Abstract
Objective
To assess the performance of rapid HIV antibody tests when used as part of a home-based community-wide counselling and testing strategy in northern Malawi.
Design
A cross-sectional population survey of HIV infection, 2007-2008.
Methods
Adults aged 15 or over in a demographic surveillance area were counselled and then offered an HIV test at their home by government certified counsellors. Two initial rapid tests (Detemine™ and Uni-Gold™) were performed on all samples, and a third, tie-breaker test (SD Bioline) used to resolve discordant results. All people who wanted to know were post-test counselled and informed of their results, with referral to local clinical services if found to be HIV positive. Laboratory quality control comprised re-testing all positive and every tenth negative venous blood sample collected.
Results
A total of 10819 adults provided venous blood samples for HIV-testing, of whom 7.5% (813) were HIV positive. The accuracy of the parallel testing strategy used was high, with 99.6% sensitivity, 100.0% specificity, 99.9% positive predictive value and 99.9% negative predictive values.
Conclusion
Face-to-face rapid testing by health personnel with minimum training, at the clients’ home performs well when used on a wide scale in the community setting.
Keywords: HIV serodiagnosis, quality control, home care, population surveillance, Malawi
Introduction
Rapid HIV antibody tests are widely used for a range of public health and clinic purposes. The rapid tests are relatively easy to perform with minimum equipment and training; and results can typically be obtained within 20 minutes, making the rapid tests well-suited for use in settings where trained laboratory technicians and specialist equipment may be limited [1,2]. In Africa, evaluation of the performance of rapid tests in the research setting, including in mobile and community-based health clinics and information centres, have shown high sensitivity and specificity [1,3,4]; however their performance in the home-based community setting is unclear. Uncertainties have, moreover, been expressed over the accuracy of rapid tests performed in mobile laboratories set up in local buildings or tents in Rakai District, Uganda [5]. In that report an unacceptably high rate of false positive results was found (43.7%, 129 of 295, total 1517 samples), attributed by the authors to the classification of weak positive bands from Determine™ HIV-1/2/O (Abbott Laboratories, Abbott Park, IL) and Uni-Gold™ Recombinant HIV-1/2 (Trinity Biotech, Bray, Ireland) tests as a positive result, when in fact they should be negative.
In Malawi, rapid tests have been incorporated into the national scale up of HIV Testing and Counselling (HTC) and the provision of anti-retroviral therapy (ART) through government services, both of which are free at the point of delivery [6]. With an increasing emphasis on universal testing and promotion of early initiation of antiretroviral therapy [7], a greater amount of HIV testing will need to be performed at, or closer to the home to increase numbers of tests and access for those least able to seek facility –based testing [8]. This requires confidence in the testing procedure. Home based testing is being introduced in several African countries [9,10,11,12] as a means to increasing uptake of testing [13]. Whilst making testing accessible by this approach appears to be feasible and desired by individuals, accuracy of this procedure will need to be carefully and regularly assessed, to ensure accuracy is equal or better than laboratory based testing. We report our experience of home-based community HIV rapid testing in Karonga District, northern Malawi.
Methods
Setting and Design
The Karonga Prevention Study (KPS) is a community-based research site, situated in Chilumba, a small lakeshore settlement in Karonga district in rural northern Malawi. The site includes a laboratory that functions to international standards. A population of approximately 33,500 individuals in an area close to Chilumba has been under continuous demographic surveillance since September 2004 [14]. HIV prevalence in adults in this area in 2006 was estimated at 11.6% [15]. Here we report on an home-based cross-sectional survey of HIV in adults, which took place in the same area between September 2007 and October 2008, as part of a four year study to assess the impact of ART on HIV transmission in the study population.
All homes in the study area were visited village-by-village; in each village, community sensitization in relation to the study took place shortly before home visits. Any person in the study population who was aged 15 years or over at the time of the household visit was eligible for inclusion in the survey. The survey interviews and HTC were conducted by counsellors who had been trained and certified by Ministry of Health staff to perform HIV counselling, whole-blood rapid testing and specimen collection by finger-prick, using standard training procedures [6]. At each home the study was introduced and explained to all members, participants who, after pre-test counselling, consented to HIV-testing were asked to provide a venous blood sample, with finger-pricks offered as an alternative if the participant preferred. The whole blood was then tested immediately for HIV at the home, all participants who wanted to know were post-test counselled and informed of their results, with referral to local clinical services if found to be HIV positive.
