Abstract
Background
Congenital secondary erythrocytoses are due to deregulation of hypoxia inducible factor resulting in overproduction of erythropoietin. The most common germline mutation identified in the hypoxia signaling pathway is the Arginine 200-Tryptophan mutant of the von Hippel-Lindau tumor suppressor gene, resulting in Chuvash polycythemia. This mutant displays a weak deficiency in hypoxia inducible factor α regulation and does not promote tumorigenesis. Other von Hippel-Lindau mutants with more deleterious effects are responsible for von Hippel-Lindau disease, which is characterized by the development of multiple tumors. Recently, a few mutations in gene for the prolyl hydroxylase domain 2 protein (PHD2) have been reported in cases of congenital erythrocytosis not associated with tumor formation with the exception of one patient with a recurrent extra-adrenal paraganglioma.
Design and Methods
Five PHD2 variants, four of which were novel, were identified in patients with erythrocytosis. These PHD2 variants were functionally analyzed and compared with the PHD2 mutant previously identified in a patient with polycythemia and paraganglioma. The capacity of PHD2 to regulate the activity, stability and hydroxylation of hypoxia inducible factor α was assessed using hypoxia-inducible reporter gene, one-hybrid and in vitro hydroxylation assays, respectively.
Results
This functional comparative study showed that two categories of PHD2 mutants could be distinguished: one category with a weak deficiency in hypoxia inducible factor α regulation and a second one with a deleterious effect; the mutant implicated in tumor occurrence belongs to the second category.
Conclusions
As observed with germline von Hippel-Lindau mutations, there are functional differences between the PHD2 mutants with regards to hypoxia inducible factor regulation. PHD2 mutation carriers do, therefore, need careful medical follow-up, since some mutations must be considered as potential candidates for tumor predisposition.
Keywords: hypoxia inducible factor, hypoxia-inducible transcription factor, PHD2, erythrocytosis
Introduction
Secondary erythrocytosis is due to external factors such as increased production of erythropoietin, the origin of which is variable and may result from germline mutations in genes encoding factors involved in the oxygen-sensing pathway. The primary cellular component implicated in oxygen homeostasis is the hypoxia-inducible transcription factor (HIF). HIF operates as a heterodimer composed of a constitutively expressed beta subunit, also known as aryl hydrocarbon receptor nuclear translocator, and an alpha subunit (1α, 2α or 3α) that is tightly regulated by oxygen via post-translational modification. The prolyl-4-hydroxylase domain (PHD) enzymes hydroxylate proline residues located in the oxygen-dependent degradation (ODD) domain of HIF-α. This hydroxylation allows the binding of the von Hipple-Lindau protein (pVHL), the substrate recognition subunit of an E3 ubiquitin ligase complex that induces ubiquitination and subsequent degradation of HIF-α by the proteasome.1,2 In the absence of oxygen, HIF-α is stabilized, heterodimerizes with HIF-1β and induces expression of hundreds of genes involved in cell survival, angiogenesis, erythropoiesis and cell proliferation.2,3 There is some restricted target gene specificity depending on the HIF-α subunit of the HIF-α/β heterodimeric transcription factor. For example, renal and hepatic erythropoietin is regulated by the HIF-2α subunit in vivo.4–7 Germline mutations in genes involved in the HIF pathway have been reported in association with syndromes that predispose patients to both neoplasms and/or congenital secondary erythrocytosis.8 The most frequent mutations involve the VHL tumor suppressor gene. Heterozygous germline mutations in this gene are responsible for von Hippel-Lindau (VHL) disease, an autosomal dominant condition predisposing to multiple tumors including central nervous system and retinal hemangioblastomas, clear-cell renal cell carcinoma, pheochromocytomas and pancreatic endocrine tumors.8 Established correlations between genotype and phenotype predict the risk of paraganglioma/pheochromocytoma, with VHL deletions or truncating mutations being associated with a low risk (VHL type 1) and VHL missense mutations being associated with a high risk (VHL type 2).9,10
In addition, a homozygous 598C>T (R200W) VHL germline mutation has been shown to account for Chuvash congenital polycythemia, an autosomal recessive disease, endemic in the Chuvash Autonomous Republic of the Russian Federation.11 Homozygous carriers of the R200W-VHL germline mutation do not develop tumors but instead have Chuvash congenital polycythemia due to high levels of erythropoietin.11 The lack of tumor development in this disorder is due to a weak defect of the mutation in terms of its HIF-α regulation (leading to delayed ubiquitination) because of its localization outside pVHL functional domains.11 Other homozygous and compound heterozygous polycythemia-associated VHL mutations have also been reported.10 Functional studies of some of these mutants have shown a weak to undetectable defect of HIF-1α regulation (unpublished data).
