-5); slight (6-20) moderate (21-40); severe/profound inflammation (41-60). In the current study no more than a medium-grade inflammation was seen, appearing as a purulent alveolar pneumonia with peribronchiolar and perivascular inflammatory infiltrates.
Lung bacterial numbers
The left lungs of the euthanized mice were ligated, resected and homogenised. Aliquots were plated and bacterial numbers of whole organs were calculated. Previous experiments showed that the distribution of bacteria is approximately equal in left and right lungs after i.t. application (data not shown).
Statistics
Each CF mouse model and its wild type controls were investigated separately by age group using non-parametric test statistics of SPSS 16 (Version 16.0.2, SPSS Inc, Chicago, USA). p-values (p < 0.05) with subsequent Bonferroni correction were calculated by 2-sided Monte Carlo simulations (100,000 simulations). Hereby groups were composed of equal numbers of mice (perfect match approach).
Results
Lung function of CF mice
To evaluate the impact of age, genetic background and the Cftr mutant construct on respiratory health, we determined lung function in young and old CF mice and their congenic wild-type littermates (Figure 1).
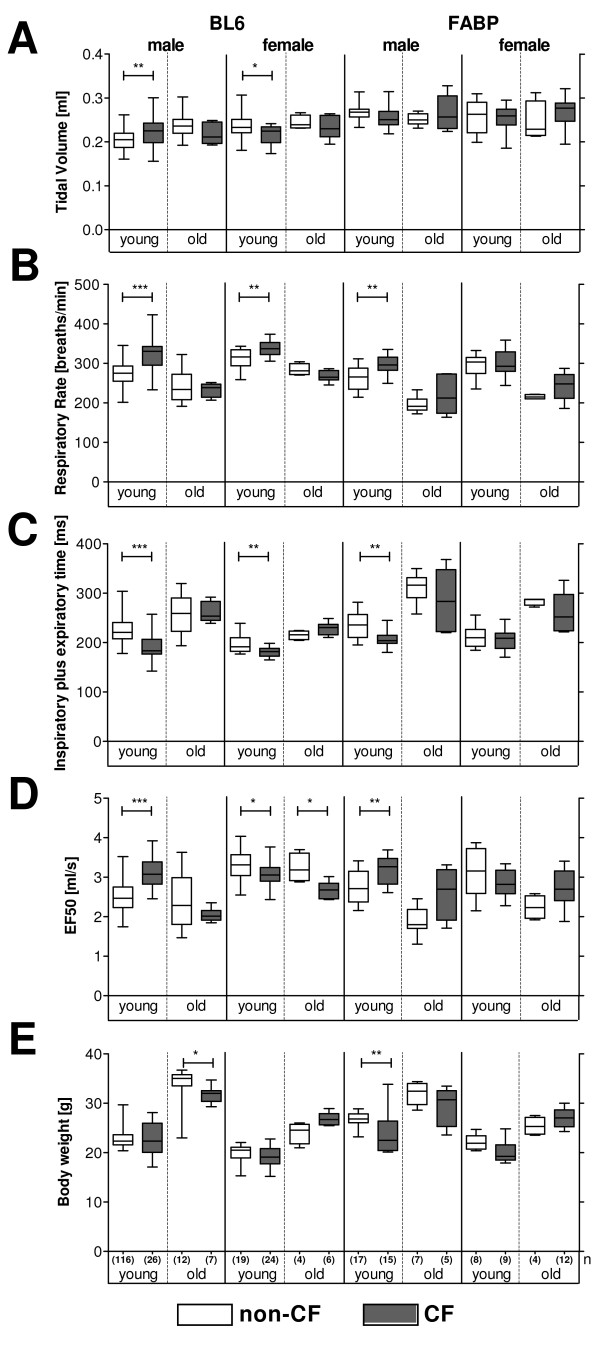
Figure 1.
Respiratory parameters and body weight in non-infected CF mice and their non-CF littermates (left panels: B6, CftrMHH; right panels: WT, CftrKO). At the day of assessment young mice were younger than 100 days and old mice elder than 179 days. The boxplots show the median, inner quartiles and range of the parameters and are clear for wildtype mice (B6, WT) and are shaded in grey for CF mice (CftrMHH, CftrKO). CF mice and non-CF littermates were compared in their lung function and body weight by Mann-Whitney rank tests (* = p < 0, 05; ** = p < 0, 01; *** = p < 0, 001). The number of investigated animals per group (n) is indicated in Figure 1E. Figure 1A shows that the tidal volume is very similar for all genotypes. The respiratory rate is higher (Figure 1B) and the time for one breath is shorter in CF mice (Figure 1C).
Tidal volume increased with age of the animals reflecting an increase in body mass. Respiration decreased, also characterized by increasing times for one breath. The slower breathing was also characterized by a decrease in the flows of expiration and inspiration.
Body weight measurements showed that CftrKO and WT mice were slightly larger and heavier than CftrMHH and B6 mice. This is mirrored in a slightly higher tidal volume for the CftrKO and WT mice.