Community-based rapid testing
In Malawi, a whole-blood testing strategy for HIV-1 and HIV-2 is recommended and can be performed by non-laboratory health personnel with minimum basic training, provided that minimum standards for quality assurance are in place [6]. Until May 2008 a parallel testing strategy was used. This strategy is based on performing two initial rapid tests simultaneously on all samples and a third rapid test used only if the results are discordant; if any two rapid test results are concordant, that result is taken as the final rapid test result and reported to the client. After May 2008 a serial testing strategy was introduced after analysis and research which determined that this approach was appropriate [6]. Under the serial testing strategy, one initial rapid test is performed on all samples, a second rapid test is only performed if the first result is positive or inconclusive, and a third performed only if the first two results are discordant. Under both testing strategies the approved rapid tests combined Determine™ HIV-1/2 (Abbott Japan Co Ltd, Tokyo, Japan) with Uni-Gold™ HIV (Trinity Biotech PLC, Bray, Ireland) as the first and second rapid tests respectively, using SD Bioline HIV 1/2 3.0 (Standard Diagnostics Inc, Kyonggi-do, Korea) as a third tie-breaker. All three rapid tests used in Malawi are a one step, visual test for the detection of antibodies to HIV types 1 and 2 in human serum, plasma or whole blood; the test kits (including test device and reagent) can be stored between 2-27°C; they have been reported to have sensitivities and specificities of, respectively, 100% and 99.4% (Determine™), 100% and 100% (Uni-Gold™), and 100% and 99.3% (SD Bioline) when evaluated under standard operational conditions [16,17].
The testing protocol followed by the HIV sero-survey followed the Malawi Ministry of Health guidelines for whole-blood parallel rapid testing, throughout the entire survey round, except that whole venous blood (taken by paramedical staff who had been certified by the Malawi Medical Council to perform phlebotomy), was used to perform the rapid test rather than finger-prick specimens, as this facilitated further laboratory-based HIV-related viral testing, including quality control testing. Determine™, Uni-Gold™ and SD Bioline test kits were stored in a cold room in the laboratory at 4-8° C until ready for use in the community setting, then carried at ambient temperatures to the field; counsellors took with them to the field sufficient supplies to meet at least one day’s needs and restocked when necessary.
At the home, counsellors were required to use a flat, dry surface for the rapid tests according to manufacturers’ guidelines, and to protect the rapid tests from environmental contamination by keeping the test devices inside a closed box while waiting for the results. The blood sample was collected in a S-Monovette® EDTA tube (Sarstedt, Nümbrecht, Germany), and from this placed immediately on the test device using a disposable plastic pipette. A mechanical timer was used, and the counsellors were instructed to read and interpret rapid test results 20 minutes after having added reagents, consistent with the manufacturers’ instructions. For all rapid test devices, counsellors were instructed that any band in the positive region was to be considered reactive to HIV irrespective of the strength of the band; if the rapid test was uninterpretable by virtue of a failed control band it was to be considered inconclusive, according to the manufacturers’ instructions. Tests were performed for one participant at a time, or together for a couple, in which latter situation the test devices were labelled with a marker pen to distinguish between the male and female partners and hence reduce the risk of muddling of test results. Test results were transcribed onto a dedicated rapid-test form, with the outcome of each interview identified by a unique study number, rather than by the participant’s name. Specimens were transported at ambient temperature to the laboratory at KPS at the end of the working day, refrigerated at 2-8°C overnight and then registered the next day for storage and confirmatory testing.
