Abstract
Cytotoxic cancer chemotherapy drugs are believed to gain selectivity by targeting cells that proliferate rapidly. However, the proliferation rate is low in many chemosensitive human cancers, and it is not clear how a drug that only kills dividing cells could promote tumor regression. Four potential solutions to this “proliferation rate paradox” are discussed for the microtubule-stabilizing drug paclitaxel: drug retention in tumors, killing of quiescent cells, targeting of noncancer cells in the tumor, and bystander effects. Testing these potential mechanisms of drug action will facilitate rational improvement of antimitotic chemotherapy and perhaps cytotoxic chemotherapy more generally.
INTRODUCTION
Cancer chemotherapy agents are broadly categorized into “targeted” and “cytotoxic” drugs. Targeted drugs work by perturbing cancer-specific pathways, for example, by inhibiting a kinase oncogene. Cytotoxic drugs, which predate them, were in most cases discovered by empirical screening for cancer cell–killing activity or by targeting metabolic pathways needed for DNA replication. Paclitaxel, the subject of much of this essay, was discovered as a cytotoxic compound in the bark of pacific yew trees (Wall and Wani, 1995). Cytotoxic drugs work by damaging DNA or microtubules and are believed to gain much or all of their specificity in the human body from their ability to preferentially kill rapidly proliferating cells (Chabner et al., 2006). This selectivity is evidenced by their biochemical mechanisms, effects in cell culture, and toxicity to proliferating tissues—bone marrow, gut, and hair follicles. The paradox in the title of this essay refers to the fact that these drugs can effectively shrink solid tumors in some patients despite proliferation rates in the tumor that are low, both in absolute terms and relative to the bone marrow. Komlodi-Pasztor et al. (2011) also emphasized the issue of low proliferation rates in a recent review of drugs that target microtubules and mitotic kinases.
There are important reasons to pursue research on cytotoxic drugs, even though targeted drugs, with their lower toxicity and basis in rational understanding of cancer, may represent the long-term future of chemotherapy. Cytotoxic drugs can be highly effective—indeed curative in a few diseases; they are often cheaper than targeted drugs (many are off patent), and targeted drugs often work best in combination with cytotoxic drugs. For these reasons cytotoxics will certainly be used for years to come. From a basic science perspective, since we do not understand how cytotoxic drugs work as medicines, we do not know the extent to which this proven approach to chemotherapy could be rationally improved. That we lack this understanding despite decades of research reflects the complexity of the human body and its response to any drug, the difficulty of making reliable, interpretable measurements in patients, and the difficulty of accurately modeling diseases and drug responses in cell culture or animals.
ANTIPROLIFERATIVE VERSUS ANTICANCER ACTIVITY OF CYTOTOXIC DRUGS
My interest in cytotoxic chemotherapy was awakened by the experience of helping move specific inhibitors of mitosis into clinical trials. In the late 1990s I was part of a collaborative group that discovered a small-molecule inhibitor of mitosis called monastrol, which inhibits kinesin-5 (also called Kif11, Eg5, and KSP; Mayer et al., 1999). When cancer cells treated with kinesin-5 inhibitors enter mitosis they build monopolar mitotic spindles instead of normal bipolar spindles, activate the spindle assembly checkpoint, arrest in mitosis, and later either die by apoptosis or arrest in a senescence-like G1 state (Orth et al., 2008). With the exception of the initial biochemical insult, these actions on dividing cells are similar to those of proven anticancer drugs that target microtubules, notably paclitaxel, which binds to microtubules and inhibits polymerization dynamics (Jordan and Wilson, 2004; Gascoigne and Taylor, 2008; Shi et al., 2008).