All mice irrespective of genotype or gender had a comparable total lung volume (tidal volume, Figure 1A). CF mice, however, achieved the comparable lung volume through an increase in respiratory rate (Figure 1B). Correspondingly the time for one breath (Time of inspiration plus expiration, Figure 1C) was smaller in CF mice. The higher respiratory rate was associated with higher flows as depicted by the EF50 parameter (Midtidal expiratory flow at 50% expiration, Figure 1D).
Outline of infection experiments
To monitor the outcome of an intratracheal instillation of P. aeruginosa in our CF mouse models and their non-CF littermates, mice of both genders were exactly matched with their corresponding wild type controls by sex, age and body weight. Mice were characterized in their global health score (Figure 2), rectal temperature (Figure 3), body weight (Figure 4) and lung function (Figure 5, Additional file 1) at 14 time points over a period of 192 h.
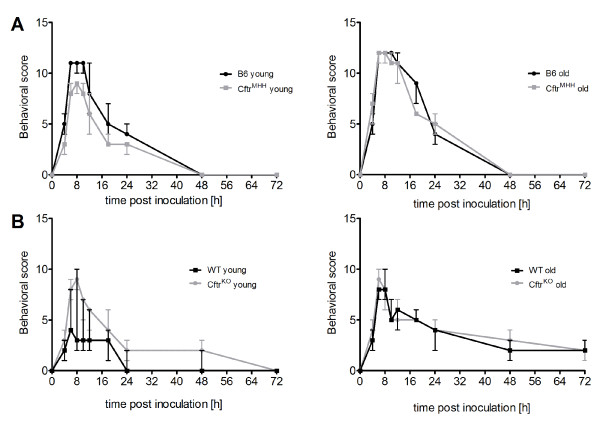
Figure 2.
Time course of the global health score during infection. The behavioral score describes the fitness of mice during infection. Mice with a C57Bl/6J background (A) were more affected than FABP mice (B). The responses of congenic non-CF and CF mice were indistinguishable (black vs. gray lines in all panels) with the single exception that young FABP WT mice were only mildly affected even during the first 24 h after challenge with P. aeruginosa (B, left panel).
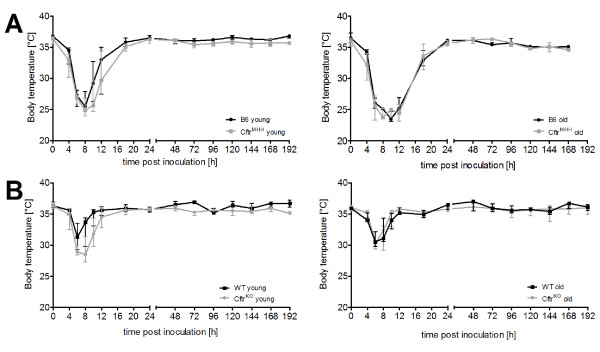
Figure 3.
Time course of rectal body temperature during infection. Body temperature of all mice showed a strong decrease, which peaked between 6 and 10 hours post inoculation. Temperature declined in C57Bl/6J mouse strains to a minimum of approximately 25°C (A), in FABP mice the minimal temperature was always higher than 30°C (B). Exceptions were the young CftrKO mice with a minimal temperature of 28.5°C at 8 h p.i. In old B6 and CftrMHH mice (A, right panel) temperature remained longer at lower values (until 12 h p.i.), although the recovery to physiological level took the same period of time as in the groups of young CftrMHH and B6 mice (A, left panel. In summary, the C57BL/6J mouse strains showed a stronger depression of body temperature upon airway exposure with P. aeruginosa than FABP mice.
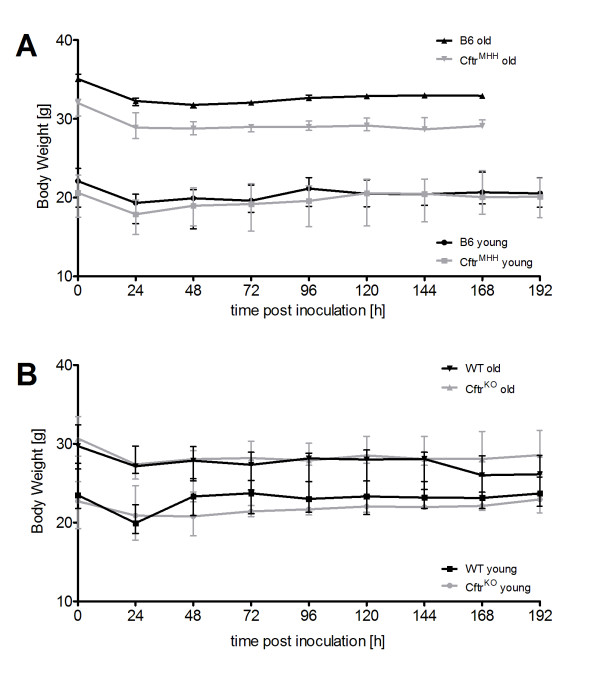
Figure 4.
