Abstract
Background
Infection with Plasmodium berghei is a widely used model of murine malaria and a powerful tool for reverse genetic and pathogenesis studies. However, the efficacy of in vitro reinvasion of erythrocytes is generally low, limiting in vitro studies.
Methods
Plasmodium berghei ANKA-infected blood obtained from a susceptible infected mouse was cultured in various conditions and in vitro parasitaemia was measured every day to evaluate the rate of reinvasion.
Results
High quality culture media were used and reinvasion rates were improved by vigorous orbital shaking of the flask and increasing density of the medium with gelatin.
Discussion
Using these settings, reinvasion of normal mouse erythrocytes by the parasite was obtained in vitro over two weeks with preservation of the infectivity in vivo.
Background
Plasmodium berghei is an African murine malaria parasite isolated by Vincke and Bafort in Katanga (PbNK for New York-Katanga) and in Kasapa (PbANKA for Antwerpen-Kasapa) [1,2]. During natural infection, the blood stages of the parasite undergo asynchronous development with a haploid cycle of 22 hours. Their preference for immature red blood cells (RBC) [1,3,4] suggests that they belong to the "vivax group". The gametocyte production is intense, with 20% of merozoites of each asexual cycle developing into gametocytes within 24 hours in phenylhydrazine-treated mice. Despite the phylogenetic distance, the conservation of housekeeping genes and of biochemical as well as genetic processes between human and murine parasites provided the first justification for their use in malaria research. Infection of mice with Plasmodium berghei and Plasmodium yoelii established the first murine models of malaria, with early studies utilizing these models to test drug efficacy (the Peter's model).
The first attempt to maintain the parasite in vitro was performed by serial passages through rats and tissue culture. These passages were associated with a sharp decrease in the virulence of the parasite in vivo [5]. Early studies developed culture conditions, which permitted the combination of in vivo and in vitro experiments with the same parasites [4-6], and methods for in vitro culture of gametocytes were rapidly described. Liver stages of P. berghei were obtained in the HepG2 cell line, which made it possible to study prophylactic drug efficacy [7]. In vitro development of Plasmodium ookinetes was achieved later on, followed by a complete in vitro development of mosquito phases. Several strains with the same genetic background [8] have been derived from the original isolates with different sensitivity to drugs. They are used for drug testing in maturation assays, molecular studies or evaluation of vaccine efficacy. Selection of mouse strains with various degrees of sensitivity for the parasite paved the way for analysis of the pathophysiology of cerebral malaria (CM) [9,10] and of severe anemia [11].
A new area of interest opened up with the establishment of protocols to knock-out specific genes in the parasite to investigate gene function. However, these reverse genetic studies (reviewed by [12]) are still limited by i) a weak transfection efficiency between 10-3 to 10-4 whereas efficiency reach 10-2 in Toxoplasma gondii), ii) the limited number of markers available to counter select transfectants iii) the mechanism of integration of foreign DNA (nearly always through homologous recombination), and iv) by the time required to select transfected parasites. Indeed the pyrimethamine treatment required to select transfectants is usually performed only 10 to 15 days after injection of the parasite into mice. Using these methods GFP fluorescent parasites were obtained, which created new areas of application. Maintaining parasites in vitro for a long period of time would allow selection of transfectants in vitro. This could pave the way for in vitro studies and facilitate infection of mice by these parasites by reducing competition between wild and mutated parasites in the mouse.
Methods
Maintenance of Plasmodium berghei
The P. berghei ANKA (PbA) strain was obtained from Josef Bafort and maintained [9] by successive infection of CBA/Ca and C57BL/6 mice. Each mouse was inoculated with 106 IRBC intra-peritoneally [10]. For each culture condition, mice were bled 7 days post-infection (when parasitaemia in blood reached more than 8%) and infected red blood cells (IRBC) were cultured after careful removal of leukocytes. Freezing of the IRBC in liquid nitrogen was done in Alsever's solution, as described [10]. All experiments complied with the Australian guidelines for animal research and protocol # K20/7-2006/3/4434 was approved by the University of Sydney Animal Ethics Committee.
