Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults. Though modern treatments are highly effective in most CLL, a challenging subgroup of patients shows poor response to standard regimens and a survival of less than two years.1–3 Identifying chemorefractory patients early, ideally before treatment, and designing therapeutic strategies tailored to overcoming chemorefractoriness remain key issues toward an optimized management of CLL. Early studies of the molecular genetics of CLL revealed that deletions of cytobands 17p13 and 11q22-q23 are major determinants of chemorefractoriness in this leukemia (Figure 1).2 Subsequent studies have reported that disrupting mutations of TP53, the tumor suppressor gene consistently affected by 17p13 deletions, predict chemorefractoriness in a fashion similar to, but independent of, 17p13 deletion, making TP53 disruption by mutation and/or deletion the predominant mechanism of chemorefractoriness in approximately 40% of CLL destined to fail treatment (Figure 1).4,5 In recent years, major improvements in sequencing technologies have provided the opportunity to comprehensively examine the CLL genome.6–8 In fludarabine-refractory CLL, this approach has allowed the identification of previously unrecognized mutated genes, including NOTCH1, the keystone of the NOTCH signaling pathway, and SF3B1, that encodes a component of the RNA splicing machinery (Figure 1).6,8 Mutations of NOTCH1 and SF3B1: i) are virtually absent in monoclonal B-cell lymphocytosis and occur at a low rate at CLL presentation, where they identify poor survival patients; ii) are recurrent in chemorefractory CLL; and iii) tend to be mutually exclusive with TP53 disruption, suggesting that they represent alternative mechanisms contributing to chemorefractoriness.6,8–10 Mutations of NOTCH1 disrupt the protein domain required to switch off NOTCH1 signaling, and may impair the cytotoxicity of fludarabine.6,7 Mutations of SF3B1 might contribute to chemorefractoriness by favoring alternative splicing of genes related to cancer, as suggested by the observation that SF3B1 regulates the production of the anti-apoptotic isoform of BCLxL.8,11 Overall, this novel information on the genetics of high-risk CLL has led to the realization that the molecular basis of fludarabine-refractoriness in this leukemia is more complex than initially thought, and might involve several alterations in addition to deletions of 17p13 and 11q23-q23 (Figure 1).6,8,10
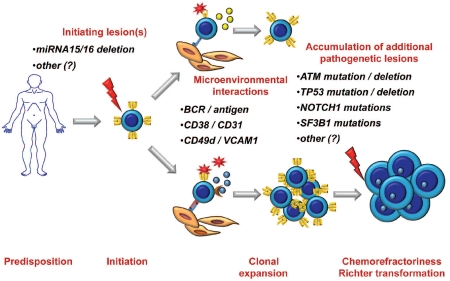
Figure 1.
A proposed model of CLL multistep pathogenesis and of its clinical implications. Although the overwhelming majority of cases do not run in families, the genetic background of the host might favor predisposition to CLL in a fraction of patients. A founding genetic lesion, conceivably represented by loss of miRNA15/16 in a substantial fraction of CLL, initiates clonal expansion, that is then favored and promoted by interactions of leukemic cells with antigens and/or the microenvironment. During their clinical course, some patients gain molecular alterations of genes (TP53, ATM, NOTCH1, SF3B1, and possibly other) that confer a higher degree of clinical aggressiveness which translates into refractoriness to conventional treatments and potential of transformation to diffuse large B cell lymphoma known as Richter’s syndrome.
Deletions of 11q23-q23 almost invariably include the ATM (for Ataxia Teleangiectasia Mutated) gene. This is regarded as the relevant tumor suppressor locus affected by this chromosomal abnormality (Figure 2).2 ATM is a large gene that consists of 66 exons spanning 146 kb of genomic DNA, and encodes a 370 kD nuclear phosphoprotein sharing homology with phosphatidylinositol 3-kinase (PI-3-K).12 Similar to other PI-3-K related proteins, ATM functions in controlling the integrity of DNA repair and recombination, and regulates cell cycle progression.12 Mutations in ATM are responsible for the autosomal recessive disorder ataxia teleangiectasia, a condition that predisposes to development of lymphoid neoplasms, with a risk for leukemia approximately 70 times higher than in the normal population.12 Mutations of ATM in CLL frequently, though not exclusively, affect the PI-3-K domain, which is highly conserved among ATM-related proteins and is crucial for the protein kinase activity of ATM.13–15 Due to the large size of the ATM gene and to difficulties in unequivocally distinguishing population polymorphisms versus pathogenetic mutations, ATM mutation studies in CLL have been challenging and have left several issues unresolved.
