Abstract
Cell polarity is important for a number of processes, from chemotaxis to embryogenesis. Recent studies suggest a new role for polarity in the orchestration of events during the final cell separation step of cell division called abscission. Abscission shares several features with cell polarization, including rearrangement of phosphatidylinositols, reorganization of microtubules, and trafficking of exocyst-associated membranes. Here we focus on how the canonical pathways for cell polarization and cell migration may play a role in spatiotemporal membrane trafficking events required for the final stages of cytokinesis.
INTRODUCTION
Cell polarity is a common feature of eukaryotic cells. A polarized cell has a single axis of asymmetry, which can be thought of as “apical” and “basal” (illustrated in Figure 1A) or “front” and “rear” (Figure 1B). This asymmetry encompasses a variety of cellular morphologies that can differ among cell types in a single organism, as well as within a single-celled organism such as Saccharomyces cerevisiae. Cells within multicellular organisms also exhibit a distinct apical–basal axis of polarity. One example is epithelial initiation, which occurs during development and involves congression of mesenchymal cells into aggregates that differentiate to form polarized apical–basal cell monolayers. At the migratory stage, mesenchymal cells set up a front–rear axis of polarity (Figure 1B). These polarity principles contribute to the higher-order organization of cell-based systems, such as shaping the embryo, wiring the developing nervous system, maintaining and regenerating tissue, and developing the immune response (reviewed in Nelson, 2009). Perturbations in cell polarity contribute to a number of tissue pathologies. Particularly striking examples include neuronal migration disorders (NMDs) such as schizencephaly, porencephaly, and lissencephaly, which are caused by defects in the polarized migration of neurons (for a review on NMDs, see Valiente and Marin, 2010).
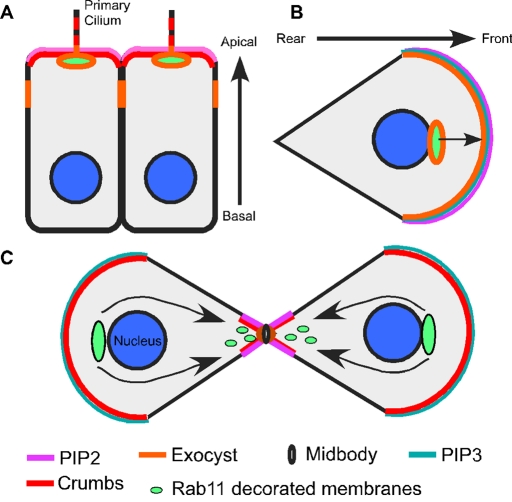
FIGURE 1:
Comparison of cellular polarity in cytokinetic cells, polarized epithelial cells, and migrating cells. (A) In polarized cells the exocyst (orange) is required for basolateral secretory-vesicle delivery and apical endosomal membrane transport. The exocyst is localized to the basal body/centrosome at the apical primary cilium. The apical and basal sides are labeled. (B) In migrating cells the exocyst (orange) is enriched at the leading edge and at recycling endosomes (lime green) for polarized membrane fusion to occur that assists in cell migration (arrow). The front and rear sides of the cell are labeled. Phosphatidylinositols (pink and teal) are also polarized in cytokinetic cells, polarized cells, and migrating cells. (C) During cytokinesis there is polarized membrane traffic (arrow) of Rab11-decorated recycling vesicles (lime green) to the cytokinetic bridge. The Rab11 recycling endosome compartment (lime green) recruits a member of the polarity complex, Crumbs, to the cytokinetic bridge (red). The exocyst complex (orange) localizes to the midbody ring (black ring) and is required for polarized membrane fusion during cytokinesis. The main theme shared between polarized cells (A, B) and cytokinetic cells (C) is that they coordinate the use of polarity proteins and polarized membrane-trafficking pathways either to construct a polarized cell or to complete cytokinesis.