Maintenance of in vitro culture
Culture medium was removed daily after centrifugation of the culture at 600 g for 10 min. The pellet was diluted at 2.5% haematocrit with fresh medium. Non-infected red blood cells (NRBC) were obtained from healthy mice, and were added twice a week after platelets had been carefully removed from the blood by two washes and centrifugation at 400 g. Buffy coat was removed following Janse et al [13]. Culture was conducted in 75 cm2 flasks, flushed with gas (5% CO2, 5% O2, 90% N2) and totally sealed. Effect of storage at 4°C of NRBC and of IRBC was checked by keeping cells in the fridge during increasing time before seeding. Selection of mature stages of the parasite was performed on an AutoMacs (Miltenyi Biotec) using the "sensitive" procedure with IRBC diluted 1:20 in PBS. Duration of the parasite life cycle and identification of the stage of the parasite in culture were evaluated by repeated Giemsa-stained thin smears on sampled IRBC.
Stability of the mouse RBC in culture
Starting culture conditions followed those described by Mons et al and Janse et al [6,13]. To avoid mild haemolysis of IRBC in culture, tests were conducted to adapt the density of the medium and the method of shaking. Concentrations of fetal calf serum (FCS) from 5 to 20% and gelatin from 0.5 to 5% were tested. IRBC were added to RPMI1640 medium supplemented by these components and maintained at 37°C for five days. Medium was changed daily and haemolysis was checked optically at each step. In parallel, several ways of agitating the culture were tried, including magnetic stirring and permanent orbital shaking to minimize haemolysis.
Reinvasion study
Rate of reinvasion was calculated by daily determination of the parasitaemia in culture, using thin Giemsa-stained smears. Parasites were counted on 100 fields at ×1, 000 magnification. Reinvasion of newly added red blood cells by the parasites after several days of culture was experimentally confirmed by labeling of i) NRBC with a green fluorescent dye (PKH-67, MINI67-IKT, Sigma) prior to addition to the culture flask and ii) parasites with hydroethidine (red fluorescence). Parasites were labeled by incubation with hydroethidine for 30 min at 37°C, two days after the addition of green-NRBC to the culture. Double-labeled IRBC were observed with a confocal microscope (Olympus FV1000).
Infectivity experiment
In vivo infectivity of the cultured parasites was evaluated by inoculating IRBC in susceptible mice (CBA/Ca or C57BL/6). After 12 days of culture 106 IRBCs were injected intra-peritoneally [10] and parasitaemia was monitored daily on thin tail vein blood smears from day 5 post-infection onwards..
Final composition of the medium
The final composition of the medium was: RPMI1640 (3/4): DMEM-F12 (1/4); bicarbonate 32 mM, HEPES 25 mM; Albumax II 0.5%; glucose 3 g/L; hypoxanthine 200 μM; calcium 2 mM; gelatin 0.1%; choline 1 mM. Chemicals and dyes were purchased from Sigma, except for AlbuMAX II (Gibco n°10021-037), DMEM/F12 (with L-glutamine, Invitrogen n°11330), hydroethidine (Polysciences n°17084) and culture flasks (75 cm2, Corning). Cultures were maintained at 32°C and medium was changed daily. Fresh mouse NRBCs were added twice a week (dilution 1:4). Haematocrit was maintained at 2.5%. Culture flasks were maintained closed and vertical, filled with no more than 30 mL and gased with a 5% CO2, 5% O2, 90% N2 gas mixture. Permanent shaking at 100 rpm on an orbital shaker was applied.
