Abstract
Background
There are limited reports of thrombosis among myelodysplastic syndrome patients exposed to erythropoiesis stimulating agents. It is not clear whether erythropoiesis stimulating agents are associated with an increased risk of thrombosis in myelodysplastic syndromes, as they are among patients with solid tumors.
Design and Methods
The association between use of erythropoiesis stimulating agent and transient thrombosis risk in patients with myelodysplastic syndromes was assessed in a case-crossover study nested within a cohort of incident myelodysplastic syndrome patients. Using the US Surveillance, Epidemiology, and End Results Medicare-linked database, cases with an incident diagnosis of deep vein thrombosis were identified. Using conditional logistical regression, the odds of exposure to erythropoiesis stimulating agents in the 12 weeks prior to the incident deep vein thrombosis (hazard period) was compared to the exposure odds in a prior 12-week comparison period.
Results
Within the cohort of eligibles with myelodysplastic syndromes (n=5,673) there were 212 incident cases of deep vein thrombosis events. Mean age was 76.2 (standard deviation=±8.6) years. Use of erythropoiesis stimulating agents was not associated with deep vein thrombosis in the crude nor the adjusted models (OR=1.21, 95% CI: 0.60, 2.43). Central venous catheter placement (OR=6.47, 95% CI: 2.37, 17.62) and red blood cell transfusion (OR=4.60, 95% CI: 2.29, 9.23) were associated with deep vein thrombosis.
Conclusions
Despite the link between use of erythropoiesis stimulating agents and thrombosis among patients with solid tumors, this study provides evidence that their safety profile may be different among patients with myelodysplastic syndromes.
Keywords: myelodysplastic syndromes, thrombosis, erythropoiesis-stimulating agents, drug safety, case-crossover
Introduction
The erythropoiesis-stimulating agents (ESA) epoetin alfa (Epogen; Amgen, Thousand Oaks, CA and Procrit: Centocor Ortho Biotech, Horsham, PA, USA) and darbepoetin alfa (Aranesp; Amgen, Thousand Oaks, CA, USA) are indicated for the treatment of anemia in chronic kidney failure patients, in patients with cancer with anemia caused by chemotherapy, in patients with HIV with anemia caused by zidovudine (AZT), and to reduce the number of transfusions in patients scheduled for major non-cardiac surgery. While ESAs do not have an FDA-approved indication for myelodysplastic syndromes (MDS), these agents are in common use to prevent or reduce the need for blood transfusions in MDS patients with anemia. Approximately 65% of 6,588 MDS patients in the Medicare population diagnosed between 2002 and 2005 used an ESA.1
A number of published meta-analyses of ESA clinical trials in solid tumor patients have shown not only increases in the risk of tumor promotion and/or mortality, but also a significant (RR < 2.0) increase in the risk of venous thromboembolism (VTE) among patients randomized to receive ESAs compared to placebo.2–7 Increased risk of thrombotic events was also reported among ESA users with chronic kidney failure and among patients preparing for major surgery.8–10 The FDA released a Public Health Advisory in 2007 and ESA product labels were updated to reflect this risk, with a recommendation that ESA therapy be suspended when hemoglobin (Hb) levels reach 12 g/dL or rise more than 1 g/dL per week.11
There are few reports of thrombosis in clinical trials of ESAs in the MDS patient population; one DVT was reported in a patient in the treatment arm in the ECOG study12 and 4 patients (2%) in a single arm treatment study of DARBO had a thromboembolitic or “related events”.13 There are also a limited number of case reports of thrombosis among MDS patients exposed to ESAs. One case report described extensive deep vein thrombosis (DVT) in an elderly male patient with the refractory anemia MDS subtype receiving 40,000 IU erythropoietin subcutaneously three times per week, with an Hb level of 15 g/dL.14 Several studies have reported VTE episodes that have occurred when ESAs were administered in conjunction with other agents known to increase the risk for VTE. A phase II study of combined treatment with thalidomide (Thalomid; Celgene, Summit, NJ, USA) and darbepoietin-alpha in low-and intermediate-risk MDS was suspended after 3 of the first 7 enrolled patients developed DVT at six, seven, and 11 weeks of therapy.15 In the single fatal case, the patient had a prior history of DVT. Hemoglobin levels rose significantly (>1 g/dL) in 3 patients in the trial, but only one of these 3 developed DVT. The authors attributed the thrombotic events to an interaction between the two drugs. Additionally, in a post-marketing signal detection analysis of adverse events among MDS patients treated with the thalidomide derivative lenalidomide (Revlimid; Celgene, Summit, NJ, USA), VTE was disproportionally reported with concomitant use of lenalidomide and an ESA, but not with lenalidomide alone.16