Specific sorting and maintenance of proteins to distinct membrane domains ensures polarity formation. The defined distribution of plasma membrane and cytoskeletal proteins in a polarized cell requires signaling networks and protein complexes comprising the Rho and Rab family GTPases, their downstream effectors, and polarity complexes (Crumbs, PAR, and Scribble). Effectors include cytoskeletal proteins and vesicle-trafficking pathways. Vesicle trafficking from the endocytic and/or exocytic membrane compartments along a polarized cytoskeleton network is required for the establishment of cellular polarity (reviewed in Nelson, 2009). This has been elegantly illustrated in the budding yeast, S. cerevisiae, in which bud growth is ensured by polarized secretion of Golgi apparatus–derived membrane vesicles to cortical actin patches under the regulation of Rho GTPases and polarity complexes at the bud tip (reviewed in Chant, 1999; Irazoqui and Lew, 2004). In multicellular eukaryotes, protein-sorting events occur at the endocytic pathway and Golgi apparatus to ensure cell polarization (Nelson, 2009). For example, plasma membrane–endocytic recycling is critical for maintaining polarized membrane protein residency to establish appropriate responses to stimuli such as nutrient internalization, junctional protein sorting (e.g., E-cadherin), and ion channel recycling (Lock and Stow, 2005; Ducharme et al., 2006). Of interest, recent evidence shows that vesicle trafficking is also required for the establishment of polarized domains during cytokinesis, the final stage of cell division (Figure 1C).
Recent studies suggest that principles of cell polarity are engaged during the process of cytokinesis. For instance, a migrating polarized cell requires constant membrane addition via secretion at the leading edge to maintain “front–rear” polarity (Nelson, 2009), just as abscission (the final stage of cytokinesis) requires membrane addition at the cytokinetic bridge via secretion and endocytic vesicle delivery (further discussed in the review by Prekeris and Gould, 2008, and depicted in Figure 2). In both cases, membrane vesicles may act as a platform for delivering essential regulators ensuring cell polarization. Another similarity between cell polarity and cytokinesis occurs within S. cerevisiae. In G1/S an actin patch is focused at the bud tip where secretory vesicles are directed, and then the patch dissipates as the bud becomes larger and the cell enters cytokinesis. Prior to spindle disassembly and cell separation, polarized-actin patch proteins and secretory vesicles are redirected to the mother bud neck, a structure analogous to the cytokinetic bridge (VerPlank and Li, 2005). The apparent importance of polarized vesicle trafficking to polarity and the final stages of cytokinesis leads one to speculate about a conserved underlying mechanism between the two processes. This notion will be discussed and proposed throughout this essay.
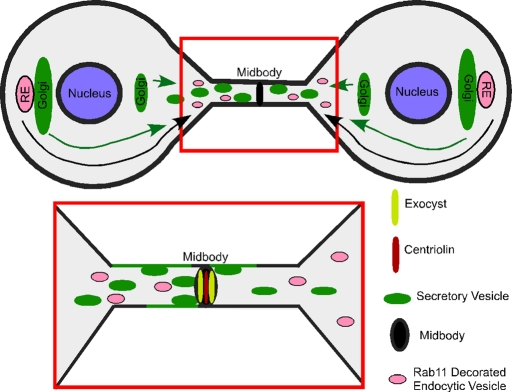
FIGURE 2:
Polarized membrane trafficking and membrane fusion at the midbody during abscission. Secretory vesicles (green) and Rab11-decorated endocytic recycling membranes (pink) undergo directed motility to the cytokinetic bridge. However, the temporal relationship between the two is unknown. Secretory vesicles (green) are known to fuse (green line) with the plasma membrane adjacent to the midbody. It is proposed that these vesicles transport to the cytokinetic bridge, dock at the midbody by an exocyst-dependent mechanism, and then fuse. Membrane addition is required for abscission, but the role of this process is unknown. One possibility is that secretory vesicles (green) or recycling endosomes (pink) bring proteins needed for abscission. Another idea is that membrane fusion is required to thin the bridge so that the fission step can occur as efficiently as possibly.