Paclitaxel and related drugs that inhibit microtubule polymerization dynamics have proven fairly effective for treating some epithelial tumors (breast, ovarian, lung, and others), although not all patients respond well (Jordan and Wilson, 2004; Chabner et al., 2006). The hope was that kinesin-5 inhibitors would have the same anticancer activity as paclitaxel but lack its neurotoxic side effects. When patients were treated with kinesin-5 inhibitors the main effect was loss of neutrophils (Purcell et al., 2010; Komlodi-Pasztor et al., 2011). This is not surprising since neutrophil progenitors in the bone marrow are among the most rapidly proliferating cells in the human body, and this toxicity is considered diagnostic of antiproliferative activity. Paclitaxel also causes loss of neutrophils, as do essentially all cytotoxic drugs. Testing whether kinesin-5 inhibitors have anticancer activity at the dose limit set by their bone marrow toxicity is difficult because patients in clinical trials often have advanced disease and have been treated with many prior drugs. Summarizing current views, it appears that kinesin-5 inhibitors stabilized tumor growth in some patients, but tumor shrinkage was rare (Purcell et al., 2010; Komlodi-Pasztor et al., 2011). Kinesin-5 inhibitors thus appear to have less anticancer activity than paclitaxel. It is too early to determine whether the same is true for other mitosis-specific drugs currently in clinical trials, which target Plk1 and Aurora kinases. Their clinical profiles are more complex, and individual drugs vary in their specificity for the target kinase. So far the more specific drugs also seem to cause strong neutropenia with weak anticancer activity at the dose limit (Katayama and Sen, 2010; Komlodi-Pasztor et al., 2011; McInnes and Wyatt, 2011). Kinesin-5, Plk1, and Aurora kinases are required for division of all human cells. If it were possible to treat patients continuously with inhibitors of any of them, cancer cells could not divide and would eventually die. Bone marrow and gut toxicity prevent such continuous dosing. To be effective, a cytotoxic drug must have anticancer activity within the dose and duration of exposure limits set by its antiproliferative activity on these tissues. Paclitaxel and other drugs that inhibit microtubule polymerization dynamics achieve this, but it is not clear that the same is true for the mitosis-specific drugs tested so far.
Why would two drugs that both arrest cells in mitosis, kill cancer cells in culture and in mice, and show strong antiproliferative activity in the bone marrow differ in their anticancer activity? Is paclitaxel just better at killing dividing cancer cells, or does it have some other activity in the human body that contributes to therapy that kinesin-5 inhibitors lack? I will return to these questions, but first I examine the basic question of proliferation rates in cancer.
PROLIFERATION RATES IN NORMAL TISSUES AND TUMORS
When cultured cancer cells are treated with paclitaxel or a kinesin-5 inhibitor, only cells that enter mitosis are killed or rendered senescent (Baguley et al., 1995; Blagosklonny et al., 2006; Gascoigne and Taylor, 2008; Orth et al., 2008; Shi et al., 2008, 2011). Quiescent cells (cells that are in a temporary state of not dividing) or cycling cells that do not reach mitosis during drug exposure are spared. In this sense both drugs are examples of M phase–specific drugs, at least in cell culture. More classic phase-specific drugs include cytarabine, a nucleoside analogue that directly inhibits DNA replication, and methotrexate, an antimetabolite that prevents dTTP synthesis, both of which are S phase specific (Chabner et al., 2006). The “fractional kill” theory for chemotherapy was developed to try to explain why a single dose of a cell cycle phase–specific cytotoxic drug only kills a fixed fraction of cancer cells, necessitating multiple doses to eradicate the tumor (Berenbaum, 1972). According to this theory, only cells that pass through the relevant cell cycle phase when drug is present above its cytotoxic threshold are killed. It predicts a strong correlation between proliferation rate and drug sensitivity in both cancers and normal tissues. This prediction holds well for paclitaxel in tissue culture (Baguley et al., 1995). Data from treatment of human solid tumors are more mixed. A positive correlation between proliferation rate and clinical response was seen in breast cancer for mostly DNA-targeted chemotherapy (Amadori et al., 1997) but not for doctetaxel (Noguchi, 2006). Fractional kill theory engendered a keen interest in measuring cancer proliferation rates. Table 1 shows some typical values.