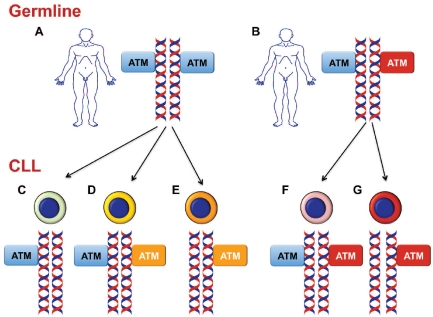
Figure 2.
Mechanisms of ATM structural alterations in CLL. Upper panel: most CLL patients carry normal (represented by blue boxes in the figure) ATM genes in their germline DNA (A), although some cases may harbor germline mutations of ATM (represented by red boxes in the figure) (B). Lower panel: at some stage during the clinical history of the disease, CLL cells may acquire somatic alterations of ATM, alternatively constituted by: ATM deletion in the presence of a residual normal ATM allele (C); somatically acquired ATM mutations (represented by orange boxes in the figure) in the presence of a residual normal ATM allele (D); biallelic ATM inactivation through deletion of one allele and somatic mutation (represented by orange boxes in the figure) of the residual allele (E); biallelic ATM inactivation through somatically acquired deletion of one allele and germline mutation (represented by red boxes in the figure) of the other allele (G). In some cases, only a germline ATM mutation (represented by red boxes in the figure) is detected in CLL cells (F).
In this issue of Haematologica, the two reports by Guarini et al.16 and by Skowronska et al.17 provide new knowledge on ATM disruption in CLL, and make an important contribution to the systematic clarification of the role of ATM mutations in the disease. In their report, Guarini et al. have systematically approached the issue of ATM mutations in CLL patients with and without deletion of 11q22-23.16 The study was based on a sizeable number of cases that were methodically screened for mutations of the entire coding sequence of ATM, represented by 62 exons, as well as for 11q22-23 deletions. The data show that, by combining mutations and deletions, genetic lesions of ATM occur in 25% of diagnostic samples of CLL.16 This frequency makes ATM alterations the most common genetic alteration predicting poor outcome at CLL presentation. Importantly, mutations of ATM occurred also in the absence of 11q22-23 deletions, indicating that ATM disruption in CLL may occur by mutation, deletion, or a combination of both events (Figure 2).16 This scenario is reminiscent of the mechanisms of TP53 disruption in CLL, and poses the diagnostic dilemma of correctly recognizing patients with mutations in the absence of deletions.4,5,16 In fact, for ATM and TP53, both mutations and deletions impact on prognosis, but whereas deletions can be easily and rapidly recognized by FISH studies, the identification of mutations requires DNA sequencing analysis that, in the absence of well defined mutational hotspots, might be particularly demanding especially for very large genes such as ATM. The pathogenicity of the ATM mutations detected by Guarini et al. in CLL was formally demonstrated by elegant model studies of the ATM protein that unambiguously localized the mutations to functionally relevant sites, including the ATP-binding pocket of ATM.16 Having identified a subset of CLL with ATM mutations in the absence of ATM deletions, Guarini et al. exploited gene expression profiling to document that ATM mutated CLL displays a common signature specifically associated with this disease genotype and characterized by the differential expression of genes potentially relevant to disease pathogenesis.16
Beside being somatically acquired, ATM mutations in CLL may be already present in the patient germline DNA (Figure 2). The true significance of germline ATM mutations in CLL pathogenesis has been a matter of longstanding debate, that is now largely solved by the new information reported by Skowrosnska et al. in this issue of the journal.17 To gain a proper understanding of the role of ATM disruption in CLL, the authors have investigated germline ATM mutations in patient cohorts with and without 11q22-q23 deletion using a highly stringent methodological approach to distinguish ATM germline pathogenic mutations versus rare population polymorphisms. Overall, the data by Skowronska et al. document that, compared to controls, the frequency of germline ATM mutations is increased in patients with 11q23-q23 deletions, but not in patients with normal 11q22-q23 