While combined ESA and thalidomide or lenalidomide therapy may be thrombogenic, it is not clear whether ESAs alone are associated with an increased risk of VTE in MDS, as they are among patients with solid tumors. It is generally believed that MDS patients are at low risk of thrombosis due to the high frequency of thrombocytopenia and severe anemia. Brandenburg and colleagues reported VTE in 14 out of 408 MDS patients treated with lenalidomide, with a cumulative incidence of 3.4% for the first VTE event. Of note, ESA users were excluded from the analyses in that study.17 Since the widespread adoption of ESAs to treat MDS-associated anemia, there has been little published on the risk of thrombosis in this patient population. Here, a large administrative database was examined to determine if the use of ESAs in MDS patients is associated with an increased risk of VTE.
Design and Methods
Study population
Patients with MDS were identified in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database. SEER is a national cancer surveillance network of 16 regional cancer registries and covers about 28% of the United States population.18 Each registry is charged with identifying all diagnosed cancers within its region and collecting detailed information including the date of cancer diagnosis, cancer site and histology, initial cancer therapies, and demographic characteristics. Once in the registry, each patient is followed to identify additional cancers (recurrences or additional primary tumors), and the date and cause of death. SEER databases are linked to Medicare enrollment and claims files at the individual level to allow tracking of cancer- and non-cancer related medical service utilization by Medicare beneficiaries before and after their cancer diagnosis. The vast majority (93%) of persons 65 years and older in SEER are successfully matched to Medicare enrollment files.19
Patients with a first diagnosis of MDS in SEER between 2001 and 2005 who were enrolled in Medicare at the time of their diagnosis were eligible. MDS diagnosis was based on the WHO classification system, including ICD-O-3 histology codes 9980 [refractory anemia (RA)], 9982 [RA with sideroblasts (RARS)], 9983 [RA with excess blasts (RAEB)], 9985 [refractory cytopenias with multilineage dysplasia (RCMD)], 9986 (MDS with 5q deletion), 9987 (therapy-related MDS), and 9989 [MDS not otherwise specified (NOS)]. Patients with a histology of 9984 [RA with excess blasts in transformation (RAEB-t)] were excluded as in the WHO classification they are classified as having acute myeloid leukemia. Patients were classified by their MDS subtype and were also categorized into low-risk (RA, RARS, 5q deletion and RCMD), high-risk (RAEB), and other/unspecified risk categories. In SEER, 87.6% of MDS cases are microscopically confirmed based on a positive histology.20
The study period began at the time of MDS diagnosis in SEER and ended on December 31, 2007. Patients were excluded if they had a gap in Medicare coverage, if the date of MDS diagnosis was unavailable or they were diagnosed upon autopsy, if they were not enrolled in Medicare (Parts A and B) or if enrolled in an HMO during the year prior to diagnosis. Patients were censored upon death, loss of Medicare Part A or B enrollment, or enrollment in an HMO. The study focused on the relationship between ESA use and incident DVT. To identify incident DVT cases, patients who had a diagnosis of DVT or pulmonary embolism in the year prior to the MDS diagnosis were excluded. Because ESAs are used to treat anemia associated with chronic renal failure, patients with a history of chronic renal failure during the year prior to their MDS diagnosis or who received dialysis during the study period were also excluded.