TABLE 1:
Cell cycle kinetics in human tumors and tissues estimated by pulse labeling with 3H-thymidine and by doubling time.
| Cell type | S phase cells (%) | Doubling time (d) |
|---|---|---|
| Breast cancer (primary) | 2–5 | ∼40–300 |
| Beast cancer (metastasis) | 3–14 (0–40 in individuals) | ∼30–90 |
| Bone marrow myeloid progenitor cells | ∼40 | ∼3 |
| Gut crypts | 10–16 | |
| Tissue culture cell line | ∼40 | ∼1 |
The ranges refer to median values reported in different studies. The range inside brackets refers to individual patient values in one large study. Data from Skipper and Perry (1970), Skipper (1971), Meyer et al. (1984), Lord (1992), Amadori et al. (1997), and Komlodi-Pasztor et al. (2011).
Table 1 and comparable data from a large literature illustrate that individual patient tumors exhibit large variation in proliferation rate, and there is also likely to be large variation between locations in a single tumor. Despite this variation, it is clear that median proliferation rates in breast tumors are much lower than in bone marrow, somewhat lower than in gut crypts, and much lower than in typical tissue culture lines. Given these data, it is not clear how a few doses of paclitaxel can “melt away” (to borrow a phrase I have heard from clinicians) a large, slow-growing tumor in a responsive patient if it kills only dividing cells. It is also unclear how any cytotoxic drug can have strong anticancer activity at the dose limit set by bone marrow and gut toxicity if relative proliferation rates are the only source of selectivity. These are not new questions—they were posed for DNA-directed drugs in the 1960s and 1970s (Skipper, 1971). I draw attention to them because they have not been answered, and in my view this means that we do not understand how cytotoxic drugs work as medicines. I also feel that many basic cell biologists have a naïve view of human cancers as proliferating as fast as HeLa cells in a dish, as I did until recently.
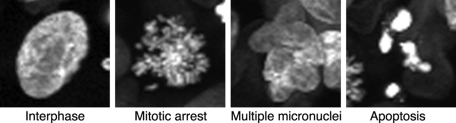
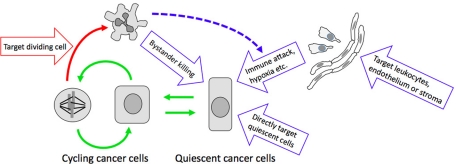
Paclitaxel has an advantage over DNA-directed drugs for discussion of the proliferation rate paradox because its actions on dividing cells generate morphological biomarkers that can be scored by microscopy (Figure 1). Mitotic arrest reports a positive response to the drug in a dividing cell. Multiple interphase micronuclei, resembling a bunch of grapes, report that a cell has passed through a defective mitosis in the presence of paclitaxel (Figure 1). This nuclear morphology arises because chromosomes are scattered when the cell slips out of mitotic arrest into G1, and separate nuclear envelopes re-form around small clusters of chromosomes. Nuclei do not fuse during interphase, so this morphology cannot be reversed unless the cell reenters mitosis. Mitotic arrest, visualized by conventional histology, was used to show that a fraction of the cells in a tumor arrest in mitosis before going on to die in diverse mouse (Milross et al., 1996) and human breast (Symmans et al., 2000) tumors treated with paclitaxel. The fraction of cells that arrest was lower in human tumors (1–6%) than in mouse tumors (5–25%), presumably reflecting lower proliferation rates. Figure 1 shows images of cells in mouse tumors responding to paclitaxel, visualized by intravital imaging of green fluorescent protein (GFP)–histone. We used this technology to track responses to a single dose of paclitaxel in a human xenograft tumor that is highly paclitaxel sensitive (Orth et al., 2011). A subset of tumor cells (<25%) underwent the typical response seen in tissue culture: mitotic arrest followed by exit into G1 with micronuclei and apoptosis either directly from mitotic arrest or following exit. The rest responded differently: proliferation and mitotic entry ceased, and mononucleated cells died out over many days, apparently without going through mitosis in drug. This proliferation block was not seen when we tracked the same cells responding to paclitaxel in culture, implying the existence of additional drug actions in the tumor environment that somehow target nondividing cells. To be optimistic, we can believe that this unexpected action of paclitaxel captures an important component of the clinical response. What might it be? We discuss four possibilities next and in Figure 2.