Recently, a similar phenotype of high erythropoietin-associated polycythemia without associated tumors has been reported in carriers of heterozygous germline mutations in the PHD2 and HIF-2A genes,12–19 with the exception of one patient carrying a H374R-PHD2 mutation.20 This particular patient simultaneously developed congenital secondary erythrocytosis and recurrent paraganglioma, a tumor originating from neural crest cells similar to pheochromocytoma but with an extra-adrenal localization.20 The analysis of the tumor showed a loss of heterozygosity including the wild-type PHD2 allele, suggesting a potential tumor suppressor role of PHD2.
PHD2 and VHL act in concert to regulate HIF-α. Based on the observation that VHL mutation carriers display different phenotypes depending on the relative capacity of the VHL mutants to regulate HIF, we sought to define the genotype-phenotype relationship regarding the capacity of PHD2 mutants to differentially regulate HIF-α. Here we report a functional study comparing five PHD2 variants associated with isolated congenital secondary erythrocytosis with the mutation identified in a patient with recurrent paraganglioma.
Design and Methods
Patients and mutation screening
Thirty-four patients who did not fulfill World Health Organization diagnostic criteria for polycythemia vera were investigated. The local ethics committee of Kremlin Bicetre Hospital approved the study and all patients provided written informed consent. Blood samples were collected from all patients and germline DNA was extracted and analyzed by direct sequencing.20 DNA from the blood of healthy donors of Caucasian origin was used as a control.
Assay of hypoxia inducible factor transcriptional activity
In order to assay HIF transcriptional activity, dual luciferase assays were performed in Hek293T cells as described previously.20 A pGL3promoter vector expressing luciferase under the control of hypoxia response elements21 was used. Cells were exposed to hypoxic conditions (1% O2) for 4 h before extraction.
Assay of hypoxia inducible factor stability
HeLa cells were transiently co-transfected with increasing amounts (50–200 ng) of an expression vector encoding the HIF-2α ODD domain (amino acids 404-569), fused to yeast Gal4 DNA-binding domain and Herpes simplex VP16-derived transactivation domain, together with the Gal4-response element driven firefly luciferase reporter, pGRE5xE1b (125 ng), and a Renilla luciferase control plasmid (4 ng).22 Twenty-four hours post-transfection, cells were cultured under either normoxic (20% O2) or hypoxic (1% O2) conditions for an additional 16 h, and firefly luciferase activity was determined and normalized to Renilla luciferase activity.
Hydroxylation assay
PHD2 and HIF-ODD proteins (plasmid pcDNA3-HA-Gal4-HIF-1α-ODD was a generous gift from WG Kaelin Jr, Dana-Farber Cancer Institute, Boston, USA)23 were produced in wheat germ extract in vitro using the TnT transcription-translation kit (Promega). The hydroxylation reaction was carried out by mixing PHD2 and HIF-1α-ODD (amino acids 536-652) proteins in a reaction buffer containing co-factors (Fe2+, ascorbic acid and 2-oxoglutarate) as described previously.24 The samples were incubated at 30°C, collected at different time points, and immunoblotted with anti-HA (Tebu) and anti-HIF-1α (Pro 564)-OH (Cell-Signaling Technology) antibodies.