Case definition
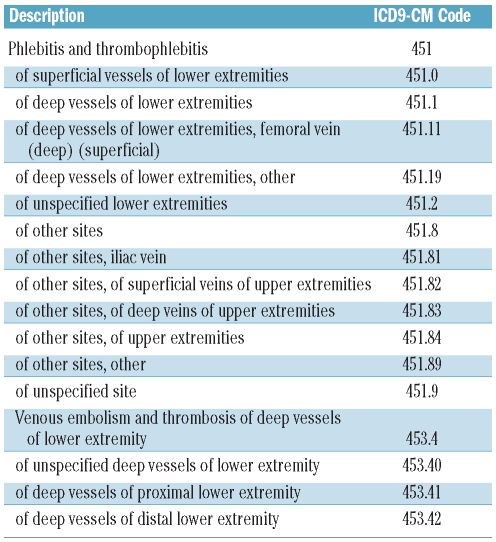
Patients were considered as cases based on a diagnosis of a deep vein thrombosis (DVT) during an outpatient visit or a hospitalization within the study period (Table 1). The event date was set at the week of the first (incident) DVT following the diagnosis of MDS. In a sensitivity analysis, we also included as cases patients who had an incident pulmonary embolism (PE).
Table 1.
Diagnostic codes used to identify a diagnosis of deep vein thrombosis
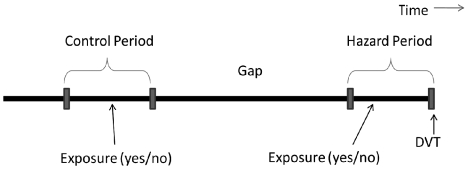
The case-crossover design is a variation on the case-control design, in which a case serves as his/her own control. This study design is suitable for the study of transient outcomes that are believed to occur in close temporal proximity to the exposure.21 Instead of a separate case and control being compared, two time periods are compared. The hazard period is a defined period of time immediately proximal to the outcome. A comparison period is typically an earlier time period equal in length to the hazard period, though it can also be a summary measure of typical experience. If a factor causes a transient increase in the risk of an event, that exposure should be more common in the time period immediately preceding the event than in a more distant comparison time period.
Here, the hazard period was defined as a 12-week period beginning the week of the DVT and counting backwards in time. The comparison period was an earlier 12-week period separated from the hazard period by a 24-week gap (Figure 1). To determine whether the results were robust to variations in the time period, we conducted a number of sensitivity analyses in which we varied the duration of the hazard/control periods and also the gap between them. For each analysis, cases needed to survive a minimum time period equal to the two exposure periods plus the gap following their initial MDS diagnosis without a thrombotic event. This ranged from 48 weeks (12 hazard weeks + 12 comparison weeks + 24 gap weeks) for the initial analysis to 16 weeks (4 weeks each hazard and comparison + 8 week gap) for the sensitivity analysis. The number of eligible cases in the analytic cohorts increased as the time period was shortened.
Figure 1.
In a case-crossover design each case serves as its own control. The exposure odds in the hazard period is compared with the exposure odds in an earlier control period.
Drug exposure
Exposure was calculated separately for the hazard and comparison periods. The exposure of interest was the use of an ESA (erythropoietin alpha or darbepoietin alpha). Weekly exposure measures were calculated based on one or more claims for either of these agents. Any exposure (yes/no) was defined as one or more claims for either agent during the 12-week period. Any exposure was also determined for the individual agents. Because darbepoietin alpha is longer-acting than erythropoietin alpha, its recommended dosing schedule is different. Therefore, cumulative exposure was calculated separately for each agent, defined as the sum of the weeks in which there was one or more claim for that agent within the 12-week period.
Covariates
Demographic characteristics of the patients were abstracted from the SEER database and conditioned on the date of the incident MDS diagnosis. These variables include patient age, gender, race, marital status, and the region of the country where they resided. A one-year look-back period from the date of MDS diagnosis was used to determine past medical history based on Medicare claims. A prior medical history was defined as either one inpatient claim with a date of service within the look-back period, or two outpatient claims (29 to 365 days apart) at least one of which was within the look-back period.