FIGURE 1:
Chromatin morphology reports responses of dividing cancer cells to paclitaxel in a tumor. HT1080 human cancer cells expressing histone H2B-GFP were grown as xenograft tumors in window chambers in nude mice, treated with paclitaxel, and imaged by laser confocal microscopy. Cells that divided in drug proceeded from mitotic arrest to multiple micronuclei and apoptosis. Next to far right and far right, sequential images of the same cell from a movie. (From Orth et al., 2011.)
FIGURE 2:
Potential actions of paclitaxel on tumor cells. Green arrows show cell cycle transitions. Red arrows show the only well-characterized drug action—killing cancer cells that enter mitosis. Blue arrows show hypothetical mechanisms for killing quiescent cancer cells that would provide solutions to the proliferation rate paradox.
Solution 1: Drug retention
Paclitaxel is retained in tumor cells for many days after it has been washed out of the circulation (e.g., Mori et al., 2006). It is thus possible that its only cytotoxic action is to kill cells that enter mitosis (Figure 2, red arrow) and that prolonged drug retention by quiescent cells is sufficient to kill them as they slowly enter mitosis. Drug retention cannot be solely responsible for the efficacy of paclitaxel in the HT1080 xenograft model discussed earlier because the majority of tumor cells appeared to die without passing through a drug-arrested mitosis (Orth et al., 2011). However, the relevance of this finding to responses in human tumors is unclear. Drug retention may be an important factor in clinical efficacy of microtubule-targeting drugs and could help resolve the proliferation rate paradox.
Solution 2: Paclitaxel kills quiescent cancer cells
This is the most straightforward explanation for death of nonproliferating cells in solid tumors. Quiescent cells contain dynamic microtubules, so they will certainly be perturbed by paclitaxel. The question is, does this perturbation kill them? Cancer cells in culture exposed to paclitaxel at therapeutically relevant concentrations rarely die unless they enter mitosis (Baguley et al., 1995; Gascoigne and Taylor, 2008), but perhaps quiescent cells are more sensitive in tumors? Some aspects of the tumor environment, such as nutrient and oxygen deprivation, promote cell death (Weinberg, 2007). Others, such as contact with extracellular matrix, promote survival (Weaver et al., 2002). Paclitaxel could act directly on quiescent cells to tip this balance toward cell death in the tumor environment. This would explain the activity of microtubule-targeting drugs and lack of activity of mitosis-specific drugs in human tumors (Komlodi-Pasztor et al., 2011). It would also explain the mitosis-independent cell death that we observed in the xenograft tumor model discussed earlier, although I am skeptical, given the lack of killing of quiescent cells by paclitaxel in tissue culture.
If a cytotoxic drug kills quiescent cells, why does it not destroy every tissue in the human body? The tumor environment could be proapoptotic, but in addition, cancer cells may be intrinsically apoptosis sensitive. Cancer cells are selected for resistance to apoptosis (Weinberg, 2007), so we might then expect cancer cells to be generally more apoptosis resistant than their normal counterparts when challenged with chemotherapy drugs. However, when responses to antimitotic drugs were compared in dividing cells in culture, most epithelial cancer cell lines underwent apoptosis more readily than noncancer immortalized lines and primary cells (Orth et al., 2008; Shi et al., 2008). Proliferating noncancer cells trend to enter a senescence-like state after exit from mitotic arrest rather than initiate apoptosis (Blagosklonny et al., 2006). By these measures, dividing cancer cells are often more apoptosis sensitive than dividing normal cells. Leukocytes, whether normal or cancer derived, are also highly prone to apoptosis during mitotic arrest, which may help account for the bone marrow toxicity of mitosis-specific drugs (Tang et al., 2011). Letai (2008) proposed the “mitochondrial priming” theory to account for enhanced apoptosis sensitivity of cancer cells. His idea is that whereas antiapoptotic pathways are often up-regulated in cancer, concurrent up-regulation of proapoptotic BH3-only BCL-2–family proteins often renders the cancer cell closer to the threshold of apoptosis than its normal counterpart. Concurrent up-regulation of proapoptotic and antiapoptotic regulators “primes” cancer cells to initiate an apoptotic response to chemotherapy drugs that inhibit antiapoptotic pathways. Some cancers evolve apoptosis resistance by mechanisms that cannot be reversed by drugs, such as complete loss of Bax and Bak, but this is less common than drug-reversible mechanisms (Deng et al., 2007). Normal cells survive in drug, or choose senescence over apoptosis, because they lack proapoptotic activation and are not primed. Pretreatment measurements of mitochondrial priming predict clinical responses to chemotherapy and might be useful for guiding treatment (Chonghaile et al., 2011).