Results
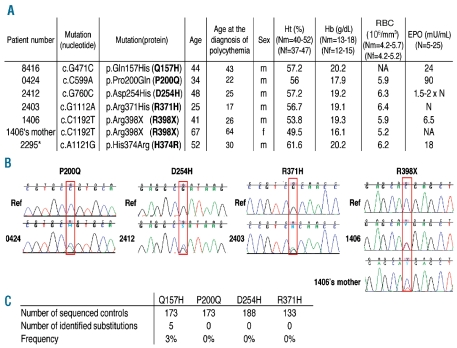
We sequenced the PHD2 gene on germline DNA from a series of Caucasian patients with unexplained polycythemia associated with normal or elevated serum erythropoietin levels (Figure 1A). Four novel heterozygous sequence variants were identified in the PHD2 gene [c.G471C, p.Gln157His (Q157H); c.C599A, p.Pro200Gln (P200Q); c.G760C, p.Asp254His (D254H); c.C1192T, p.Arg398X (R398X)] as well as the already described c.G1112A, p.Arg371His (R371H) mutation16 (Figures 1A and B). Genetic testing was performed on available parents and relatives of the PHD2 mutation carriers, but no mutation was found except in the mother of the PHD2-R398X carrier who presented characteristics of polycythemia (Figure 1A). She harbors the PHD2 mutation but with a mosaic status as demonstrated by the reduced height of the peak on the sequence chromatogram (Figure 1B). The low proportion of the mutated allele was confirmed by a quantitative allele-specific oligonucleotide method (data not shown).
Figure 1.
Identification of PHD2 mutations in patients with polycythemia. (A) Table of patients diagnosed with erythrocytosis and a PHD2 variation. Ht: hematocrit; Hb: hemoglobin; RC: number of red cells; EPO: erythropoietin; m: male; f: female; N: normal; NA: not available; *: previously described.20 (B) Sequence chromatogram of PHD2 in the area of the mutated nucleotide. Wild-type DNA was used as reference (Ref) (top) and compared to germline DNA of the patient (bottom). Only one relative of a patient was available for genetic testing (the mother of the PHD2-R398X carrier). She harbors the PHD2 mutation but with a mosaic pattern as demonstrated by the reduced height of the peak on the sequence chromatogram. (C) Frequency of the PHD2 variations in a control population.
Concerning the other families, only a few relatives were available for further genetic and clinical investigations but there was no history of familial polycythemia. Briefly, the brother, the sister and the son of patient #2295 and the father of patient #2403 agreed to genetic testing and were not carriers of PHD2 mutation. Other parents had died previously, of known causes in two cases: esophageal cancer for the mother of patient #2403 and colon cancer for the mother of patient #2412. Finally, patient #0424 was an adopted child, without children.
The frequencies of the different variants were evaluated in a control population (Figure 1C). Only the Q157H variant was found and was, therefore, classified as a polymorphism. Analysis of amino acid conservation supports this conclusion, as the Q157 amino acid is not conserved either between species or within the PHD protein family (PHD1 and 3) (Online Supplementary Table S1). In contrast, amino acids P200 and R371 are highly conserved and the D254 amino acid is fully conserved, similar to H374 described in our previous study.20 As far as concerns the location of the amino acids in the protein, D254, like H374, is part of the PHD2 catalytic site.25 The P200 amino acid is located within the nuclear localization signal (NLS) implicated in the shuttling of PHD2 between the cytoplasm and the nucleus which plays a crucial role in HIF regulation.26 We tested the shuttling of the P200Q mutation by immunofluorescence but we did not observe any impact of the mutation on the capacity of PHD2 to shuttle (data not shown).
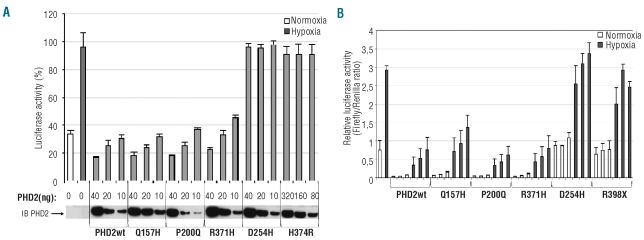
The functional consequences of these mutations on PHD2 activity were first evaluated using a hypoxia reporter assay based on the transcriptional activity of endogenous HIF (accumulated during 4 h of hypoxia). A luciferase reporter gene driven by a hypoxia response element derived from the erythropoietin 3′ enhancer was used. Adding wild-type PHD2 led to a dose-dependent suppression of HIF-α-mediated luciferase expression (Figure 2A). As expected, the PHD2 variant with the Q157H polymorphism reduced luciferase activity to the same extent as wild-type PHD2 (Figure 2A). Within the different missense mutations, we observed two types of mutants: one type (including PHD2-P200Q and R371H) did not affect the capacity of PHD2 to regulate HIF-α transcriptional activity, whereas a second type (including PHD2-D254H and H374R) completely abrogated PHD2 activity (Figure 2A).