Laboratory-based quality control
Laboratory-based quality control at KPS was conducted by systematically re-testing plasma from all samples that were positive, inconclusive or for which the initial two rapid tests were discordant using the parallel testing strategy, and from every tenth negative sample collected. The first 500 negative community samples collected in 2007 were re-tested, in view of findings from Rakai, Uganda reported early the same year. The quality control “gold standard” HIV tests comprised two parallel HIV antibody tests: the Edgware modification [18] of the Serodia® HIV-1/2 (Fujirebio Inc, Tokyo, Japan) particle agglutination test and the Vironostika® HIV Uni-Form II plus O (bioMérieux bv, Boxtel, The Netherlands) EIA test, and was performed blind to the community rapid-test results. Initial discordance in the laboratory between the Edgware and Vironostika® tests was resolved by repeating the Edgware and Vironostika® tests for that sample. Discordance between the laboratory quality control outcome and the community rapid-test results was addressed by laboratory-based repeat rapid-testing testing of first that sample, and then all samples collected on the same day for any clinical or research reason, in order to assess the likelihood of results being wrongly transcribed or of specimen mix-ups caused by mislabelling the specimens or data forms in the field or laboratory i.e. ensuring no positive samples from that day which had been reported negative and could therefore account for a false positive result on laboratory QC. All results were reviewed, if necessary arranging a follow-up visit to the participant and, in the case of samples that could not be resolved locally, sending the sample to a reference laboratory before assigning the final HIV summary result.
External quality assurance at KPS was based on retesting a panel of six specimens, provided thrice annually by the United Kingdom National External Quality Assurance Service for Microbiology (UK NEQAS). Ministry of Health staff and national reference-laboratory personnel were also invited to observe testing practices, and retest a sample of specimens. Re-testing of finger-prick samples was not undertaken.
Statistical Analyses
We assessed the performance of the rapid tests by comparing the results of community-based parallel testing with those from laboratory-based quality control. Confidence intervals were calculated using exact methods. All analyses were conducted in STATA 9.2 software (StataCorp, College Station, Texas, USA).
Results
Between September 2007 and October 2008, a total of 11172 of 16894 eligible adults agreed to HIV testing. Of these, 10819 provided a venous blood sample for HIV testing and are included in subsequent analyses; data for 331 participants who preferred to give finger-prick samples and 22 for whom the original rapid-test results or specimen were missing, are excluded from analyses and not considered further here.
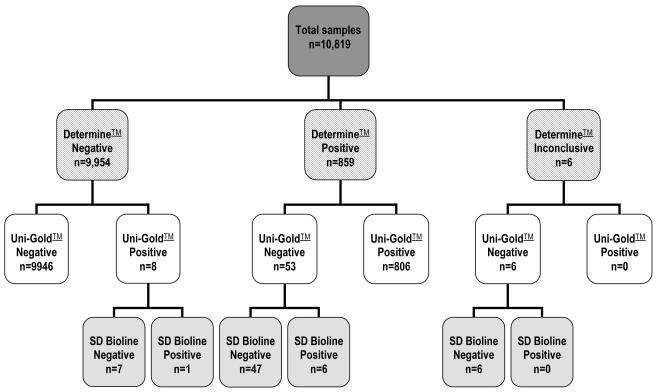
The rapid test results using the parallel strategy are shown in Figure 1. Overall, 7.5% (813 of 10819) of the clients tested were HIV positive; the final outcome of all rapid tests was positive or negative; with any inconclusive initial test results resolved through applying the testing algorithm. In over 99.4% of samples (10752 of 10819) there was concordance between the Determine™ and Uni-Gold™ test result. Overall, a total of 9954 samples tested negative by Determine™, of which eight were positive with the Uni-Gold™ test result; only one remained positive after the SD Bioline tie-break was used. None of the 9954 would have been further tested after the initial Determine™ test had a serial testing strategy been used, so one HIV positive case would have been missed.
Figure 1.