DISCUSSION
During cell division the front–rear polarity of phosphatidylinositols is reestablished in cytokinesis
Asymmetry in an epithelial, neuronal, or migrating cell is reflected in its structural, molecular, and functional polarity. For example, in a migrating cell the broad leading edge (front) of the cell defines the direction of movement, and the more-focused rear trails behind. When a migrating cell divides, it first rounds up, thus eliminating its preexisting polarity. This involves vesiculating and dispersing sorting compartments such as the Golgi apparatus and the endosomal system. During cytokinesis, furrow ingression gives rise to the intercellular bridge, a thin cytoplasmic connection between the two nascent daughter cells that is later resolved in the process of abscission. Each nascent daughter assumes an intracellular organization roughly similar to an interphase cell, where the Golgi apparatus and endocytic system have partially reorganized over the mother and daughter centrosomes (Figure 2). At this time, cytokinetic cells have started to reestablish front–rear polarity such that they need only to sever the bond between them to gain the freedom to migrate.
The morphological front–rear polarity observed between migration and cytokinesis is further defined by molecular reorganization—specifically, changes in phosphotidylinositol concentration within the plasma membrane (Figure 1). However, little is known about how lipid domain polarization occurs during polarization or cell division. Possibilities include 1) remodeling of existing lipid domains by membrane traffic (endocytosis, directed secretion); 2) cortical flow of specific phosphatidylinositols to and immobilization at a specific region; and 3) targeting of lipid-modifying enzymes to a specific region. Cytokinetic proteomic and RNA interference screens have led to the identification of intracellular transport genes required for cytokinesis (Echard et al., 2004; Skop et al., 2004) and subsequently for lipid domain polarization. These genes included Rab GTPase family members and inositol-modifying enzymes, such as phosphatidylinositol 3-kinases (PI3Ks), which are Rab effectors (e.g., Rab5; Vieira et al., 2003) that connect membrane traffic with lipid modification.
Cytokinesis is accompanied by polarized organization of phosphatidylinositols. For instance, phosphatidylinositol (3,4,5)-triphosphate (PI(3,4,5)P3) localizes to the plasma membrane near the spindle poles. On the other hand, phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) predominantly localizes to the cleavage furrow and then to the intracellular bridge between mother and daughter cells analogous to the rear of the migrating cell (Figure 1C; Janetopoulos et al., 2005; Toyoshima et al., 2007). In a migrating cell, polarity is established by local PI(3,4,5)P3 accumulation at the cell's leading edge (Figure 1B; Parent and Devreotes, 1999), which is achieved through localization of PI3 kinases and the tumor suppressor PTEN (Kolsch et al., 2008). This reorganization facilitates pseudopodia extension (Wang et al., 2002) through actin polymerization induced by actin-binding proteins (WASP, profilin, cofilin, capping protein) binding to membranous PI(3,4,5)P3 and PI(4,5)P2 (Yin and Janmey, 2003) and subsequent membrane addition by vesicle transport. Mislocalization of phosphatidylinositols disorganizes polarity of the aforementioned actin-binding proteins (reviewed in Gassama-Diagne and Payrastre, 2009; Nelson, 2009) and also inhibits cytokinesis by a similar mechanism (Janetopoulos et al., 2005). For example, cells lacking PTEN and PI3K are unable to create a stable actin/myosin–based contractile ring, thus failing to form a cleavage furrow between dividing mother and daughter cells (Janetopoulos et al., 2005). This finding suggests that polarization of phosphatidylinositols at the midzone is required to initiate cytokinesis.
The exocyst may function similarly in polarity formation and cytokinesis
Secretion from the Golgi apparatus has been suggested as the primary trafficking route for both cell polarization and cytokinesis (Gromley et al., 2005; Nelson, 2009). However, recent evidence links the secretory and endocytic recycling pathways, making it difficult to dissect their individual functions. Originally, the exocyst vesicle-tethering complex was found at the apex of the lateral membrane domain in polarized epithelial cells (Figure 1A), where it specified basolateral secretory-vesicle delivery (Grindstaff et al., 1998). More recently, the exocyst was also found at the apical recycling endosome compartment (Oztan et al., 2007; Bryant et al., 2010) and at the basal bodies of the primary cilium (Babbey et al., 2010; Figure 1A). In addition, the exocyst subunit Sec15 was identified as a Rab GTPase-Rab11 effector that colocalizes at the recycling endosome in both Drosophila and mammals (Zhang et al., 2004; Wu et al., 2005). Thus the exocyst has distinct localizations that include the plasma membrane, secretory vesicles, the basal body, and recycling endosomes, which suggests not only roles in secretory vesicle fusion at the plasma membrane and during endocytic recycling, but perhaps various other functions.