Time course of body weight during infection. After challenge with P. aeruginosa all animal groups lost weight during the first 24 hours p.i. Young B6 and CftrMHH mice started to regain weight directly thereafter, although this increase was small and both groups had not reached their initial weight by the end of the experiments (A). Weight loss was more pronounced in the old B6 and CftrMHH mice that also did not regain their initial weight by 192 hours p.i. In both CF mouse models CF mice younger than 3 months displayed a reduced body weight compared to their wild type littermates. This tendency became more pronounced in adult C57BL6/J, but not FABP mice thus showing an anthropometric CF phenotype in the former, but not in the latter strain. Please note the different initial values of young and old mice and the stronger impact of gender in the old mice, reflected in the large error bars of old CftrMHH, old CftrKO and WT mice (A, B). Since the group of old CftrMHH mice was exclusively formed by male animals, less pronounced intragroup differences were noted (A, grey line with triangles).
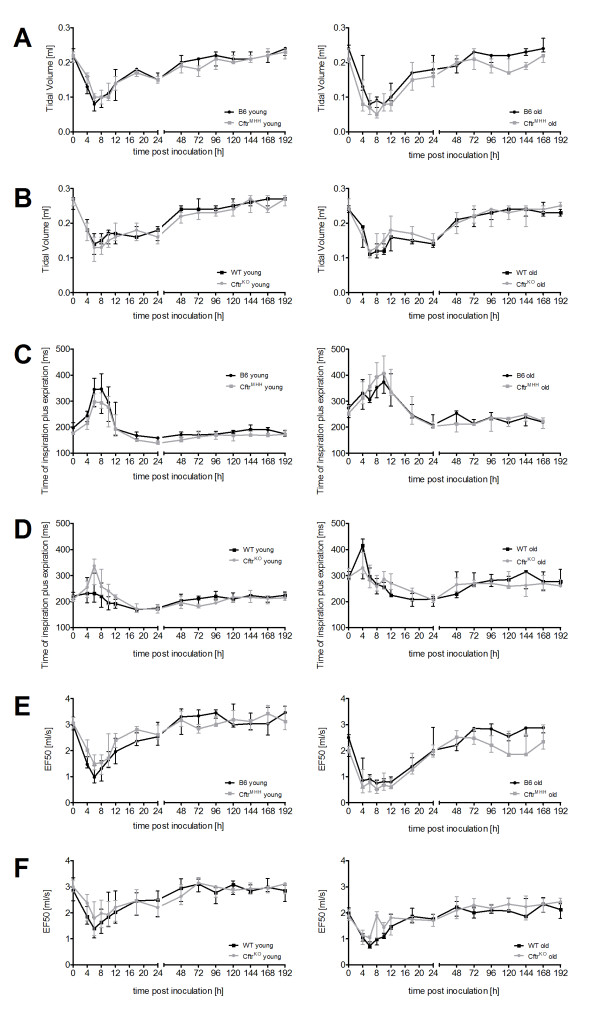
Figure 5.
Time course of selected lung function parameters during infection. Spirometric curves depicting the time course of Tidal Volume (Total volume inspired and expired in one breath) (A, B), Inspiratory plus Expiratory time (Time required for one breath) (C, D) and Expiratory flow at 50% Expiration (EF50) (E, F) of CftrMHH mice and CftrKO and wild type mice after the intratracheal instillation of P. aeruginosa. There are no marked differences for none of the parameters between CF and wild type mice of either genotype. C57BL6/J mice responded stronger to the bacterial infection than FABP mice as indicated by the larger decrease in lung volume (A). As young mice breathe faster than old mice, the overall time for one breath increases with age (C, D).
Global body condition
B6 mice were more affected by P. aeruginosa in their body condition than the CftrKO and their non-CF littermates (Figure 2). With the exception of young CftrKO mice the corresponding CF and non-CF mouse lines exhibited a similar time course of disease symptoms. Animals were notably affected between 6-12 h after inoculation and recovered within the next 48 h. Young WT mice were least affected by the instillation of P. aeruginosa into their lungs.
Rectal body temperature
Upon exposure to bacteria mice did not react with fever but with a drop of body temperature. The temperature profile mimicked the time course of the global health score. CftrMHH and the B6 mice experienced a stronger drop of temperature than the CftrKO and WT mice, the latter being minimally affected with a maximal reduction of rectal temperature of 5°C (Figure 3).
Body Weight
Within the first 24 h post inoculation the mice lost 8% (old B6) to 13% (young CftrMHH) of their initial body weight (Figure 4). By the end of the experiment the young mice had almost completely regained their initial weight, whereas an irreversible weight loss was observed in all old mice. Consistent with an intestinal CF phenotype, old CftrMHH mice were significantly lighter than their congenic B6 mice [24].