Outcome of rapid HIV testing by Determine™ test result
A total of 2911 blood samples collected were subjected to internal laboratory-based quality control; these comprised all 813 positive samples and 2098 negative samples identified by the community-based parallel testing strategy, and included 67 samples for which the initial two rapid tests were discordant. The performance of community-based rapid HIV testing is presented by rapid test strategy in Table 1, using the parallel Edgware and Vironostika® laboratory quality control tests as the gold standard. The parallel rapid testing strategy actually used gave a high sensitivity and specificity, and high positive and negative predictive values. In only four samples there was discordancy between the overall laboratory and the community HIV test result; for one of these samples the specimen was brought back to the office for testing as the participant did not wish to know their results; re-testing of all specimens collected on the same day did not reveal any specimen mix-up arising from mislabelling but it is possible that the interviewer tested the wrong specimen at the office or incorrectly transcribed the rapid test results. For the remaining three samples discordancy is more likely to have arisen as a result of sample peculiarities; there having been discordancy between the initial Determine™ and Uni-Gold™ rapid tests conducted at the household, between the initial Edgware and Vironostika® quality control tests conducted in the laboratory, and between the laboratory rapid re-tests. In terms of rapid test performance a similar outcome would have been reached had the same samples that were selected for laboratory quality-control been tested using the national serial testing strategy.
Table 1.
Performance of community-based rapid HIV testing
| Laboratory quality control result | Performance % (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|
| Rapid test result (community) |
Negative | Positive | Total (%) | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
|
| |||||||
| Parallel testing a | |||||||
| Negative | 2095 | 3 | 2098 | 99.6(98.9-99.9) | 100.0(99.7–100.0) | 99.9(99.3–100.0) | 99.9(99.6–100.0) |
| Positive | 1 | 812 | 813 | ||||
| Serial testing b | |||||||
| Negative | 2095 | 4 | 2099 | 99.5(98.7-99.9) | 100.0(99.7–00.0) | 99.9(99.3–100.0) | 99.8(99.5–99.9) |
| Positive | 1 | 811 | 812 | ||||
| Uni-Gold™ alone | |||||||
| Negative | 2089 | 8 | 2097 | 99.0(98.1-99.6) | 99.7(99.3–99.9) | 99.1(98.2–99.7) | 99.6(99.2-99.8) |
| Positive | 7 | 807 | 814 | ||||
| Determine™ alone | |||||||
| Negative | 2043 | 3 | 2046 | 99.6(98.9-99.9) | 97.5(96.7–8.1) | 94.5(92.8–96.0) | 99.9( 99.6–100.0) |
| Positive | 47 | 812 | 859 | ||||
| Inconclusive | 6 | 0 | 6 | ||||
| Total | 2096 | 815 | 2911 | ||||
Parallel testing strategy: actual strategy, tests used Determine™, Uni-Gold™ and SD Bioline, result negative defined as non-reactive for at least two of the three rapid tests; result positive defined as reactive for at least two of the threerapid tests
Serial testing strategy: theoretical strategy, tests used Determine™, Uni-Gold™ and SD Bioline tests, result negative defined as non-reactive for Determine™ alone or nonreactive for both Uni-Gold™ and SD Bioline; result positive defined as reactive for Determine™ and Uni-Gold™ or reactive for Determine™ and SD Bioline
We estimated the cost of rapid test kits in the first survey round at just over $26,019.20, based on a per-test median international transaction price of $0.80 for Determine™ and SD Bioline, and $1.60 for Uni-Gold™ [19]; the equivalent cost under a serial testing strategy, had this been applied would have been $10,086.40. Alternatively in our setting the cost of test kits per HIV-infection diagnosed would be $32.0 under the parallel system and $12.4 under the serial test system.
Discussion
Our results provide evidence that rapid-testing conducted in a “point-of-service” community-setting by non-laboratory health personnel with adequate training, combined with a system that ensures quality control, accurately determines the presence or absence of antibodies to HIV. At the level of the individual tests discordance rate between Determine™ and Uni-Gold™ was low; when used together in a parallel or serial strategy, with SD Bioline as a tie-breaker test, we have found that in this setting, the positive predictive value of the rapid tests (whether in a parallel or serial strategy) when compared to the parallel Edgware and Vironostika® laboratory testing strategy as gold standard is high. We are confident that a result in the community can be reported to the client, and that there is no need to discriminate between light and dark bands, which is difficult to do objectively and therefore has practical limitations.