Results
Stability of red blood cells
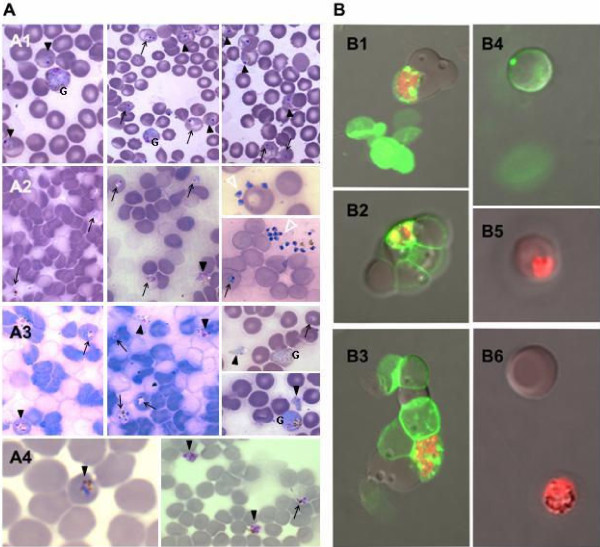
The stability of mouse RBC in culture was the first parameter that needed improving. As previously described, mouse IRBC maintained in culture at 37°C [14] presented mild haemolysis. During this study addition of 20% FCS to the medium significantly reduced this haemolysis, but consequently decreased maturation rate of the parasites after several days of culture. Haemolysis was totally abolished by addition of gelatin. Along the same line, permanent orbital shaking of the flask at 100 rpm achieved substantial mechanical disruption of the late schizonts (Figure 1) without inducing red cell alterations as seen with the magnetic stirrer. Using this setting, large numbers of well-separated merozoites were observed in culture (Figure 1A2) and the position of the merozoites on the RBC as well as merozoites inside RBC was readily detected (Figure 1A2).
Figure 1.
Aspect of in vitro culture of P. berghei in mouse erythrocytes. A/Follow up of the cultures. Schizonts burst and reinvasion is followed up on Giemsa-stained thin smears. Late stage (arrow head), early stage (arrow), merozoites (empty arrow head), gametocytes (G) A1: aspects of culture at day 3 after seeding showing early and late stages and gametocytes; A2: aspects of culture at day 4 with early and late stages and release of merozoites; A3: aspects of culture at day 5 showing early and late stages and release of merozoites; A4: aspects of culture at day 7 showing trophozoites. B/Double fluorescent labelling of erythrocytes and parasites To confirm reinvasion of red blood cells (RBC) by merozoites, RBC are labeled with a green fluorescent dye (PKH-67) prior to addition to the culture flask. They are added to the parasite culture at a ratio of 1:4. After two days of culture, parasites are labeled using hydroethidine (red fluorescent dye). Double-labeled IRBC are observed with a confocal microscope (Olympus FV1000, ×60). B1-3: aspect of IRBC after co-culture with green labelled RBC (bright field, red and green fluorescence merged). B4: green labelled RBC before adjunction to culture; B5-6: hydroethidine labelled parasites in culture (before adjunction of green RBC).
Merozoite production and reinvasion
In RPMI1640 medium supplemented in the same way as for Plasmodium falciparum [15], a decrease in the number of merozoites produced by parasites, i.e. the number of nuclei in schizonts, was observed after three life cycles of the parasite. This suggested insufficiency in nutrient levels, which was not overcome by increasing FCS concentration to more than 5% [13]. Medium composition was modified by addition of DMEM/F12 to RPMI1640. This medium is also compatible with endothelial cell culture. Based on the follow up of in vitro parasitaemia, numerous experiments were conducted, allowing optimization of the culture medium mixture to one part DMEM/F12 and three parts RPMI1640. Higher proportion of DMEM/F12 induced a reduction of the parasitaemia. Similarly, ionic concentration in the medium was adapted in agreement with the mouse serum concentrations [16]. Addition of calcium improved their maintenance in culture. Due to a high rate of parasite multiplication and acidification of the medium, the bicarbonate concentration was increased and culture media was changed daily. The temperature was also decreased to 32°C without apparent change in the duration of the life cycle of the parasite. Reinvasion of newly added red blood cell by the parasites was confirmed by dual labeling of NRBC and parasites with hydroethidine (Figure 1B).