Lung function
The time course during infection is shown exemplarily for tidal volume (the total volume inspired and expired in one breath) (Figure 5A, B), Time of inspiration plus expiration (Figure 5C, D) and EF50 (Figure 5E, F). The data of all 14 measured lung function parameters are shown in the online supplement (Additional file 1). The old non-CF and CF mice showed a similar response in lung function towards the instillation of P. aeruginosa into their lungs. Young KO mice differed from their WT FABP littermates in a shorter 'time of pause' prior to and after inoculation, but not in any other of the 14 parameters. In contrast, non-infected young CftrMHH mice showed another breathing pattern than their congenic B6 mice (see above). CftrMHH mice differed from their non-CF congenics in seven lung function parameters with a high respiratory rate as the leading symptom. This characteristic pattern of non-infected CftrMHH mice was also seen in the challenged mice at the late time points of 144, 168 and 192 h when they had recovered from infection. Thus, by the end of experiment the CftrMHH mice had regained the initial lung function phenotype. Besides these CF-genotype-driven differences between congenic mice that were apparently independent of bacterial infection, a differential response of CftrMHH and B6 mice was noted at time points 4 and 6 hours after challenge with P. aeruginosa. Lung function slightly, but significantly differed in seven flow or volume parameters (Additional file 1). In summary, differences in lung function between congenic non-CF and CF mice were not detectable or subtle, and if they were more prominent as in the case of CftrMHH mice, they were also existent in non-infected animals.
Endpoint analyses
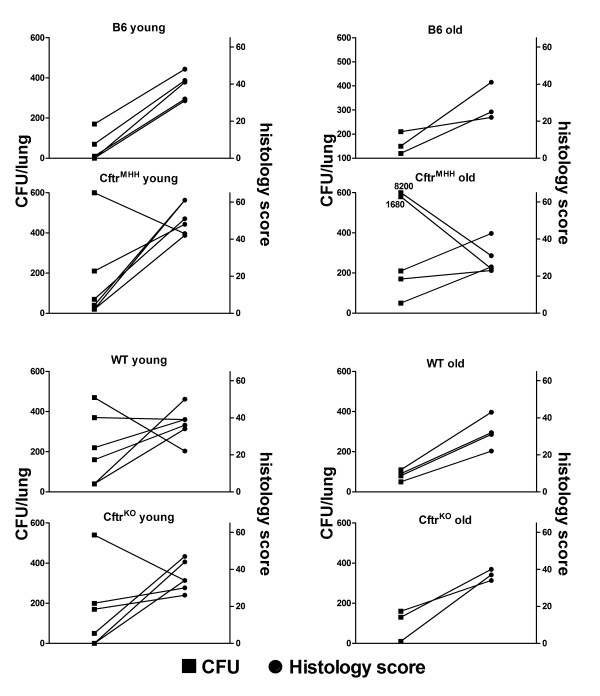
Forty-eight hours after challenge the numbers of viable P. aeruginosa TBCF10839 in the murine lungs ranged from zero to a maximum of 8.2 × 103 CFU (Figure 6). The mice had cleared 99% or more of the initial inoculum. No differences could be observed between young and old and between CF and non-CF mice.
Figure 6.
Bacterial load and inflammation. Differentiated by age and genotype, the graphs depict the histology inflammation score of the right lung (circles) and the number of viable bacteria recovered from the left lung (squares) at 48 hours after intratracheal instillation of P. aeruginosa TBCF10839.
Examination of lung histology (Figure 7) revealed most signs of inflammation in the lung parenchyma such as alveolar histiocytosis, PMNs in the alveoli and partially even necrosis of the alveolar septae. In the most strongly affected animals approximately 20-30% of the tissue showed signs of an acute catarrhalic-suppurating alveolar pneumonia. Inflammatory cell infiltrates of bronchi and perivascular edema were seen in less than 10% of examined sections.
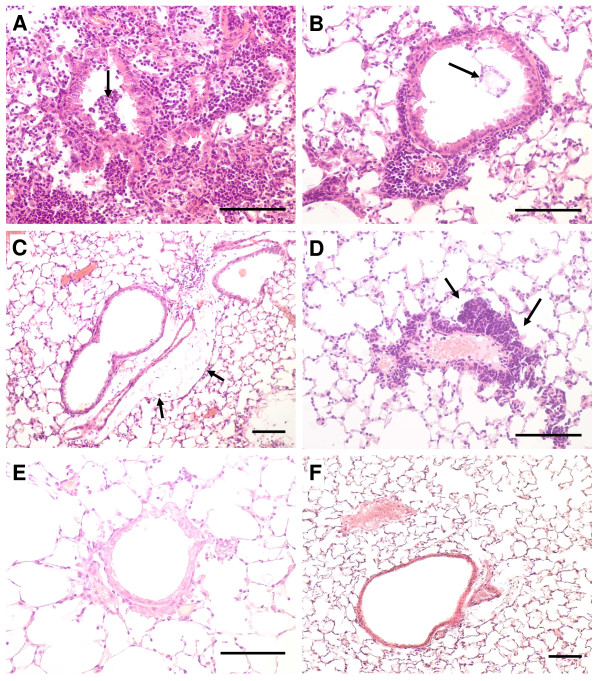
Figure 7.