Because each person serves as his/her own control, static factors such as gender, medical and family history, underlying risk factors and, to some extent, age are matched between cases and controls. Only factors that change over the study period can be evaluated for their temporal association with DVT risk. These transient covariates included the use of granulocyte colony-stimulating factor (G-CSF) and chemotherapy. Unlike oral drugs, these drugs are covered under Medicare part B and can be identified through procedure codes specific to their administration. Patients were also classified according to the presence or absence of an inpatient hospitalization, red blood cell transfusion, platelet transfusion, and central venous catheter placement during the hazard and comparison periods.
Statistical analysis
The MDS patients who had an incident DVT during follow up were characterized according to their baseline characteristics and past medical history. Among these cases, the proportion of patients who had an ESA exposure, transfusions, and other key exposures and events were compared between the hazard and comparison periods using χ2 tests. We constructed a conditional logistical regression model to compare the transient odds of ESA exposure in the hazard and the comparison period, controlling for time-dependent variables. Two sets of time-variant factors were highly correlated (red blood cell transfusions with platelet transfusions and central venous catheterization with hospitalization). The factor with the greater predictive value in a separate interim model was selected for inclusion in the final regression model. The main model was based on exposure periods (hazard and comparison) of 12 weeks each and a gap between exposure periods of 24 weeks.
To determine if the results were robust to the length of the exposure period, the models were rerun after recalculated exposures and all transient variables using exposure periods of both eight and four weeks. The effect of disease progression was assessed by reducing the gap between exposure periods from 24 to eight weeks. Each adjusted model was tested for important statistical interactions between the exposure and covariates, defined as a 10% or greater change in the beta coefficient for ESA when comparing models with and without the interaction term. To maintain confidentiality, cell sizes of less than 11 patients (<5.2% of the cohort) were noted and the actual cell values suppressed. This study has been approved by the Institutional Review Board of the University of Maryland, Baltimore, USA.
Results
Patients’ characteristics
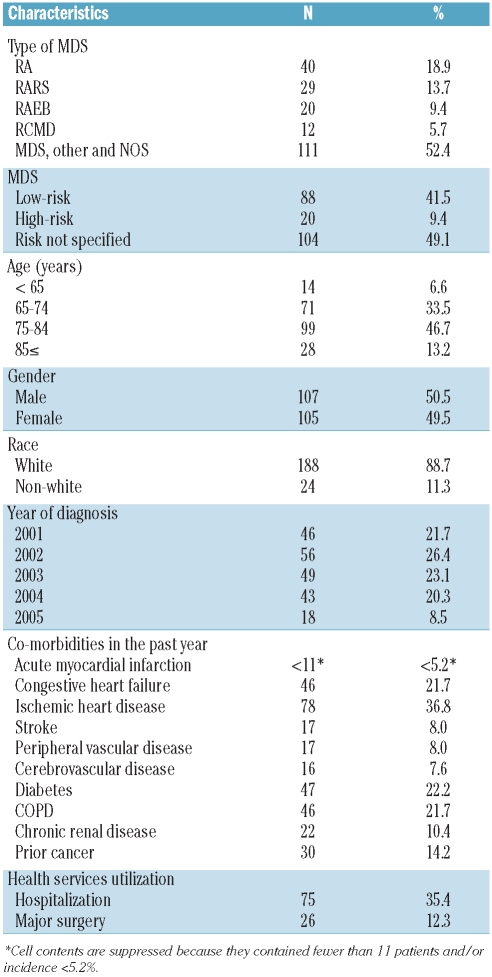
A total of 5,673 MDS patients met eligibility criteria for this study, among whom 212 had an incident DVT within the required time frame. Among the 212 incident DVT cases, the most common type of MDS was the category ‘not otherwise specified’ (49.1%), followed by refractory anemia (RA) (18.9%) and refractory anemia with ringed sideroblasts (RARS) (13.7%), with only 20 cases (9.4%) categorized as refractory anemia with excess blasts (RAEB) (Table 2). The majority of patients (46.7%) were 75–84 years of age at diagnosis of MDS, with approximately equal distributions of males and females.
Table 2.
Characteristics of MDS patients with incident DVT in the case-crossover study cohort (n=212).