Figure 2.
Functional study of PHD2 mutants using luciferase reporter assays. (A) PHD2-dependent regulation of endogenous HIF in an assay based on a hypoxia response element reporter gene. Cells were co-transfected in a 12-well format with various amounts of pcDNA3-HA-PHD2 expression vectors (to enable the expression of the same amount of PHD2 proteins) in addition to pGL3 reporter vectors encoding firefly luciferase under the control of a sensitive hypoxia response element and Renilla luciferase as a control of transfection efficiency. Cells were placed in hypoxic conditions (1% O2) for 4 h in order to accumulate endogenous HIFα before being collected. Results are given in percentage of firefly luciferase activity normalized to Renilla luciferase activity. The amount of HA-PHD2 transfected (PHD2) was quantified by immunoblotting using an anti-HA antibody. (B) The effect of PHD2 effect on HIF-2α protein stability in a one-hybrid reporter assay. Cells were cotransfected in a 6-well format with various amounts of pcDNA3-HA-PHD2 expression vectors (200, 100 and 50 ng), a Gal4-VP16-HIF-2αODD (amino acids 404-569) construct as well as a Gal4 response element-driven firefly luciferase reporter and a Renilla luciferase control plasmid. Twenty-four hours post-transfection cells were incubated for 16 h in normoxic or hypoxic conditions. Results are mean values of three independent experiments performed in triplicate.
We next studied the PHD2 mutants using a one-hybrid reporter assay based on the capacity of PHD2 to induce HIF-α protein instability. Cells were co-transfected with expression vectors containing the different PHD2 mutants (50 to 200 ng), the oxygen-dependent degradation domain of HIF-2α (HIF-2α-ODD) fused to yeast Gal4 DNA-binding domain and Herpes simplex VP16-derived transactivation domain, together with a reporter vector expressing luciferase under the control of a Gal4-response element. In this assay, luciferase expression reflects the stability of HIF-2α-ODD. A trend similar to that in the previous test was obtained: there was no detectable effect of the P200Q and R371H substitutions on PHD2 activity (which was comparable to that of the wild-type PHD2) and there was total abolition of the D254H mutant activity (comparable to that of the truncated PHD2 variant R398X) (Figure 2B). Identical results were obtained using a HIF-1α-ODD stability reporter (data not shown). The experiment was repeated with the P200Q-PHD2 mutant in oxygen-regulated erythropoietin-expressing cells (including the human hepatoma cell line Hep3B and renal erythropoietin-producing cells, a new cell model isolated from the tumor-free tissue of a patient with renal carcinoma) but no significant difference was observed (data not shown).27
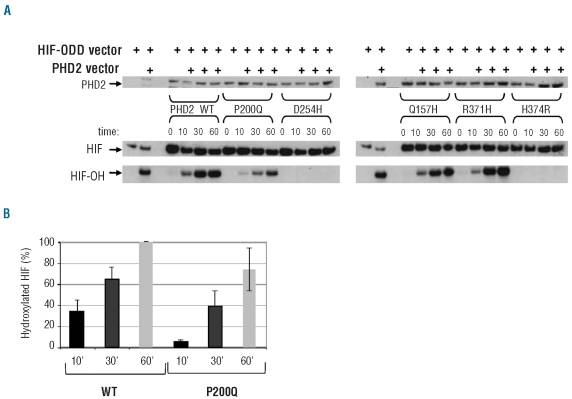
We next performed a sensitive in vitro assay in order to test the ability of the PHD2 mutants to hydroxylate HIF-1α in a time-dependent manner. In this assay, in vitro-translated PHD2 proteins were mixed with HIF-1α-ODD in the presence of co-factors necessary for the hydroxylation reaction. The capacity of the different PHD2 mutants to hydroxylate HIF-1α was measured by immunoblotting using an antibody specific for the hydroxylated HIF-1α-ODD (HIF-OH, Figure 3A). The H374R and D254H substitutions totally impaired HIF-1α hydroxylation (Figure 3A). By contrast the R371H mutant behaved like the wild-type PHD2 and the P200Q mutant, although capable of hydroxylating HIF-1α (Figure 3A), showed a reproducible and consistent delay (Figure 3B).