Lung pathohistology photomicrographs. Representative examples of the stained specimen slides show the inflammation in the murine lungs 48 h after an intratracheal infection with 6.0 × 105 CFU of P. aeruginosa. Slides A-E represent different degrees of inflammation as they were assessed in the semi-quantitative pathohistological score; control (F). Hematoxilin-eosin staining; scale bar: 100 μm. A) Profound purulent pneumonia in an old FABP WT mouse, massive inflammatory infiltrates within the alveolae and intrabronchiolar (arrow), B) Old FABP WT mouse showing moderate inflammation with peri- and minor intrabronchiolar (arrowhead) leucocyte accumulation, C) Peribronchiolar and perivascular inflammation accompanied by inflammatory edema (arrow) in a young CftrMHH mouse D) Old CftrKO mouse: Strong leucocyte accumulation (arrow) around a lung vessel, E) Only faint peribronchiolar inflammation in an old CftrMHH F) Control: normal lung parenchyma.
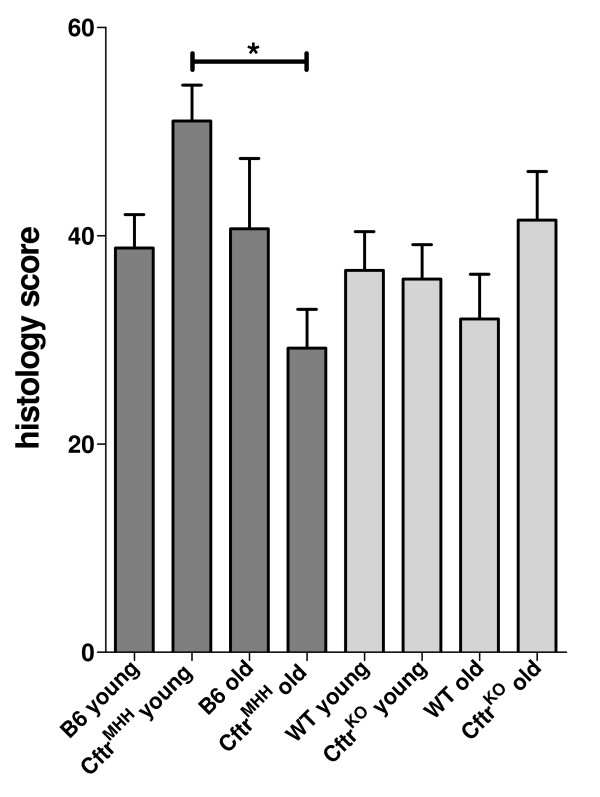
Among the different animal groups the young CftrMHH mice exhibited a significantly higher inflammation than the old CftrMHH mice in the lung parenchyma, but not in other lung compartments. Correspondingly young CftrMHH mice had the highest mean histology score (Figure 8). At the chosen endpoint of 48 hours the number of viable bacteria in the lungs did not significantly correlate with the severity of airway inflammation classified by the histology score (Figure 6).
Figure 8.
Mean histology scores of lung inflammation 48 hours after intratracheal instillation of P. aeruginosa. The bars show the mean and the standard error of the mean. *, P < 0.05; Kruskall-Wallis rank test and Dunn's multiple comparison test of the individual scores.
Discussion
This study shows for the first time lung function data of CF mice and demonstrates the impact of age, cftr genotype, genetic background and an acute airway infection with P. aeruginosa on lung function. Lung function measurements in uninfected CF and non-CF mice showed general trends for the investigated age groups. Tidal volume increased slightly with increased body weight. Respiratory rate decreased as mice breathe slower, which is also observed in the time required for one breath. In general the flows also decreased concordant with the slower respiration. CF and wild type mice had approximately the same tidal volume. However, when further lung function parameters were taken into account, it could be observed that the tidal volume levels of the CF mice were only achieved through faster breathing as characterized by the times for breathing and the respiratory rate. Interestingly these variations between CF and non-CF mice of the same genetic background did not withstand under P. aeruginosa airway infection.
Chronic airway infections with P. aeruginosa contribute substantially to morbidity and mortality in individuals with CF [33,34]. Numerous infection models have been established in rodents to mimick the P. aeruginosa infection in CF, but only the rather artificial bead models with encapsulated P. aeruginosa partially succeeded to mimick bacterial persistence in lungs that is typical for human CF airways [35-37]. No chronic P. aeruginosa infection model has yet been established in CF mice [10,14,15]. This fact may be ascribed to differences in lung morphology or lung physiology such as the lack of submucosal glands in the lower conducting airways of the mouse or to the endogeneous expression of alternative chloride channels in murine lungs that may at least in part rescue loss-of-function Cftr [11,38]. Although the former argument is probably true and hence calls for alternative infection models in the recently developed CF pigs and CF ferrets [39-45], the latter argument is less convincing because we meanwhile know of many CF patients who express residual amounts of CFTR or alternative ion conductances and still become chronically colonized with P. aeruginosa in their airways [46,47]. Hence we propose that we should revisit the CF mouse infection models and try to pinpoint concordant and discordant mechanisms that are operating in CF mice and CF patients.