The exocyst may be involved in regulating polarized-vectorial membrane traffic toward the leading edge of a migrating cell during interphase (Figure 1B) or in the opposite direction toward the midbody during cytokinesis (Figure 1C). A migratory cell requires de novo plasma membrane addition at the leading edge that is driven by membrane traffic (Lim et al., 2005). This occurs through use of the small-GTPase RalB to confine vesicle trafficking to the leading edge, achieving directional cell movement (Camonis and White, 2005; Rosse et al., 2006). These vesicles then tether at the leading edge via the RalB-recruited-exocyst complex (Rosse et al., 2006), suggesting that localized membrane addition is required to create a polarized migrating cell. Of interest, this same process seems to be similar, if not the same, for membrane addition at the cytokinetic bridge. As in cell migration, RalB recruits the exocyst to the cytokinetic bridge during abscission (Cascone et al., 2008). In addition, two independent groups showed that secretory vesicles dock via the exocyst and fuse within the cytokinetic bridge (Gromley et al., 2005; Goss and Toomre, 2008). Most important, these studies found that vesicle fusion near the midbody ring is required for abscission. One interesting point that these findings highlight is that vectorial membrane transport toward the midbody is opposite that of membrane transport in a migrating cell, suggesting that cytokinetic cells can reorganize the direction of polarized membrane traffic.
During both cell migration and cytokinesis, plasma membrane addition driven by the exocyst complex is likely coordinated with Rab-dependent and soluble N-ethylmaleimide–sensitive fusion protein attachment protein receptor (SNARE)–dependent machinery. One of the best examples of this possible coordination is with the mother-centriole-localized protein centriolin. During cytokinesis centriolin localizes to the midbody ring within the cytokinetic bridge (Figure 2). At the bridge, centriolin can interact with both the exocyst complex and a SNARE-associated protein, Snapin, which can mediate secretory vesicle fusion (Gromley et al., 2005). In a separate study, exocyst depletion not only caused inhibition of secretory vesicle fusion at the midbody, but also impaired Rab11 localization to the cytokinetic bridge (Fielding et al., 2005). These results suggest that the exocyst is required for both endosomal and secretory membrane-trafficking steps during cytokinesis (Fielding et al., 2005; Gromley et al., 2005), which is similar to the requirement of the exocyst complex for endocytic recycling and secretory traffic to the leading edge in polarized cells (Grindstaff et al., 1998; Zhang et al., 2004; Wu et al., 2005; Rosse et al., 2006; Oztan et al., 2007; Nelson, 2009).
Rab GTPase family members are required for cytokinesis
Rab GTPases are key regulators of membrane traffic, with more than 60 members in mammalian cells that define particular routes within the secretory and endocytic pathways. Rab11 is a well-established participant in recycling endosomal trafficking. In polarized epithelial cells Rab11 is associated with membranes in the apical portion near the centrosome and beneath the apical plasma membrane (Casanova et al., 1999). This localization enables Rab11 to regulate efficient endosomal recycling to apical plasma membrane domains (Prekeris et al., 2000). Rab11 is required for cytokinesis in Caenorhabditis elegans and Drosophila and was the first Rab GTPase implicated in cytokinesis in human cells (reviewed in Strickland and Burgess, 2004).
Drosophila embryogenesis has provided a unique system for studying Rab11-dependent membrane trafficking (discussed in Strickland and Burgess, 2004). Molecular elements required for cellularization of the Drosophila embryo are often homologous to those that drive cytokinesis in mammalian cells. Of interest, Rab11 activity is essential for cellularization of the Drosophila embryo. Dominant-negative Rab11 caused defects in membrane addition and furrow morphology (Ullrich et al., 1996). These phenotypes were very similar to Drosophila Nuclear Fallout mutants (Rothwell et al., 1998), suggesting that both genes may be involved in common pathways. In fact, Nuclear Fallout is closely related to the mammalian Rab11-binding protein FIP3 (Hickson et al., 2003), which is required for mammalian cytokinesis (Fielding et al., 2005; Wilson et al., 2005).