As might be expected given the age distribution, there was a high prevalence of chronic conditions. Within the year prior to their MDS diagnosis, 36.8% of the DVT patients had a health care service claim with a diagnosis of ischemic heart disease. High rates of congestive heart failure (21.7%), diabetes (22.2%) and chronic obstructive pulmonary disease (COPD) (21.7%) were also noted. Thirty patients (14.2%) had a history of cancer prior to their diagnosis of MDS.
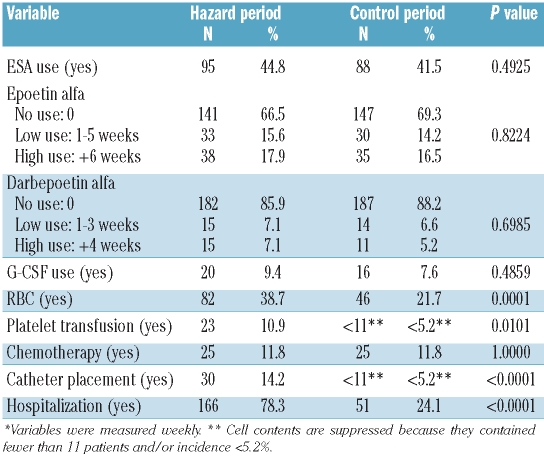
There were similar rates of ESA utilization in the hazard period and the comparison period (44.8% vs. 41.5%) (Table 3). The length of exposure was similarly distributed across time periods regardless of the choice of agent, epoetin alpha or darbepoetin alpha. Factors that differed significantly across the two exposure periods included red blood cell transfusions, platelet transfusions, insertion of a central venous catheter, and hospitalizations. Each of these factors was more common in the hazard period than in the comparator period. There was significant overlap in the patients who received transfusions of red blood cells and platelets (73.9% in the hazard period, 100% in the comparator period). Approximately 97% of the central venous catheter insertions in the hazard period were per-formed during hospitalization.
Table 3.
Distribution of time-dependent factors in the hazard and control time periods* (n=212).
Thombosis risk factors
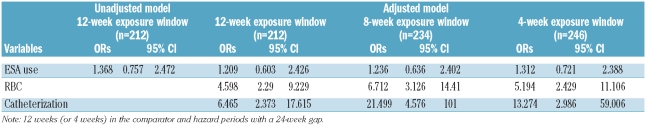
In the crude conditional logistical regression model, the odds ratio (OR) for ESA use was slightly elevated, but did not vary significantly between time periods (OR=1.37, 95% CI:0.76, 2.47) (Table 4). Controlling for red blood cell transfusions and central venous catheter insertion in an adjusted model resulted in a smaller OR which remained non-significant (OR=1.21, 95% CI: 0.60, 2.43).
Table 4.
Results of the conditional logistical regression models in the primary analysis (12-week exposure window) and secondary analysis (8-and 4-week exposure windows), all with a 24 week gap between case and control exposure windows.
In contrast to ESA use, both central venous catheterization and red blood cell transfusions were temporally associated with increased odds of DVT. In a model that contained all three of these covariates, central venous catheterization and red cell transfusions were associated with a 6.5-fold (OR=6.47, 95% CI: 2.37, 17.62) and a 4.6-fold (OR=4.60, 95% CI: 2.29, 9.23) increase in the odds of DVT.
There was no significant difference in the use of ESA between the hazard and comparison periods regardless of their length and gap: 41.9% vs. 39.3% for 8-week periods with a 24-week gap between them and 37.2% vs. 39.2% for 4-week periods with an 8-week gap. While varying the lengths of the hazard and control periods, as well as the gap between them, had no effect on the association between ESA use and DVT, it did have a major impact on the association of catheterization and DVT and an important but somewhat lesser impact on that between red blood cell transfusions and DVT. Reducing the exposure periods from 12 to eight weeks, while retaining a 24-week gap, resulted in an increase in the OR for catheter placement from 6.5 to 21.5 (95% CI: 4.58, 101.00) and for red blood cell transfusions from 4.6 to 6.7 (95% CI: 3.12, 14.4). The OR for catheter placement showed the greatest variability. When the exposure periods were reduced to just four weeks, with an 8-week gap, the OR for catheter placement increased to 30.1 (95% CI: 4.06, 223.49), while the OR for transfusions dropped to 2.1 (95% CI: 1.25, 1.87) (data not shown) The increase in OR for catheter placement as the exposure time period is decreased suggests a high risk of thrombosis at the time of implantation of a venous catheter which, while remaining elevated, declines rapidly in the weeks following the procedure.