Solution 3: Paclitaxel targets noncancer cells in the tumor
Because microtubules are present in most cells, paclitaxel must act on noncancer cells in tumors, including endothelial cells, stromal fibroblasts, and leukocytes (Figure 2). Some of these actions might be important for, and even central to, the therapeutic response.
Endothelium: Tumors depend on blood vessels for oxygen and nutrients, and microtubules play important roles in endothelial cells. Drugs that depolymerize microtubules, notably combretastatin, can promote tumor regression by damaging established blood vessels, with unexplained tumor selectivity (Kanthou and Tozer, 2009). Paclitaxel lacks comparable damaging effects on established endothelial barriers (Verin et al., 2001) but might still cause some kind of endothelial damage in tumors.
Stroma: We know little about how paclitaxel might affect tumor stromal cells and the signals they send.
Leukocytes: These cells depend on microtubules for many aspects of their biology, including organelle transport and chemotaxis. Paclitaxel has complex effects on leukocytes, and there is some evidence that it enhances the ability of the immune system to kill cancer cells (Javeed et al., 2009). Tumor-associated macrophages are very common in solid tumors, where they are believed to mostly help the cancer by enhancing tumor growth and metastasis (Coussens and Werb, 2002). Paclitaxel and other chemotherapy drugs could change this situation. Paclitaxel acts directly on mouse macrophages, in concert with inflammatory cytokines, to induce expression of inducible nitric oxide synthase (iNOS), which can act as a weapon to kill cancer cells (Manthey et al., 1994). Nitric oxide (NO) can react with superoxide (O2−) to form peroxynitrite (ONOO−) when both iNOS and NADPH oxidase are activated in macrophages (Brown and Neher, 2010). Peroxynitrite is more cytotoxic than either nitric oxide or superoxide alone and could play a role in chemotherapy responses. Activated macrophages also secrete inflammatory cytokines that could kill tumor cells directly or summon cytotoxic NK cells. Induction of a cytotoxic phenotype in tumor macrophages by direct action of microtubule-targeting drugs could explain why they show greater antitumor activity than mitosis-specific drugs.
Solution 4: Bystander killing
By this I mean that a drug acts on a subset of cancer cells in the expected way, which for paclitaxel is to damage or kill cells that enter mitosis. Damaged or dead cells then send some new signal or cause some environment change that causes damage or death to neighboring cancer cells. Bystander killing could occur by direct cancer cell–to–cancer cell signaling. It could also occur indirectly, via the immune system or blood vessels (Figure 2, dotted blue line). Bystander effects are well known in radiation biology (Little, 2006). Irradiated cells can damage unirradiated neighbors by signals that are transmitted through gap junctions and by secreted factors. DNA damage triggers secretion of inflammatory cytokines, which comprise an important class of bystander signal that can act directly or indirectly via leukocytes (Prise and O'sullivan, 2009). All cytotoxic drugs, including mitosis-arresting drugs (Orth et al., 2011), cause DNA damage, so we should expect bystander effects in chemotherapy. Bystander killing cannot obviously explain differences between paclitaxel and kinesin-5 inhibitors unless paclitaxel is simply better at killing dividing cells or damaging their DNA, for which there is some cell culture evidence (Shi et al., 2008, 2011).