In our study population there has been no indication of the problems with rapid test performance with Determine™ and Uni-Gold™ that had been observed elsewhere. The reasons for this are unclear; the performance of rapid tests for HIV antibody might be affected by early or acute HIV infection, during the period preceding the emergence of antibody, but it is unlikely this could have played a major role when incidence is substantially less than 1% per year. Variation in the test accuracy between different HIV subtypes (such as the dominant HIV-1 subtype C in Malawi, compared with subtypes A and D, and AD recombinants found in Uganda) and in the potential for cross-reactions with a different spectrum of endemic infections may in part be responsible [5]. However a more likely explanation for general variation in rapid test performance in different African settings, may relate to transport and storage of test kits and specimens and conditions under which the rapid tests were performed. In Malawi, rapid test kits were used for home-based whole blood HIV testing, contributing to optimal conditions for the collection, labelling and testing of specimens and confirmation of results; the use of whole blood from a venous specimen rather than a direct finger-prick guaranteed the required volume of whole blood. There is little in the literature comparing finger prick with whole blood for HIV rapid testing in situations of service delivery, however it would seem prudent to ensure adequate attention is paid to training staff in the optimal performance of finger pricks with correct lancets to ensure adequate volumes of blood. The performance of CD4 T-cell counts has been demonstrated to be reproducible when done in this way [20]. We did not incorporate a direct comparison of finger prick versus venous blood as this was not the primary purpose of this study, but subsequent work will need to consider this. In contrast, situations involving the transportation of the clients’ specimens away from the point of specimen collection can increase the opportunity for mislabelling of specimens, and where the testing process relies on the prior preparation of laboratory serum samples under field conditions, this may also affect the technical performance of the rapid tests used. Overall, given appropriate attention to the detail of storing and transporting of test kits, which are reasonably robust and temperature stable, and to the collection and testing of an appropriate volume of blood, we feel it likely that our results can be reproduced in service settings.
Our consideration of the performance of home-based community-testing under a serial testing strategy indicates that one participant out of the 10819 specimens tested would have been given a different result had the parallel testing strategy been used. While we found that in our population a parallel testing strategy provides HIV test results with high sensitivity, specificity and predictive values; a serial testing strategy affords very nearly the same level of accuracy at approximately 40% of the test kit cost. Our study has demonstrated that rapid HIV tests perform well when used on a wide scale in the home-based community setting. The recorded prevalence in this study of 7.5% was lower than the estimated 11.6%, but this was primarily explained by refusals to test in those who already new their status to be positive from previous rounds of testing, thus lowering the measured prevalence form previous surveys [15]. The high sensitivity of Determine™ and high specificity of Uni-Gold™ make them very well suited as screening and confirmatory tests respectively; when combined in series, with a system that ensures the appropriate storage of test devices and reagents, adherence to protocol when conducting rapid tests, and regular supervision of operator procedures to ensure that sample mix-ups and the potential reporting of wrong results do not occur, face-to-face rapid testing by government trained staff at the clients’ home can be of high accuracy. Laboratory based quality-control of field results should also be an essential component of home based testing, as it should of any system that is relying on remotely performed rapid HIV tests. This helps to ensure the quality of the tests by direct confirmation of accuracy and also by the indirect effects of ensuring operators are subjected to supervision of their testing accuracy and are motivated by this. The intensity of laboratory quality control in routine practice is not immediately obvious from our results. Our approach of all positives and a random selection of similar numbers of field test negatives is probably unnecessarily intense. The actual numbers of quality control tests may be less important in a public health based testing initiative than ensuring random sampling and feedback is done on a regular basis and on random samples to ensure that counsellors can be confident that this process is on-going and helpful.
As an initiative to increase access to and uptake of “point-of-service” HIV testing and consequent referral for appropriate care, we can be reassured that home-based counselling and rapid HIV testing is acceptable to both counsellors and their clients in the community setting.
Acknowledgements
This paper was written by AMM, RN, EB, JS, BN, JRG, ACC, NF. AMM prepared the manuscript, did statistical analysis and with NF was responsible for the scientific leadership and management of the study in Malawi. RN was responsible for laboratory assays and quality control. EB and BN supervised the field work and data collection. JS was responsible for data management and with ACC provided key input into design and implementation of data systems for laboratory testing in Malawi. JRG and NF obtained funding for the study, and with ACC provided critical comment on the manuscript. We thank all the people living in Chilumba and the surrounding area who participated in the study; we also thank the Traditional Authority Wasambo and his village headmen for their support of the study, and KPS field, laboratory, data-office, administrative and support staff for their work. Funding for this study was provided by the Wellcome Trust, United Kingdom.