Discussion
ESAs are an important, albeit off-label, therapy in the management of MDS. Systematic reviews and meta-analyses of studies of ESAs in MDS patients and a recent phase III trial showed ESAs to have potentially clinically important anemia-ameliorating activity in subsets of MDS patients.12,22,23 Unlike anemic patients with epithelial malignancy, ESA-treated MDS patients rarely achieve normal levels of hemoglobin. Few studies raised a question of safety in terms of increased risk of thrombosis as MDS patients have a low baseline risk of thrombosis. Given the severity of anemia in MDS patients and a typically low baseline risk of thrombosis, ESAs were expected to be associated with a lesser risk of thrombosis in this population. The lack of a significant association between recent ESA use and DVT in MDS patients in this observational study provides evidence that this theory may be correct.
The validity of observational studies of the benefits and risks of pharmaceutical products may be compromised by numerous biases and confounding factors which may not be fully identified or adjusted. Because the decision to use ESAs may have been related to the patient’s perceived risk of thrombosis as well as factors that are not observable in a claims database, such as family history or laboratory test results, we employed a case-crossover design to overcome this limitation. By having each case serve as its own control, observable and unobservable factors that remain static during the study period are matched in cases and controls. Even though the study is based on the national SEER-Medicare linked database, MDS is a rare disease and the rate of DVT is low among MDS patients. Therefore, our study had limited statistical power and could not rule out a small increase in risk of the magnitude seen here (1.21-fold).
Factors that were found associated with transient DVT risk were implantation of a central venous catheter and red blood cell transfusions. The odds ratio for catheter placement increased drastically as the exposure window was shortened. This suggests that the risk is greatest at or immediately following the procedure and that this excess risk drops off quickly. However, there is also the potential that this finding is partially or wholly attributable to surveillance bias. Not all DVTs are symptomatic and, additionally, patients may not present for treatment even if they have a symptomatic DVT. The incidence of DVT in clinical trials varies significantly based on the protocol used to identify them and the length of the study.24 Hospital medical staff are likely to closely monitor a patient for DVT following procedures such as central venous catheterization that are known to be associated with increased thrombosis risk. This increased vigilance could create an artificial increase in DVT immediately following the procedure.
Current guidelines from the National Comprehensive Cancer Network suggest that anemic MDS patients be pre-screened for clinical factors predictive of potential response to ESAs prior to embarking on a trial of ESA therapy. The guidelines further recommend that patients who do not respond to ESAs after eight or nine weeks have the ESA therapy altered or discontinued.25 Clinical responses to ESA therapy persist for a median of two years.26 The current study provides important reassurance that continuing administration of ESAs to clinically responding MDS patients does not put such patients at increased risk of severe thromboembolic disease. This study does not address the safety of combinations of ESAs with other potentially thrombogenic therapies, such as thalidomide or lenalidomide. Additionally, to what extent ESA therapy impacts progression of MDS and overall survival of MDS patients remains unclear.
Footnotes
Funding: this research was supported by a grant from the National Cancer Institute at the National Institutes of Health (5RC1CA145831-2).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Davidoff AJ, Weiss Smith S, Baer MR, Ke X, Bierenbaum JM, Hendrick F, et al. Access to Care, Race and Education Are Key Determinants of Erythropoietin Stimulating Agent (ESA) Use In Myelodysplastic Syndromes (MDS) Blood (ASH Annual Meeting Abstracts) 2010;116(21):3815. [Google Scholar]
- 2.Berndt E, Kallich J, McDermott A, Xu X, Lee H, Glaspy J. Reductions in anaemia and fatigue are associated with improvements in productivity in cancer patients receiving chemotherapy. Pharmaco Economics. 2005;23(5):505–14. doi: 10.2165/00019053-200523050-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst. 2005;97(7):489–98. doi: 10.1093/jnci/dji087. [DOI] [PubMed] [Google Scholar]
- 4.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–42. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 5.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–14. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 6.Glaspy J, Crawford J, Vansteenkiste J, Henry D, Rao S, Bowers P, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301–15. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Hemmelgarn B, Reiman T, Manns B, Reaume MN, Lloyd A, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. Cmaj. 2009;180(11):E62–71. doi: 10.1503/cmaj.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 9.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369(9559):381–8. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 10.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Oncologic Drugs Advisory Committee briefing document: Continuing Reassessment of the Risks of Erythropoiesis-Stimulating Agents (ESAs) Administered for the Treatment of Anemia associated with Cancer Chemotherapy. [Accessed February 12, 2010]. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4301b2-02-FDA.pdf.