Only recently a link between Cftr genotype and the airway infection with P. aeruginosa in mice could be established. CF mice elder than 16 weeks became susceptible to airway colonization with P. aeruginosa when infected by the intranasal route [19]. This phenotype was associated with an age-dependent accumulation of ceramide in airway epithelial cells. When ceramide accumulation was prevented by pharmacological or genetic means, the CF mice lost their increased susceptibility to colonization with P. aeruginosa.
For the present study we selected exactly the same CF mouse lines and their congenic or transgenic controls, but inoculated P. aeruginosa by intratracheal instillation [23]. The congenic B6 mice showed more pronounced symptoms of acute infection than the transgenic mice, but non-CF and CF animals with the same genetic background behaved more or less similar. The old CF mice were not more susceptible to P. aeruginosa infection than their age-matched wild type controls.
Since the present and the previous studies primarily differ in their mode of bacterial infection (Figure 9), we hypothesize that the divergent infection routes explain the inconsistent outcome of the two studies of how and to which extent P. aeruginosa colonizes the airways of old CF mice. The intranasal route deposits the bacteria in the upper airways from which they spread to the intestine and the lower airways. In contrast the intratracheal route inoculates the lower airways, preferentially the smaller conducting airways [23]. The intratracheal instillation thus bypasses the initial steps of any lung infection, i.e. the colonization of the upper airways and the largest lower airways with the (opportunistic) pathogen (Figure 9).
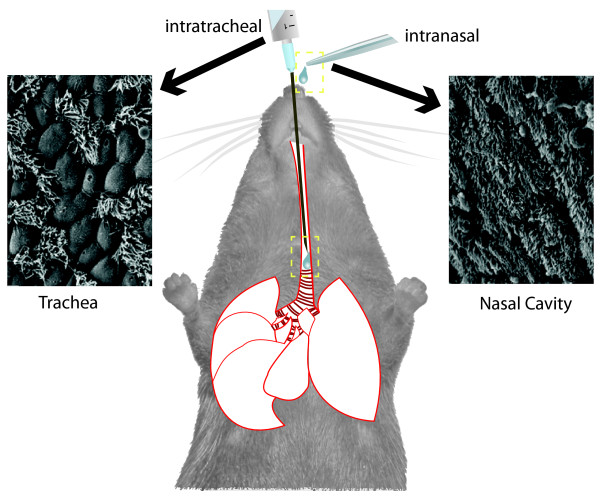
Figure 9.
Experimental lung infection - Impact of infection route. The sketch highlights the differential experimental set-up for intranasal and intratracheal infection in mice. Compared to an intranasal infection route the intratracheal instillation bypasses the initial host immune response conducted by the respiratory epithelium of the upper airways and deposits more bacteria into the distal airways. The basic defect in CF impairs the mucociliary clearance [48]. Ciliated respiratory epithelium is abundant in the upper and conducting airways, decreasing with the increasing branching of the airways. Correspondingly mucociliary clearance plays a prominent role for the elimination of the pathogen from these compartments, but not from the alveolar space. Please note the differential abundance of respiratory epithelium in the upper airways (right photograph) and the lower conducting airways of mice (left photograph).
We have previously investigated the CF phenotype of trachea and upper airways of CftrMHH mice [21,25]. Like in humans, the nasal epithelium of the CF mice exhibited the basic defect of Na+ hyperabsorption and Cl- hyposecretion [25] and the trachea had accumulated ceramide [21]. The basic defect of perturbed electrolyte transport across the apical epithelial membrane translates into airway surface dehydration and impaired mucociliary clearance [48], and we have indeed measured impaired clearance in CftrMHH mice [49]. Correspondingly, if CF mice were exposed intranasally with P. aeruginosa the bacterial clearance did not work efficiently in the upper airways of CF mice and the bacterial load increased within the first hours [19]. In contrast, if the murine lung was inoculated with P. aeruginosa by intratracheal instillation, the bacterial clearance from the upper airways and the large conducting lower airways was bypassed and the host response to the intratracheal infection route was indistinguishable between congenic CF and non-CF mice. Thus we would like to conclude that the CF condition undermines the first barrier of host defense, i.e. bacterial clearance, but does not compromise the subsequent host responses. This conclusion fits with our current knowledge of how the basic defect in CF patients predisposes to infection in conducting airways [40,48,50,51]: CFTR-deficiency impairs ciliary clearance and slows down mucus transport thus facilitating bacterial colonization, particularly if the airways are injured by an acute viral infection [52].