Funding: Funding for this study was provided by the Wellcome Trust, United Kingdom.
Footnotes
Conflicts of interest: None
Conflict of interest. All the authors confirm that they have no conflict of interest.
Ethical considerations. This study was conducted in full accordance with ethical principles. Blood samples were obtained by venepuncture or fingerprick. Data and blood samples were obtained with the voluntary informed consent of the subjects, and ethical approval for this study was given by the Malawi National Health Sciences Research Committee (protocol number 419), and the ethics committee of the London School of Hygiene and Tropical Medicine (protocol number 5081).
References
- 1.Plate DK. Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS Res Hum Retroviruses. 2007;23:1491–1498. doi: 10.1089/aid.2007.0020. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation . Rapid HIV tests: guidelines for use in HIV testing and counselling services in resource-constrained settings. WHO; Geneva (Switzerland): 2004. [Google Scholar]
- 3.Everett DB, Baisley K, Changalucha J, Vallely A, Watson-Jones D, et al. Suitability of simple human immunodeficiency virus rapid tests in clinical trials in community-based clinic settings. J Clin Microbiol. 2009;47:1058–1062. doi: 10.1128/JCM.01998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayhood MK, Afwamba IA, Odhiambo CO, Ndanu E, Thielman NM, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and determine HIV-1/2 rapid human immunodeficiency virus antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–3951. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RH, Makumbi F, Serwadda D, Lutalo T, Nalugoda F, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ. 2007;335:188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Government of Malawi . Guidelines for HIV Testing and Counselling (HTC) 3rd edition Ministry of Health; Lilongwe (Malawi): 2009. [Google Scholar]
- 7.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 8.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 10.Bateganya MH, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counseling and testing in developing countries. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD006493.pub2. CD006493. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009;6:217–223. doi: 10.1007/s11904-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14:849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 13.Colombet I, Saguez C, Sanson-Le Pors MJ, Coudert B, Chatellier G, et al. Diagnosis of tetanus immunization status: multicenter assessment of a rapid biological test. Clin Diagn Lab Immunol. 2005;12:1057–1062. doi: 10.1128/CDLI.12.9.1057-1062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath N, Kranzer K, Saul J, Crampin AC, Malema S, et al. Estimating the need for antiretroviral treatment and an assessment of a simplified HIV/AIDS case definition in rural Malawi. AIDS. 2007;21(Suppl 6):S105–113. doi: 10.1097/01.aids.0000299417.69432.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organisation . HIV assays: operational characteristics. Report 12: simple/rapid tests, whole blood specimens. WHO; Geneva (Switzerland): 2002. [Google Scholar]
- 17.World Health Organisation . HIV assays: operational characteristics. Report 14: simple/rapid tests. WHO; Geneva (Switzerland): 2004. [Google Scholar]
- 18.Sterne JA, Turner AC, Fine PE, Parry JV, Lucas SB, et al. Testing for antibody to human immunodeficiency virus type 1 in a population in which mycobacterial diseases are endemic. J Infect Dis. 1995;172:543–546. doi: 10.1093/infdis/172.2.543. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organisation . Transaction prices for antiretroviral medicines and HIV diagnostics from 2008 to October 2009. Summary Report from the Global Price Reporting Mechanism November 2009. WHO; Geneva (Switzerland): 2009. [Google Scholar]
- 20.MacLennan CA, van Oosterhout JJ, White SA, Drayson MT, Zijlstra EE, et al. Finger-prick blood samples can be used interchangeably with venous samples for CD4 cell counting indicating their potential for use in CD4 rapid tests. AIDS. 2007;21:1643–1645. doi: 10.1097/QAD.0b013e32823bcb03. [DOI] [PMC free article] [PubMed] [Google Scholar]