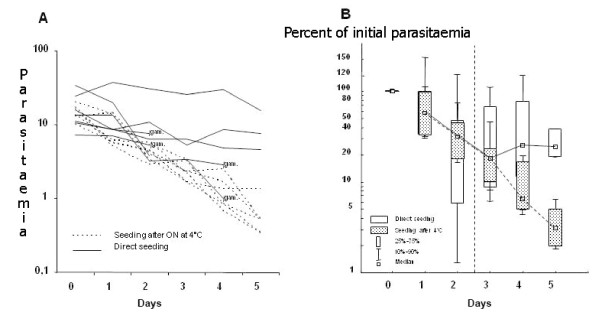
After seeding in culture, parasitaemia could be maintained between 5 to 15% for 5 to 7 days (Figure 2) with fresh blood being added twice a week. After 12 days most of the cultures had still 0.5 to 1% parasitaemia despite a dilution of the IRBC 1:4 with NRBC twice a week. Storage of the normal RBC at 4°C for up to one week before use in culture had no effect on in vitro invasion. In contrast, storage of the IRBC at 4°C, either before seeding or after a couple of days of culture, had a dramatic effect on reinvasion (Figure 2) as already described for merozoites [17]. In most flasks, gametocyte production was rapidly observed and associated with a sharp decrease of the asexual parasitaemia (Figure 2A).
Figure 2.
In vitro reinvasion rate of mouse erythrocytes by P. berghei. A/Effect of the storage of P. berghei ANKA before seeding. Isolates from 6 mice are seeded immediately after bleeding (direct seeding) and 6 after overnight storage at 4°C. Depending on the mouse, some isolates turned rapidly to gametocyte production (gam). No adjunction of RBC was done in the cultures. B/Follow up of parasitaemia after seeding of 18 separate isolates Isolates from 9 mice are seeded immediately after bleeding (direct seeding) and 9 after overnight storage of the infected blood at 4°C (ON). RBC are added after 2 days of culture, as marked by the dotted line (dilution 1:1 RBC:IRBC). After five days of culture most of the isolates seeded immediately after bleeding still harboured more than 30% of their initial parasitaemia (at the seeding day). After 12 days of culture parasitaemia of the isolates (direct seeding) are usually between 1 to 5% (data not shown). This is a different set of mice from Figure 2A.
Selection of mature stage of the parasite, from infected blood was easily obtained using the AutoMacs in the same conditions as those used for P. falciparum. Following separation, parasitaemia could reach up to 90%.
Infectivity
Infectivity of the cultured parasites for the host was assessed by re-injecting IRBC in mice either directly from cultures or after freezing cultured parasites in liquid nitrogen. Repeated cycles of in vitro culture of PbA did not affect the infectivity of the parasite for mice. After 12 days of culture, injection of 106 IRBC in susceptible CBA/J mice readily induced 2% to 6% of parasitaemia within 7 days. Mice with high parasitaemia displayed clear signs of CM.
Discussion
In most protocols, early culture conditions for P. berghei closely resembled those of P. falciparum [13], as the maturation of young blood stages into schizonts is straightforward [18]. Long-term in vitro cultivation of P. berghei was claimed to be obtained by Ramaiya et al [18] at 27°C, and by Smalley [14] who achieved a low multiplication rate at 15°C. However, at 37°C, a decrease in parasite density was always observed as a consequence of the instability of the red blood cells (RBC). During all these studies, re-invasion appeared to be the bottleneck of the long-term cultivation of P. berghei. This was attributed to two main points: i) a restricted ability to invade mature RBC, which required adding blood with a high percentage of reticulocytes to the cultures, and ii) release of merozoites staying packed together or surrounded by RBC membrane preventing them from invading new cells. Schizonts can indeed survive for more than 20 hours in culture and most of the authors used a magnetic stirrer to increase the disruption of the IRBC. During this study, conditions of culture were adjusted to allow an average of 10% in vitro parasitaemia during the first week after seeding and at least 0.5 to 1% after 10 days. Mature stages of the parasite were easily purified using the AutoMacs procedure.
Preference of P. berghei for mouse reticulocytes has been widely reported and in "CM resistant" mice parasitaemia can reach 80% of RBC [10,11] supposed to be mostly reticulocytes. This has implications for in vitro studies of drug sensitivity or cell/cell interactions. In this study, mice used to provide NRBC for culture were not treated before bleeding to increase reticulocytosis. Nevertheless their blood allowed regular reinvasion of RBC by parasites. This protocol for culture of PbA-IRBC in vitro provides significant advantages particularly in allowing in vitro studies of co-culture with endothelial cells or leukocytes in conditions similar to human malaria. Cultivation of the parasites in this setting does not induce any loss of infectivity for mice.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RJ designed the study and performed all the adaptation of the in vitro system and the confocal analysis; FEA and VC performed the in vivo studies; GEG provided support for the study, the scientific background on the Plasmodium berghei ANKA model and helped in the writing of the paper. All authors read and approved the final manuscript.