Figure 3.
Functional analysis of PHD2 mutants using an in vitro hydroxylation assay. (A) Immunoblot estimation of HIF-1α-ODD protein hydroxylation in vitro. PHD2 and HIF-1α-ODD proteins were synthesized separately by in vitro transcription-translation reactions. The hydroxylation reaction was then processed by mixing HA-PHD2 and HA-HIF-1α-ODD proteins in a reaction buffer containing PHD2 enzymatic co-factors. The reaction was carried out at 30°C and samples were collected after 0, 10, 30, and 60 min of incubation. For immunoblotting, 10 μL aliquots of the hydroxylation reaction assays were separated by SDS-PAGE, blotted, and incubated with an anti-HA antibody [to quantify PHD2 and total HIF-ODD (HIF)] and anti-hydroxylated HIF-1 antibody [to quantify hydroxylated HIF-ODD (HIF-OH)]. (B) Quantification of hydroxylated HIF-1α. The proportion of hydroxylated HIF-1α was measured and related to total HIF-1α. The 100% value corresponds to the quantity of HIF-1α hydroxylated by the PHD2-WT protein after 1 h. Means were obtained with three independent experiments.
Discussion
Taken together, these results show that PHD2 mutations can be divided into several different classes in terms of their effects on HIF regulation. Genotype/phenotype correlations cannot be established for PHD2 mutations because they are rare events that have been reported in only ten families to date (including those in the present study), in contrast to the 945 families described with a VHL mutation.10 In addition, the parents of the PHD2 mutation carriers reported in the literature were either dead or not available for further genetic and clinical investigations (including one parent who died of esophageal cancer18). In our study, only one parent was genetically tested and diagnosed as a mosaic carrier which prevents any conclusion regarding the developed phenotype. Nonetheless, regarding the close functional relation between VHL and PHD2 in the regulation of HIF and the implication of the HIF pathway in the genesis of pheochromocytoma,28–31 we can hypothesize similarities between the various types of mutants and raise the question of a possible risk of development of paraganglioma/pheochromocytoma in PHD2 mutation carriers. Subjects with one category of mutantion (P200Q and R371H) display features similar to those with the VHL-R200W mutantion responsible for Chuvash polycythemia without any increased risk of neoplasia.11 Like the VHL-R200W mutant, which is located outside functional domains, the PHD2-P200Q and R371H mutations are not located in the catalytic domain of the enzyme and have a moderate impact on HIFα regulation. In addition, the VHL-R200W mutant only induces delayed ubiquitination of HIFα which may be comparable to the delayed hydroxylation of HIFα observed with the PHD2-P200Q mutant. Interestingly, Pro200 is only one residue N-terminal to Cys201 which has been shown to chelate zinc and cadmium ions, providing evidence for the existence of a second metal binding site on PHD2.32,33 This Cys201 affects PHD2 hydroxylation activity and appears to be implicated in redox signaling in vitro.34 The very close location to the functionally important Cys201 residue could be the cause of the delayed hydroxylation of HIF-1α by the PHD2-P200Q mutant. Intriguingly, concerning the R371H mutant, the previously reported loss-of-function effect of this mutant16 could not be confirmed by any of the three tests of our study. The R371H mutation segregates with erythrocytosis in two different families (described herein and by Percy et al.16) and is unequivocally involved in this pathology. We currently cannot explain why, in our hands, the R371H mutation failed to abolish PHD2 catalytic activity. The PHD2 expression vectors were re-sequenced and their functions confirmed by immunoblotting. Parallel experiments with other PHD2 mutants confirmed the validity of our assays. For this category of mutants which have a moderate impact on HIFα regulation, we cannot rule out potential indirect regulation on the oxygen sensing pathway via PHD2-interacting proteins. Indeed, during the past decade a large number of PHD2-interacting proteins have been discovered, including both upstream regulators and downstream targets of PHD2, substantially increasing the complexity of the PHD/HIF oxygen-sensing regulation pathway.7 Moreover, PHD2 has been reported to have hydroxylation-independent gene regulatory functions.35–37
Another category, including the PHD2-R398X mutation and three other PHD2 truncated mutations described previously,12 can be compared to the VHL truncation mutations (VHL disease type 1) which are not associated with the development of pheochromocytomas. Subject with these first two categories could be considered at low risk of developing paraganglioma/pheochromocytoma.