- 12.Greenberg PL, Sun Z, Miller KB, Bennett JM, Tallman MS, Dewald G, et al. Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996) Blood. 2009;114(12):2393–400. doi: 10.1182/blood-2009-03-211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilove J, Paquette R, Lyons RM, Mushtaq C, Sekeres MA, Tomita D, et al. Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodys-plastic syndromes. Br J Haematol. 2008;142(3):379–93. doi: 10.1111/j.1365-2141.2008.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niazy MN, Neyyarapally TI, Chattopadhyay A. Erythropoietin-induced deep vein thrombosis in myelodysplastic syndrome. J Assoc Physicians India. 2008;56:195–6. [PubMed] [Google Scholar]
- 15.Steurer M, Sudmeier I, Stauder R, Gastl G. Thromboembolic events in patients with myelodysplastic syndrome receiving thalidomide in combination with darbepoietin-alpha. Br J Haematol. 2003;121(1):101–3. doi: 10.1046/j.1365-2141.2003.04252.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Brandenburg NA, Freeman J, Salomon ML, Zeldis JB, Knight RD, et al. Venous thromboembolism in myelodys-plastic syndrome patients receiving lenalidomide: results from postmarketing surveillance and data mining techniques. Clin Drug Investig. 2009;29(3):161–71. doi: 10.2165/00044011-200929030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Brandenburg NA, Weiss L, Bwire R, Schmidt M, Knight R, List AF. Venous thromboembolism in patients with myelodysplastic syndrome treated with lenaliomide: Incidence and risk factors. Presented at: 44th American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2008; Chicago, IL. 2008. Abstract 7084. [Google Scholar]
- 18.National Cancer Institute. The Surveillance, Epidemiology, and End Results (SEER) Medicare Linked Database. http://healthservices.cancer.gov/seer-medicare/
- 19.National Cancer Institute. SEER-Medicare: How the SEER & Medicare Data are Linked. [Accessed September 3, 2011]. http://healthservices.cancer.gov/seer-medicare/overview/linked.html.
- 20.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 21.Delaney JA, Suissa S. The case-crossover study design in pharmacoepidemiology. Stat Methods Med Res. 2009;18(1):53–65. doi: 10.1177/0962280208092346. [DOI] [PubMed] [Google Scholar]
- 22.Ross SD, Allen IE, Probst CA, Sercus B, Crean SM, Ranganathan G. Efficacy and safety of erythropoiesis-stimulating proteins in myelodysplastic syndrome: a systematic review and meta-analysis. Oncologist. 2007;12(10):1264–73. doi: 10.1634/theoncologist.12-10-1264. [DOI] [PubMed] [Google Scholar]
- 23.Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol. 2008;87(7):527–36. doi: 10.1007/s00277-008-0450-7. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds MW, Shibata A, Zhao S, Jones N, Fahrbach K, Goodnough LT. Impact of clinical trial design and execution-related factors on incidence of thromboembolic events in cancer patients: a systematic review and meta-analysis. Curr Med Res Opin. 2008;24(2):497–505. doi: 10.1185/030079908x261050. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cancer- and chemotherapy-induced anemia (version 2.2011) [Accessed May 27, 2011]. http://www.nccn.org/professionals/physi-cian_gls/pdf/anemia.pdf.
- 26.Jadersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodys-plastic syndrome. J Clin Oncol. 2008;26(21):3607–13. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]