alleles.17 Most CLL carrying germline ATM mutations presented with advanced stage at diagnosis, and carried other unfavorable prognostic markers, including unmutated IGHV genes.17 The model proposed by Skowronska et al. is consistent with a multistep process of ATM disruption in CLL, and shows that patients with germline ATM mutations may subsequently acquire 11q22-q23 deletions encompassing the ATM locus on the other allele, leading to a complete loss of ATM function and a full blown aggressive clinical phenotype.17 According to this model, Skowronska et al. argue that ATM germline mutations predispose to rapid disease progression through ATM loss, rather than being involved in disease initiation.17
The two reports by Guarini et al.16 and Skowronska et al.17 clarify and unequivocally and substantiate the role of ATM mutations in CLL pathogenesis and in determining the clinical aggressiveness of the disease, but also pose new questions that need to be addressed. The first question stems from the observation that only 20–40% of 11q22-q23 deletions associate with ATM mutations on the remaining allele. What happens to the remaining allele in CLL with 11q22-q23 deletions but without ATM mutations is still unknown. Although ATM haploinsufficiency might be a putative explanation, it is also possible that genes other than ATM might be affected on the remaining allele. A search for structural alterations of alternative genes mapping to 11q22-q23 should thus be encouraged to address this issue. A second unresolved question concerns the exact prognostic role of ATM mutations in fit CLL patients treated with immunochemotherapy, since clinical trials have shown that FCR (fludarabine, cyclophosphamide, rituximab), but not FC (fludarabine, cyclophosphamide), is able to overcome the chemorefractoriness associated with 11q22-q23 deletions.18,19
Thanks to the application of next generation sequencing to CLL investigations, research on the molecular pathogenesis of high-risk CLL has advanced at a sustained pace during the last few months and hopefully will progress further in the near future. In addition to ATM and TP53, cancer genes recurrently affected by mutations in high-risk CLL now also include NOTCH1 and SF3B1 (Figure 1).6–8,10 Mutations of all these genes predict poor prognosis in consecutive CLL series, mainly because of refractoriness to standard treatment.6,7,8,10 The occurrence of TP53 mutations in CLL is a well codified indication for treating patients with alemtuzumab-containing regimens followed by transplant consolidation.2 But will mutations of ATM, NOTCH1 and SF3B1 translate into clinically meaningful molecular markers for a personalized approach to CLL management? Or will they remain one of the many (and perhaps dispensable) biological prognosticators of CLL? It might still be too early to say. One remarkable feature of ATM, NOTCH1 and SF3B1 mutations, however, is that these molecular markers are true structural alterations of the CLL genome that conceivably have exerted a direct causative role at some stage of the leukemogenesis process. The example of many other tumors, both hematologic and solid, has taught us that biological markers, whose nature is to be cancer genetic lesions, harbor an added value in terms of clinical relevance. In fact, cancer genetic lesions not only are frequently robust prognosticators revealing an otherwise undetectable clini-co-biological heterogeneity of the disease, but might also provide a suitable target for molecular therapy. In the case of ATM disruption, ATM mutant cells exhibit an impaired DNA double strand break repair.12 Inhibition of poly (ADP-ribose) polymerase (PARP) imposes the requirement for DNA double strand break repair, and selectively sensitizes ATM-deficient tumor cells to killing. On these grounds, PARP inhibitors have been proposed as appropriate agents for treating refractory ATM mutant lymphoid malignancies.20 These clinical trials are currently ongoing. If successful, mutations of ATM will provide a potentially important target for novel therapeutic strategies devoted to CLL patients who are refractory to currently available treatments.