The formation and targeting of Rab11 vesicles to membrane domains within the cytokinetic bridge may be analogous to the mechanism for targeting Rab11 vesicles to the apical plasma membrane. In fact, Rab11-positive recycling endosomes, together with the Rab11 effectors FIP3 and FIP4, are required for membrane delivery during cytokinesis and abscission, although the site of targeting is unknown (Wilson et al., 2005). Both FIP3 and FIP4 bind the small GTP-binding protein ARF6, which binds to the exocyst subunit Exo70 and is required for cytokinesis (Fielding et al., 2005). One proposed role for these complex interactions is that FIP3 and FIP4 couple Rab11-positive vesicle traffic from the recycling endosome to the midbody, where they are tethered via interactions with ARF6 and the Exocyst (Fielding et al., 2005). Of interest, polarized migrating cells rely on ARF6-regulated endosomal trafficking to concentrate active Cdc42 at the leading edge and recruit the Par6-aPKC polarity complex (Osmani et al., 2010).
Rab35 is a newly discovered GTPase required for cytokinesis. Like Rab11, Rab35 localizes to the endocytic recycling pathway and regulates cytokinesis. However, during interphase Rab35 does not colocalize exactly with the Rab11 compartment. Rab35 functions at an early (fast recycling) endosome, prior to the relatively slow recycling endosome step regulated by Rab11 (Kouranti et al., 2006). Whether a role exists for Rab35 in regulating either polarity in migratory cells or epithelial cells is not yet known. One argument that it may regulate polarity is that Rab35 is required during cytokinesis to concentrate PI(4,5)P2 at the cytokinetic bridge (Figure 1C; Kouranti et al., 2006). In addition, like Rab11, Rab35 is required during cytokinesis after furrow ingression to provide polarized delivery of membrane vesicles derived from recycling endosomes to the cytokinetic bridge. This membrane could be required during abscission for nascent daughter cell separation (Wilson et al., 2005). Because Rab11 and Rab35 are localized to different subcellular compartments and control distinct endocytic recycling pathways (Zerial and McBride, 2001), it is likely that there are multiple endocytic routes that are individually essential for cytokinesis and, hypothetically, polarity.
The role for Crumbs during cytokinesis
Crumbs proteins are important determinants of apical membrane identity (McCaffrey and Macara, 2009) and may be required for cytokinesis. A recent study found that the apical membrane is established during and after cytokinesis through the delivery of Crumbs3-positive membranes from a Rab11-regulated recycling endosome compartment at the spindle poles that move centripetally along microtubules toward the plasma membrane (Schluter et al., 2009). Live-cell imaging revealed Crumbs3 localization between the two daughter cells within the cytokinetic bridge (Schluter et al., 2009). Although these data strongly suggest that polarity is initiated during cytokinesis, a requirement for polarity complexes in cytokinesis has yet to be determined. Of interest, the studies of Schluter et al. (2009) and others (reviewed in Shivas et al., 2010) have shown that the endocytic pathway can play an important role in the localization of polarity proteins during cytokinesis and polarity formation; there is also evidence for a reciprocal role for polarity proteins in the regulation of endocytic machinery (Balklava et al., 2007). Taken together, these studies suggest a synergistic model in which polarity and endocytosis regulators may cross-regulate one another and mutually contribute to the completion of cytokinesis and the formation and function of polarized cells.
CONCLUSION
Establishment of polarity is important for cell migration and partitioning an organism into external and internal compartments. The discussion here suggests that polarity also functions in cell division. This is suggested by the organization of polarity complexes to cytokinetic sites and defects in cell division following loss or mutation of polarity complex proteins (Albertson and Doe, 2003; Humbert et al., 2006). Similarly, although the localization of polarity components such as Crumbs and phosphatidylinositols to mitotic structures, as well as directed vesicular traffic, highlight the initial stages of polarity during the final stages of cytokinesis, the functional relevance of this reorganization is unknown. Therefore comparing components that are involved in cytokinesis, such as Rab35, that are not yet known to be involved in cell polarity and investigating their possible conserved function between cytokinesis and polarity may shed light on novel molecular mechanisms. The same approach could be used in a reciprocal manner to address whether known polarity proteins not yet shown to be involved in cytokinesis have cytokinetic functions.