Bystander effects caused by inflammatory signaling are probably important in chemotherapy. A recent paper examined the response of a mouse tumor to a DNA-alkylating drug, where the primary drug-induced cell death mechanism was necrosis (Guerriero et al., 2011). Necrotic cell death caused release of the proinflammatory chromatin molecule HMGB1 (Andersson and Tracey, 2011). This induced massive recruitment and activation of innate immune cells, which was required for tumor regression. Because the drug was ineffective at promoting tumor regression in the absence of inflammatory signaling, tumor cells were presumably killed in large part by leukocytes as a bystander response. The primary drug-induced death mechanism in paclitaxel is apoptosis, which is less proinflammatory than necrosis. However, inflammatory signaling might result if the rate of apoptotic death overwhelmed phagocytic clean-up mechanisms (“secondary necrosis”; Silva, 2010) or if tumor cells exit mitotic arrest into a senescence-like state (Blagosklonny et al., 2006) and this triggers cytokine release and immune clearance (Xue et al., 2007). Taxane treatment induces massive leukocyte recruitment to drug-responsive tumors in mice (Schimming et al., 1999) and humans (Demaria et al., 2001), but it is not clear whether the leukocytes are just cleaning up corpses or playing an active role in therapy. Combining these ideas with solution 3, I speculate that bystander signals from damaged cancer cells could combine with direct action of microtubule-targeting drugs on leukocytes to induce a tumor-killing inflammatory reaction (Figure 2, dotted blue arrow).
An extreme form of bystander effect is induction of adaptive immunity by dying cells. Kroemer, Zitvogel, and colleagues argue that the most effective cytotoxic agents may work by promoting “immunogenic cell death,” a sequence of cellular events that render dying cancer cells highly immunogenic (Kepp et al., 2011). Oxaliplatin is an effective trigger of immunogenic death in mice; how paclitaxel acts in this respect is unclear. This exciting potential mechanism harnesses the full power of the immune system to reject the cancer, but its role in current chemotherapy is unclear.
An interesting aspect of bystander effects is their potential for positive feedback. If damage to one tumor cell triggers damage to more than one neighbor, a snowball effect could be set in motion that ends up killing entire tracts of the tumor. Getting this snowball rolling would presumably require some threshold level of direct cell killing by drug, so one could imagine that success versus failure of therapy is balanced on a knife edge. This could help explain large differences in response among patients with similar disease and the need for maximally tolerated drug doses.
FUTURE DIRECTIONS
Each of the nonmitotic mechanisms for killing quiescent cells shown as blue arrows in Figure 2 would, if true, have important implications for enhancing chemotherapy with existing drugs and designing better future drugs or combinations. They also predict drug resistance mechanisms quite distinct from those usually considered for taxanes, such as tumor cells evolving lack of signaling to immune cells in response to drug or resistance to killing by activated immune cells. Immune-mediated resistance mechanisms could reduce responses to all chemotherapeutics and play a role in the general chemoresistance often seen in advanced cancers. Which, if any, of the mechanisms in Figure 2 operates in a particular drug/tumor model could be tested in mice. A confounding issue is that proliferation rates are much higher in mouse models than in human tumors. The response to microtubule-targeting drugs will thus be more dominated by mitosis-dependent mechanisms in mice (Komlodi-Pasztor et al., 2011). Evaluating these mechanisms in humans will be much more challenging but very important. We do not know how cytotoxic chemotherapy drugs work as medicines, and until we do it will be difficult to rationally improve their use and design better ones.
The mechanisms in Figure 2 provide an interesting context for addressing two important questions in antimitotic chemotherapy research: why do different microtubule-stabilizing drugs exhibit different clinical activities, and could we develop a mitosis-specific drug with strong anticancer activity? New taxane derivatives (e.g., carbazitaxel) and formulations (e.g., NAB-paclitaxel), as well as different drugs, that inhibit microtubule polymerization dynamics (e.g., ixabepilone, eribulin) have gained approval for cancer treatment recently because they work better than paclitaxel in some indications, and in no case is it clear why (Ribeiro et al., 2011). Expression of the drug efflux pump ABCB1 causes paclitaxel resistance in cell culture, and some of the new drugs were developed in part as poor substrates for this pump, but it is not clear that this can explain all the clinical differences. Perhaps these drugs also differ in effects on noncancer cells in tumors.