Conclusions
Hence, the bottom-line of our previous and present studies is that CF mice are suited to investigate of how the basic defect translates into an increased susceptibility to airway colonization with P. aeruginosa. The first line of host defense, i.e. the removal of bacteria from the airways by mucociliary clearance is deficient in CF mice. However, the subsequent steps of host-pathogen interaction during an acute infection with P. aeruginosa are not compromised in CF mice. In other words, CF mice are appropriate models to study the very early host defense mechanisms.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AM designed and carried out the infection experiments and drafted the manuscript. FW participated in the infection experiments, did the spirometry measurements and performed the statistical analysis. TKM participated in the infection experiments. DW supervised the breeding of the utilized mouse models and did the genotyping of the CftrKO mice and their respective littermates. UB was responsible for the make-up of the spirometry unit. EG assisted in the concept of the study. BT conceived the study, participated in its design and coordination and in the writing of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Longitudinal values for spirometric parameters. The comprehensive table shows the courses of each single spirometric parameter. Significant differences are marked in grey.
Contributor Information
Antje Munder, Email: munder.antje@mh-hannover.de.
Florian Wölbeling, Email: flowoelbeling@web.de.
Tanja Kerber-Momot, Email: tanitamo@gmx.de.
Dirk Wedekind, Email: wedekind.dirk@mh-hannover.de.
Ulrich Baumann, Email: baumann.ulrich@mh-hannover.de.
Erich Gulbins, Email: erich.gulbins@uni-due.de.
Burkhard Tümmler, Email: tuemmler.burkhard@mh-hannover.de.
Acknowledgements
The authors are indebted to Achim Gruber's laboratory for the preparation and staining of the histological samples. We also thank Damaris Leemhuis and Sylwia Wiehlmann for excellent technical assistance and to Dagmar Stelte for her excellent editing of the sketch.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft to BT (SFB 587, project A9). FW received a predoctoral stipend from the DFG-supported IRTG ‚Pseudomonas: Pathogenicity and Biotechnology' (GRK 653/3 and 653/4). Publication was supported by the 'Open Access Publishing' project of the Deutsche Forschungsgemeinschaft.
References
- Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Tata F, Stanier P, Wicking C, Halford S, Kruyer H, Lench NJ, Scambler PJ, Hansen C, Braman JC, Williamson R. Cloning the mouse homolog of the human cystic fibrosis transmembrane conductance regulator gene. Genomics. 1991;10:301–307. doi: 10.1016/0888-7543(91)90312-3. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Lemna WK, Ballard CF, Rosenbloom CL, Rozmahel R, Plavsic N, Tsui LC, Beaudet AL. Molecular cloning and sequence analysis of the murine cDNA for the cystic fibrosis transmembrane conductance regulator. Genomics. 1991;10:547–550. doi: 10.1016/0888-7543(91)90434-G. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:193–214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Rozmahel R, Wilschanski M, Matin A, Plyte S, Oliver M, Auerbach W, Moore A, Forstner J, Durie P, Nadeau J, Bear C, Tsui LC. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- Haston CK, McKerlie C, Newbigging S, Corey M, Rozmahel R, Tsui LC. Detection of modifier loci influencing the lung phenotype of cystic fibrosis knockout mice. Mamm Genome. 2002;13:605–13. doi: 10.1007/s00335-002-2190-7. [DOI] [PubMed] [Google Scholar]
- Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Radzioch D, O'Brodovich H, Tsui LC, Buchwald M, Tanswell AK. Lung disease in mice with cystic fibrosis. J Clin Invest. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Iraj E, Schwab U, Gilmour MI, Koller BH. A murine model of cystic fibrosis. Am J Respir Crit Care Med. 1995;151:59–64. doi: 10.1164/ajrccm/151.3_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- Dorin JR, Dickinson P, Alton EWFW, Smith SN, Geddes DM, Stevenson BJ, Kimber WL, Fleming S, Clarke AR, Hooper ML, Anderson L, Beddington RSP, Porteous DJ. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992;359:211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- Dorin JR, Stevenson BJ, Fleming S, Alton EW, Dickinson P, Porteous DJ. Long-term survival of the exon 10 insertional cystic fibrosis mutant mouse is a consequence of low level residual wild-type Cftr gene expression. Mamm Genome. 1994;5:465–472. doi: 10.1007/BF00369314. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- Teichgräber V, Ulrich M, Endlich N, Riethmüller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kürthy G, Schmid KW, Weller M, Tümmler B, Lang F, Grassme H, Döring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Tümmler B, Gulbins E, Grassmé H. Accumulation of ceramide in the trachea and intestine of cystic fibrosis mice causes inflammation and cell death. Biochem Biophys Res Commun. 2010;403:368–74. doi: 10.1016/j.bbrc.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Wölbeling F, Munder A, Stanke F, Tümmler B, Baumann U. Head-out spirometry accurately monitors the course of Pseudomonas aeruginosa lung infection in mice. Respiration. 2010;80:340–346. doi: 10.1159/000319442. [DOI] [PubMed] [Google Scholar]