Acknowledgments
Work by the authors quoted in this article was supported by AIRC, Special Program Molecular Clinical Oncology, 5 × 1000, No. 10007, Milan, Italy (to GG), Progetto FIRB-Programma “Futuro in Ricerca” 2008 (to DR), PRIN 2008 (to GG), and PRIN 2009 (to DR), MIUR, Rome, Italy, Progetto Giovani Ricercatori 2008 (to DR) and Ricerca Sanitaria Finalizzata 2008 (to GG), Ministero della Salute, Rome, Italy, and Novara-AIL Onlus, Novara, Italy (to GG).
Footnotes
( Related Articles on pages 47 and 142)
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
Note
Since the acceptance of this manuscript, two novel studies (Quesada et al, Nat Genet. 2011 Dec 11. doi: 10.1038/ng.1032; Wang et al, N Engl J Med. 2011 Dec 12) have appeared in the literature reporting SF3B1 mutations in chronic lymphocytic leukemia
References
- 1.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:481–8. doi: 10.1182/asheducation-2010.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117(12):3391–401. doi: 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 4.Zenz T, Kröber A, Scherer K, Häbe S, Bühler A, Benner A, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112(8):3322–9. doi: 10.1182/blood-2008-04-154070. [DOI] [PubMed] [Google Scholar]
- 5.Rossi D, Cerri M, Deambrogi C, Sozzi E, Cresta S, Rasi S, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. doi: 10.1158/1078-0432.CCR-08-1630. [DOI] [PubMed] [Google Scholar]
- 6.Fabbri G, Rasi S, Rossi D, Trifonov V, Khiabanian H, Ma J, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208(7):1389–40. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–5. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M, et al. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood. 2011 Oct 28; doi: 10.1182/blood-2011-08-373159. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasi S, Monti S, Spina V, Foa’ R, Gaidano G, Rossi D. Analysis of NOTCH1 mutations in monoclonal B cell lymphocytosis. Haematologica. 2011;97(1):153–4. doi: 10.3324/haematol.2011.053090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi D, Rasi S, Fabbri G, Spina V, Fangazio M, Forconi F, et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2011 Nov 10; doi: 10.1182/blood-2011-09-379966. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24(21):2343–64. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability-an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–8. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 13.Bullrich F, Rasio D, Kitada S, Starostik P, Kipps T, Keating M, et al. ATM mutations in B-cell chronic lymphocytic leukemia. Cancer Res. 1999;59(1):24–7. [PubMed] [Google Scholar]
- 14.Schaffner C, Stilgenbauer S, Rappold GA, Döhner H, Lichter P. Somatic ATM mutations indicate a pathogenetic role of ATM in B-cell chronic lymphocytic leukemia. Blood. 1999;94(2):748–53. [PubMed] [Google Scholar]
- 15.Stankovic T, Weber P, Stewart G, Bedenham T, Murray J, Byrd PJ, et al. Inactivation of ataxia teleangiectasia mutated gene in B-cell chronic lymphocytic leukemia. Lancet. 1999;353(1):26–9. doi: 10.1016/S0140-6736(98)10117-4. [DOI] [PubMed] [Google Scholar]
- 16.Guarini A, Marinelli M, Tavolaro S, Bellacchio E, Magliozzi M, Chiaretti S, et al. ATM gene alterations in chronic lymphocytic leukemia patients induce a distinct gene expression profile and predict disease progression. Haematologica. 2011;97(1):47–55. doi: 10.3324/haematol.2011.049270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skowronska A, Austen B, Powell JE, Weston V, Oscier DG, Dyer MJ, et al. ATM germline heterozygosity does not play a role in CLL initiation but influences rapid disease progression through loss of the remaining ATM allele. Haematologica. 2011;97(1):142–6. doi: 10.3324/haematol.2011.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsimberidou A-M, Tam C, Abruzzo LV, O’Brien S, Wierda WG, Lerner S, et al. Chemoimmunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer. 2009;115(1):373–80. doi: 10.1002/cncr.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 20.Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer JS, et al. The PARP inhibitor olaparib induces significant killing of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116(22):4578–87. doi: 10.1182/blood-2010-01-265769. [DOI] [PubMed] [Google Scholar]