Mitosis-specific drugs such as kinesin-5 inhibitors have so far lacked strong anticancer activity at the dose limit set by their antiproliferative activity (discussed earlier). If paclitaxel works as a medicine because it both arrests cancer cells in mitosis and, for example, acts directly on tumor macrophages or endothelium, then any drug with only mitosis-specific actions will likely fail. Two exceptions could be imagined—a drug that is delivered specifically to the tumors, and a drug that exploits some cancer-specific defect in mitotic mechanism to cause synthetic lethality while lacking strong antiproliferative activity on normal cells. Identifying exploitable, cancer-specific defects needed for a synthetic lethal antimitotic drug is an exciting goal for mitosis research.
Acknowledgments
I thank the members of my group, whose efforts have shaped my thoughts on chemotherapy, especially James Orth, who initiated this line of research, which is supported by National Cancer Institute Program Grant CA139980. I also thank the many colleagues who have educated me and provided feedback on this essay, especially Bruce Chabner, Anthony Letai, Neal Rosen, Gary Schwartz, and Ralph Weissleder.
Abbreviations used:
- GFP
green fluorescent protein
- iNOS
inducible nitric oxide synthase
Footnotes
REFERENCES
- Amadori D, Volpi A, Maltoni R, Nanni O, Amaducci L, Amadori A, Giunchi DC, Vio A, Saragoni A, Silvestrini R. Cell proliferation as a predictor of response to chemotherapy in metastatic breast cancer: a prospective study. Breast Cancer Res Treat. 1997;43:7–14. doi: 10.1023/a:1005780107879. [DOI] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley BC, Marshall ES, Whittaker JR, Dotchin MC, Nixon J, McCrystal MR, Finlay GJ, Matthews JH, Holdaway KM, van Zijl P. Resistance mechanisms determining the in vitro sensitivity to paclitaxel of tumour cells cultured from patients with ovarian cancer. Eur J Cancer. 1995;31A:230–237. doi: 10.1016/0959-8049(94)00472-h. [DOI] [PubMed] [Google Scholar]
- Berenbaum MC. In vivo determination of the fractional kill of human tumor cells by chemotherapeutic agents. Cancer Chemother Rep. 1972;56:563–71. [PubMed] [Google Scholar]
- Blagosklonny MV, Demidenko ZN, Giovino M, Szynal C, Donskoy E, Herrmann RA, Barry JJ, Whalen AM. Cytostatic activity of paclitaxel in coronary artery smooth muscle cells is mediated through transient mitotic arrest followed by permanent post-mitotic arrest: comparison with cancer cells. Cell Cycle. 2006;5:1574–1579. doi: 10.4161/cc.5.14.3113. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Chabner BA, et al. Antineoplastic agents. In: Brunton LL, editor. Goodman and Gilman's The Pharmacological Basis for Therapeutics. 11th. New York: McGraw-Hill; 2006. pp. 1315–1403. [Google Scholar]
- Chonghaile T, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, Crawford HC, Zong WX. DNA alkylating therapy induces tumor regression through an HMGB1-mediated activation of innate immunity. J Immunol. 2011;186:3517–3526. doi: 10.4049/jimmunol.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38:283–290. doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. Int J Exp Pathol. 2009;90:284–294. doi: 10.1111/j.1365-2613.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Sen S. Aurora kinase inhibitors as anticancer molecules. Biochim Biophys Acta. 2010;1799:829–839. doi: 10.1016/j.bbagrm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Little JB. Cellular radiation effects and the bystander response. Mutat Res. 2006;597:113–118. doi: 10.1016/j.mrfmmm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lord BI. Myeloid cell kinetics in response to haemopoietic growth factors. Baillieres Clin Haematol. 1992;5:533–550. doi: 10.1016/s0950-3536(11)80006-5. [DOI] [PubMed] [Google Scholar]