- Munder A, Krusch S, Tschernig T, Dorsch M, Lührmann A, van Griensven M, Tümmler B, Weiss S, Hedrich HJ. Pulmonary microbial infection in mice: Comparison of different application methods and correlation of bacterial numbers and histopathology. Exp Toxicol Pathol. 2002;54:127–133. doi: 10.1078/0940-2993-00240. [DOI] [PubMed] [Google Scholar]
- Charizopoulou N, Jansen S, Dorsch M, Stanke F, Dorin JR, Hedrich HJ, Tümmler B. Instability of the insertional mutation CftrTgH(neoim)Hgu cystic fibrosis mouse model. BMC Genetics. 2004;5:6. doi: 10.1186/1471-2156-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B, Wilke M, Stanke F, Dorsch M, Jansen S, Wedekind D, Charizopoulou N, Bot A, Burmester M, Leonhard-Marek S, de Jonge HR, Hedrich HJ, Breves G, Tümmler B. Very mild disease phenotype of congenic CftrTgH(neoim)Hgu cystic fibrosis mice. BMC Genetics. 2008;9:28. doi: 10.1186/1471-2156-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Cassell MA, Sargent EE, Donnelly CJ, Robinson P, Crew V, Asquith S, Haar RV, Wiles MV. Development of a SNP genotyping panel for genetic monitoring of the laboratory mouse. Genomics. 2004;83:902–11. doi: 10.1016/j.ygeno.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Hedrich HJ, Rapp KG, Zschege C. Genetic constancy, respectively subline drifting of inbred strains of rats and mice. Z Versuchstierkd. 1975;17:263–74. [PubMed] [Google Scholar]
- The JAX® Mice Database. http://jaxmice.jax.org/strain/002364.html
- Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. FELASA (Federation of European Laboratory Animal Science Associations Working Group on Health Monitoring of Rodent and Rabbit Colonies). FELASA, London UK. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim. 2002;36:20–42. doi: 10.1258/0023677021911740. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8. National Academies Press (US), Washington (DC); 2011. [PubMed] [Google Scholar]
- Tümmler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp D, von der Hardt H. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J Clin Microbiol. 1991;29:1265–1267. doi: 10.1128/jcm.29.6.1265-1267.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder A, Zelmer A, Schmiedl A, Dittmar KE, Rohde M, Dorsch M, Otto K, Hedrich HJ, Tümmler B, Weiss S, Tschernig T. Murine pulmonary infection with Listeria monocytogenes: differential susceptibility of BALB/c, C57BL/6 and DBA/2 mice. Microbes Infect. 2005;7:600–611. doi: 10.1016/j.micinf.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Saiman L. Microbiology of early CF lung disease. Paediatr Respir Rev. 2004;5(Suppl A):367–369. doi: 10.1016/s1526-0542(04)90065-6. [DOI] [PubMed] [Google Scholar]
- Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- Cash HA, Woods DE, McCullough B, Johanson WG Jr, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- O'Reilly T. Relevance of animal models for chronic bacterial infections in humans. Am J Respir Crit Care Med. 1995;151:2101–2107. doi: 10.1164/ajrccm.151.6.7767564. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Shand GH, Hansen BL, Hansen GN. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS. 1990;98:203–211. doi: 10.1111/j.1699-0463.1990.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010;120:3137–3148. doi: 10.1172/JCI42992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120:3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, Zabner J, Fredericks DC, McCray PB Jr, Welsh MJ, Stoltz DA. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci USA. 2010;107:20571–5. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–23. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubesch P, Dörk T, Wulbrand U, Kälin N, Neumann T, Wulf B, Geerlings H, Weissbrodt H, von der Hardt H, Tümmler B. Genetic determinants of airways' colonisation with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993;341:189–193. doi: 10.1016/0140-6736(93)90062-L. [DOI] [PubMed] [Google Scholar]
- Kumar V, Becker T, Jansen S, van Barneveld A, Boztug K, Wölfl S, Tümmler B, Stanke F. Expression levels of FAS are regulated through an evolutionary conserved element in intron 2, which modulates cystic fibrosis disease severity. Genes Immun. 2008;9:689–696. doi: 10.1038/gene.2008.63. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–70. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Larbig M, Jansen S, Dorsch M, Bernhard W, Bellmann B, Dorin JR, Porteous DJ, Von Der Hardt H, Steinmetz I, Hedrich HJ, Tuemmler B, Tschernig T. Residual cftr expression varies with age in cftr(tm1Hgu) cystic fibrosis mice: impact on morphology and physiology. Pathobiology. 2002;70:89–97. doi: 10.1159/000067308. [DOI] [PubMed] [Google Scholar]
- Choi JY, Joo NS, Krouse ME, Wu JV, Robbins RC, Ianowski JP, Hanrahan JW, Wine JJ. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest. 2007;117:3118–3127. doi: 10.1172/JCI31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120:3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EEL, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Longitudinal values for spirometric parameters. The comprehensive table shows the courses of each single spirometric parameter. Significant differences are marked in grey.