A last category could be compared to the VHL missense mutations involved in VHL disease type 2, associated with a high risk of paraganglioma/pheochromocytoma. This category includes the previously described PHD2-H374R mutation identified in a 43-year-old patient with paraganglioma. A loss of the PHD2 wild-type allele was demonstrated in the patient with this tumor, arguing for a tumor suppressor role of PHD2.20 No PHD2 mutations have been identified in other series of patients affected by pheochromocytomas (73 patients with hereditary paraganglioma and pheochomocytoma syndrome, Gimenez-Roqueplo, Hôpital Européen Georges Pompidou, Paris, unpublished data) and/or renal carcinoma,38 but the risk that germline PHD2 mutation carriers have of developing tumors should not be underestimated. Indeed, like His374, Asp254 is highly conserved, and located in the catalytic site of PHD2.25 Moreover, the D254H mutation results in a severe loss of function. Therefore, although no cases of paraganglioma or pheochromocytoma have yet been detected in the D254H-PHD2 mutation carrier, this mutation may be considered as a potential candidate for tumor predisposition.
In conclusion, using three different approaches we demonstrated that distinct PHD2 mutations have differential effects on HIF regulation. We suggest that, by analogy to VHL mutations, carriers of particular PHD2 mutations may be prone to tumor development. These patients would then require screening for tumor prevention and early detection.
Acknowledgments
The authors thank Christophe Marzac, Bruno Varet, Aurélie Hummel and Bruno Cassinat for their precious help in recruiting patients and Sylvie Hermouet for scientific discussions.
Footnotes
Funding: this work was supported by grants from the “Association pour la Recherche sur le Cancer” (ARC), the French National Cancer Institute (“INCa”, PNES Rein, and Réseau National Prédispositions héréditaires au cancer du rein) and the French Ligue Nationale contre le Cancer (Comités du Cher et de l’Indre) as well as by the HypoxiaNet COST Action TD0901. DH is supported by a postdoctoral Marie Curie IEF Fellowship from the European Commission and the Sassella Stiftung. DH and RHW are supported by grants from the State Secretariat of Education and Research (C10.0106) and from the NCCR Kidney.CH, funded by the SNF.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–73. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 2.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–43. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 3.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005(306):re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 4.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117(4):1068–77. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13(7):1721–32. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 6.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. Faseb J. 2004;18(12):1462–4. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 7.Wenger RH, Hoogewijs D. Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol. 2010;298(6):F1287–96. doi: 10.1152/ajprenal.00736.2009. [DOI] [PubMed] [Google Scholar]
- 8.Richard S, Graff J, Lindau J, Resche F. Von Hippel-Lindau disease. Lancet. 2004;363(9416):1231–4. doi: 10.1016/S0140-6736(04)15957-6. [DOI] [PubMed] [Google Scholar]
- 9.Maher ER, Webster AR, Richards FM, Green JS, Crossey PA, Payne SJ, et al. Phenotypic expression in von Hippel-Lindau disease: correlations with germline VHL gene mutations. J Med Genet. 1996;33(4):328–32. doi: 10.1136/jmg.33.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordstrom-O’Brien M, van der Luijt RB, van Rooijen E, van den Ouweland AM, Majoor-Krakauer DF, Lolkema MP, et al. Genetic analysis of von Hippel-Lindau disease. Hum Mutat. 2010;31(5):521–37. doi: 10.1002/humu.21219. [DOI] [PubMed] [Google Scholar]
- 11.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32(4):614–21. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Prehu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008;40(2):160–5. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Gale DP, Harten SK, Reid CD, Tuddenham EG, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood. 2008;112(3):919–21. doi: 10.1182/blood-2008-04-153718. [DOI] [PubMed] [Google Scholar]