We conclude that there is a likely requirement for spatiotemporal orchestration of membrane trafficking and polarity-complex machinery to construct new polarized membranes during both cytokinesis and apical surface formation. However, the precise spatiotemporal relationships and functional relevance of these factors have yet to be determined. Future insights into how endocytic recycling, secretory, and polarity pathways act together during cytokinesis will require careful spatiotemporal analysis of these components at the cytokinetic bridge.
Acknowledgments
We thank David Lambright (University of Massachusetts Medical School), Charles Yeaman (University of Iowa, Iowa City, IA), Alison Bright (Doxsey lab, University of Massachusetts Medical School), and Benedicte Delaval (Doxsey lab, University of Massachusetts Medical School) for critical reading of the manuscript. The National Institutes of Health, the Ellison Medical Foundation, and the W. M. Keck Foundation supported the work in S.D.'s laboratory. H.H. is supported by a National Research Service Awards Postdoctoral Fellowship.
Abbreviations used:
- aPKC
atypical protein kinase C
- ARF6
ADP-ribosylation factor 6
- FIP3 and FIP4
Rab11 family interacting protein
- PAR6
partitioning defective 6 homologue alpha
- PI(3,4,5)P3
phosphatidylinositol (3,4,5)-triphosphate
- PI3Ks
phosphatidylinositol 3-kinases
- PI(4,5)P2
phosphatidylinositol (4,5)-bisphosphate
- PTEN
phosphatase and tensin homologue
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor
- WASP
Wiskott-Aldrich syndrome protein
Footnotes
REFERENCES
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–170. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- Babbey CM, Bacallao RL, Dunn KW. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299:F495–F506. doi: 10.1152/ajprenal.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–332. doi: 10.1016/j.tcb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone I, Selimoglu R, Ozdemir C, Del Nery E, Yeaman C, White M, Camonis J. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 2008;27:2375–2387. doi: 10.1038/emboj.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J. Cell polarity in yeast. Annu Rev Cell Dev Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Hales CM, Lapierre LA, Ham AJ, Oztan A, Apodaca G, Goldenring JR. MARK2/EMK1/Par-1Balpha phosphorylation of Rab11-family interacting protein 2 is necessary for the timely establishment of polarity in Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3625–3637. doi: 10.1091/mbc.E05-08-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard A, Hickson GR, Foley E, O'Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Payrastre B. Phosphoinositide signaling pathways: promising role as builders of epithelial cell polarity. Int Rev Cell Mol Biol. 2009;273:313–343. doi: 10.1016/S1937-6448(08)01808-X. [DOI] [PubMed] [Google Scholar]
- Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Hickson GR, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Lew DJ. Polarity establishment in yeast. J Cell Sci. 2004;117:2169–2171. doi: 10.1242/jcs.00953. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8:467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harb Perspect Biol. 2009;1:a001370. doi: 10.1101/cshperspect.a001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–1269. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan A, Silvis M, Weisz OA, Bradbury NA, Hsu SC, Goldenring JR, Yeaman C, Apodaca G. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Gould GW. Breaking up is hard to do—membrane traffic in cytokinesis. J Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol. 2006;26:727–734. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell WF, Fogarty P, Field CM, Sullivan W. Nuclear-fallout, a Drosophila protein that cycles from the cytoplasm to the centrosomes, regulates cortical microfilament organization. Development. 1998;125:1295–1303. doi: 10.1242/dev.125.7.1295. [DOI] [PubMed] [Google Scholar]
- Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–4663. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop AR, Liu H, Yates J 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland LI, Burgess DR. Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 2004;14:115–118. doi: 10.1016/j.tcb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Toyoshima F, Matsumura S, Morimoto H, Mitsushima M, Nishida E. PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev Cell. 2007;13:796–811. doi: 10.1016/j.devcel.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- VerPlank L, Li R. Cell cycle-regulated trafficking of Chs2 controls actomyosin ring stability during cytokinesis. Mol Biol Cell. 2005;16:2529–2543. doi: 10.1091/mbc.E04-12-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]