Contributor Information
Ronan Jambou, Email: rjambou@pasteur.fr.
Fatima El-Assaad, Email: fatima.el-assaad@sydney.edu.au.
Valery Combes, Email: valery.combes@sydney.edu.au.
Georges E Grau, Email: ggrau@med.usyd.edu.au.
Acknowledgements and Funding
This work was supported by funds from the National Health and Medical Research Council of Australia (NHMRC Project Grant 464893), the Australian Research Council (DP0774425) and the Rebecca L. Cooper Medical Research Foundation, Sydney, Australia. The authors also declare that they do not have a commercial or other association that might pose a conflict of interest.
References
- Vincke LH, Lips H. Ann Soc Belg Med Trop. 1948. pp. 97–104. [PubMed]
- Vincke LH, Bafort F. Results of 2 years of observation of the cyclical transmission of Plasmodium berghei. Ann Soc Belges Med Trop Parasitol Mycol. 1968;48:439–454. [PubMed] [Google Scholar]
- Gaillard G. Concours Medical. 1949. p. 2395. [PubMed]
- Garnham PC. The structure of early sporogonic stages of Plasmodium berghei. Ann Soc Belges Med Trop Parasitol Mycol. 1965;45:259–264. [PubMed] [Google Scholar]
- Weiss ML, Degiusti DL. Modification of a malaria parasite (Plasmodium berghei) following passage through tissue culture. Nature. 1964;201:731–732. doi: 10.1038/201731a0. [DOI] [PubMed] [Google Scholar]
- Mons B, Janse CJ, Boorsma EG, Van der Kaay HJ. Synchronized erythrocytic schizogony and gametocytogenesis of Plasmodium berghei in vivo and in vitro. Parasitology. 1985;91:423–430. doi: 10.1017/S0031182000062673. [DOI] [PubMed] [Google Scholar]
- Strome CP, De Santis PL, Beaudoin RL. The cultivation of the exoerythrocytic stages of Plasmodium berghei from sporozoites. Vitro. 1979;15:531–536. doi: 10.1007/BF02618155. [DOI] [PubMed] [Google Scholar]
- Saul A, Prescott N, Smith F, Cheng Q, Walliker D. Evidence of cross-contamination among laboratory lines of Plasmodium berghei. Mol Biochem Parasitol. 1997;84:143–7. doi: 10.1016/S0166-6851(96)02779-X. [DOI] [PubMed] [Google Scholar]
- Rest JR. Cerebral malaria in inbred mice, a new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986;137:2348–2354. [PubMed] [Google Scholar]
- Evans KJ, Hansen DS, van Rooijen N, Buckingham LA, Schofield L. Severe malarial anemia of low parasite burden in rodent models results from accelerated clearance of uninfected erythrocytes. Blood. 2006;107:1192–1199. doi: 10.1182/blood-2005-08-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Mons B, Croon JJ, van der Kaay HJ. Long-term in vitro cultures of Plasmodium berghei and preliminary observations on gametocytogenesis. Int J Parasitol. 1984;14:317–320. doi: 10.1016/0020-7519(84)90083-3. [DOI] [PubMed] [Google Scholar]
- Smalley ME, Butcher GA. The in vitro culture of the blood stages of Plasmodium berghei. Int J Parasitol. 1975;5:131–132. doi: 10.1016/0020-7519(75)90018-1. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Mouse Phenotype data base. http://phenome.jax.org
- McAlister RO. Time-dependent loss of invasive ability of Plasmodium berghei merozoites in vitro. J Parasitol. 1977;63:455–463. doi: 10.2307/3280000. [DOI] [PubMed] [Google Scholar]
- Ramaiya ML, Kamath VR, Renapurkar DM. Long-term in vitro cultivation of Plasmodium berghei. Int J Parasitol. 1987;17:1329–1331. doi: 10.1016/0020-7519(87)90099-3. [DOI] [PubMed] [Google Scholar]