- 14.Martini M, Teofili L, Cenci T, Giona F, Torti L, Rea M, et al. A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica. 2008;93(7):1068–71. doi: 10.3324/haematol.13210. [DOI] [PubMed] [Google Scholar]
- 15.Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, et al. Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood. 2008;111(11):5400–2. doi: 10.1182/blood-2008-02-137703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110(6):2193–6. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358(2):162–8. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci USA. 2006;103(3):654–9. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Wijk R, Sutherland S, Van Wesel AC, Huizinga EG, Percy MJ, Bierings M, et al. Erythrocytosis associated with a novel missense mutation in the HIF2A gene. Haematologica. 2010;95(5):829–32. doi: 10.3324/haematol.2009.017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359(25):2685–92. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 21.Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1alpha. Cancer Res. 2006;66(7):3688–98. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- 22.Koditz J, Nesper J, Wottawa M, Stiehl DP, Camenisch G, Franke C, et al. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood. 2007;110(10):3610–7. doi: 10.1182/blood-2007-06-094441. [DOI] [PubMed] [Google Scholar]
- 23.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2(7):423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277(42):39792–800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonough MA, Li V, Flashman E, Chowdhury R, Mohr C, Lienard BM, et al. Cellular oxygen sensing: crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc Natl Acad Sci USA. 2006;103(26):9814–9. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhoff A, Pientka FK, Mockel S, Kettelhake A, Hartmann E, Kohler M, et al. Cellular oxygen sensing: importins and exportins are mediators of intracellular localisation of prolyl-4-hydroxylases PHD1 and PHD2. Biochem Biophys Res Commun. 2009;387(4):705–11. doi: 10.1016/j.bbrc.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 27.Frede S, Freitag P, Geuting L, Konietzny R, Fandrey J. Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC. Blood. 2011;117(18):4905–14. doi: 10.1182/blood-2010-07-298083. [DOI] [PubMed] [Google Scholar]
- 28.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- 30.Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, et al. Expression of HIF-1alpha, HIF-2alpha (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91(11):4593–8. doi: 10.1210/jc.2006-0920. [DOI] [PubMed] [Google Scholar]
- 32.Mecinovic J, Chowdhury R, Flashman E, Schofield CJ. Use of mass spectrometry to probe the nucleophilicity of cysteinyl residues of prolyl hydroxylase domain 2. Anal Biochem. 2009;393(2):215–21. doi: 10.1016/j.ab.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Mecinovic J, Chowdhury R, Lienard BM, Flashman E, Buck MR, Oldham NJ, et al. ESI-MS studies on prolyl hydroxylase domain 2 reveal a new metal binding site. ChemMedChem. 2008;3(4):569–72. doi: 10.1002/cmdc.200700233. [DOI] [PubMed] [Google Scholar]
- 34.Nytko KJ, Maeda N, Schlafli P, Spielmann P, Wenger RH, Stiehl DP. Vitamin C is dispensable for oxygen sensing in vivo. Blood. 2011;117(20):5485–93. doi: 10.1182/blood-2010-09-307637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bordoli MR, Stiehl DP, Borsig L, Kristiansen G, Hausladen S, Schraml P, et al. Prolyl-4-hydroxylase PHD2- and hypoxia-inducible factor 2-dependent regulation of amphiregulin contributes to breast tumorigenesis. Oncogene. 2011;30(5):548–60. doi: 10.1038/onc.2010.433. [DOI] [PubMed] [Google Scholar]
- 36.Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15(6):527–38. doi: 10.1016/j.ccr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao Z, Zhang Y, Powell-Coffman JA. Two distinct roles for EGL-9 in the regulation of HIF-1-mediated gene expression in Caenorhabditis elegans. Genetics. 2009;183(3):821–9. doi: 10.1534/genetics.109.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astuti D, Ricketts CJ, Chowdhury R, McDonough MA, Gentle D, Kirby G, et al. Mutation analysis of HIF prolyl hydroxylases (PHD/EGLN) in individuals with features of phaeochromocytoma and renal cell carcinoma susceptibility. Endocr Relat Cancer. 2010;18(1):73–83. doi: 10.1677/ERC-10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]