- Manthey CL, Perera PY, Salkowski CA, Vogel SN. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994;152:825–831. [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- McInnes C, Wyatt MD. PLK1 as an oncology target: current status and future potential. Drug Discov Today. 2011;16:619–625. doi: 10.1016/j.drudis.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Meyer JS, McDivitt RW, Stone KR, Prey MU, Bauer WC. Practical breast carcinoma cell kinetics: review and update. Breast Cancer Res Treat. 1984;4:79–88. doi: 10.1007/BF01806389. [DOI] [PubMed] [Google Scholar]
- Milross CG, Mason KA, Hunter NR, Chung WK, Peters LJ, Milas L. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88:1308–1314. doi: 10.1093/jnci/88.18.1308. [DOI] [PubMed] [Google Scholar]
- Mori T, Kinoshita Y, Watanabe A, Yamaguchi T, Hosokawa K, Honjo H. Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer Chemother Pharmacol. 2006;58:665–672. doi: 10.1007/s00280-006-0209-6. [DOI] [PubMed] [Google Scholar]
- Noguchi S. Predictive factors for response to docetaxel in human breast cancers. Cancer Sci. 2006;97:813–820. doi: 10.1111/j.1349-7006.2006.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 2011;71:4608–4616. doi: 10.1158/0008-5472.CAN-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Loewer A, Lahav G, Mitchison TJ. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-09-0781. [MBoC In Press, doi:10.1091/mbc.E11-09-0781] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Tang Y, Shi J, Loy CT, Amendt C, Wilm C, Zenke FT, Mitchison TJ. Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate. Mol Cancer Ther. 2008;7:3480–3489. doi: 10.1158/1535-7163.MCT-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, O'sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JW, et al. Activity of the kinesin spindle protein inhibitor ispinesib (SB-715992) in models of breast cancer. Clin Cancer Res. 2010;16:566–576. doi: 10.1158/1078-0432.CCR-09-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JT, et al. Cytotoxic drugs for patients with breast cancer in the era of targeted treatment: back to the future? Ann Oncol. 2011 doi: 10.1093/annonc/mdr382. Sep 26 PMID: 21896541 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chemother Pharmacol. 1999;43:165–172. doi: 10.1007/s002800050879. [DOI] [PubMed] [Google Scholar]
- Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–3276. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhou Y, Huang HC, Mitchison TJ. Navitoclax (ABT-263) accelerates apoptosis during drug-induced mitotic arrest by antagonizing Bcl-xL. Cancer Res. 2011;71:4518–4526. doi: 10.1158/0008-5472.CAN-10-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 2010;584:4491–4499. doi: 10.1016/j.febslet.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Skipper HE. Kinetics of mammary tumor cell growth and implications for therapy. Cancer. 1971;28:1479–1499. doi: 10.1002/1097-0142(197112)28:6<1479::aid-cncr2820280622>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Skipper HE, Perry S. Kinetics of normal and leukemic leukocyte populations and relevance to chemotherapy. Cancer Res. 1970;30:1883–1897. [PubMed] [Google Scholar]
- Symmans WF, et al. Paclitaxel-induced apoptosis and mitotic arrest assessed by serial fine-needle aspiration: implications for early prediction of breast cancer response to neoadjuvant treatment. Clin Cancer Res. 2000;6:4610–4617. [PubMed] [Google Scholar]
- Tang Y, Orth JD, Xie T, Mitchison TJ. Rapid induction of apoptosis during kinesin-5 inhibitor-induced mitotic arrest in HL60 cells. Cancer Lett. 2011;310:15–24. doi: 10.1016/j.canlet.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L565–L574. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- Wall ME, Wani MC. Camptothecin and Taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1995;55:753–760. [PubMed] [Google Scholar]
- Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. New York: Garland Sciences; The Biology of Cancer. [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]