Abstract
Background
This phase I trial was conducted to determine the safety and pharmacokinetics of monoclonal antibody 216, a human monoclonal Immunoglobulin M antibody targeting a linear B-cell lactosamine antigen, administered alone and in combination with vincristine in patients with relapsed or refractory B-cell acute lymphoblastic leukemia, and to preliminarily assess tumor targeting and efficacy.
Design and Methods
Three cohorts of patients received escalating doses of monoclonal antibody 216 administered as an intravenous infusion. In the case of poor response to the first dose of monoclonal antibody 216 alone, defined as less than 75% reduction in peripheral blood blast count, a second dose of the antibody with vincristine was given between days 4 and 7. Responses were assessed weekly until day 35. Serum concentration of monoclonal antibody 216 was measured before and after infusion. Monoclonal antibody 216 targeting was determined with an anti-idiotypic antibody to monoclonal antibody 216 and preliminary efficacy was analyzed by changes in peripheral blood blasts.
Results
Thirteen patients were enrolled. One episode of grade 3 epistaxis was the only dose-limiting toxicity observed. All patients showed a poor response to the first monoclonal antibody 216 infusion with a decrease in peripheral blasts from 6–65% in 9 patients. In 8 patients, addition of vincristine to monoclonal antibody 216 resulted in an average reduction of the peripheral blasts of 81%. One patient without peripheral blasts achieved a hypoplastic marrow without evidence of leukemia after one infusion of monoclonal antibody 216 and monoclonal antibody 216/vincristine each. Monoclonal antibody 216 was detected on peripheral blasts in all patients.
Conclusions
Treatment with monoclonal antibody 216 in combination with vincristine is feasible and well tolerated in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Binding of monoclonal antibody 216 to leukemic blasts was efficient, and favorable early responses were observed.
Keywords: antibody 216, relapsed, refractory, B-cell acute lymphoblastic leukemia
Introduction
Over recent decades, there has been a steady improvement in treatment outcome of patients with ALL. This progress is due to factors including intensification of chemotherapy and risk-adapted therapy, as well as better supportive care.1 As a result, cure rates now approach 90% and 40% for pediatric and adult ALL, respectively.2 While the risk of relapse is much lower in the pediatric population, both pediatric and adult patients face significantly inferior outcomes if the disease recurs. Less than one third of children and few adults with relapsed ALL survive, despite the use of aggressive salvage regimens and stem cell transplantation.3,4 Novel therapies are, therefore, needed that have both activity against ALL and a toxicity profile distinct from conventional chemotherapy. Targeted therapy using monoclonal antibodies such as rituximab and trastuzumab has become an integral part of standard treatment for certain malignancies.5,6 For ALL, there is pre-clinical and early clinical experience with a variety of monoclonal antibodies including rituximab, alemtuzumab, epratuzumab and gemtuzumab,7–10 suggesting that the use of monoclonal antibodies alone or in combination with standard chemotherapy is a viable treatment option.
Human mAb216 is a naturally occurring human monoclonal IgM antibody. The variable heavy chain of mAb216 is derived from the VH4-34 gene encoding cold agglutinin-IgM antibodies against human fetal or adult red blood cells (RBC).11,12 A subset of anti-fetal RBC antibodies also binds and kills human B lymphocytes.13–15 The epitope on human B cells is a linear lactosamine determinant similar to the fetal RBC i-antigen that is not expressed on more than 90% of CD34-positive cells in normal bone marrow and is rarely expressed on normal adult RBC. A branching transferase enzyme converts i to I-antigen following birth. Rare individuals do not branch their i-antigen due to inheritance of an autosomal recessive mutation in the transferase gene.16,17
In a library of 30 VH4-34 encoded antibodies, mAb216 was found to have high binding and cytotoxicity against normal B lymphocytes, B-cell lymphomas and B-progenitor lymphoblasts.18–20 Binding of mAb216 to its linear lactosamine ligand leads to disruption of the plasma membrane and formation of large membrane pores resulting in cell lysis (Online Supplementary Figure S1).14,18 This non-classical apoptosis occurs in the absence of complement fixation but in vitro cytotoxicity is increased in assays using human complement.20 In vitro binding and cytotoxicity studies with B-cell ALL bone marrow specimens demonstrated that mAb216 binds and kills blasts from patients with B-progenitor ALL.20 Using flow cytometry, the cytotoxic effect of mAb216 and chemotherapeutic drugs (daunomycin 4ng/mL, L asparginase 0.8 U/mL and vincristine 2ng/mL) used to treat B-progenitor ALL either alone or in combination was assessed in pre-B ALL cell lines. The combination of vincristine and mAb216 caused an enhanced degree of cytotoxicity when compared to the additive effect of each single agent alone.20
The development of mAb216 for clinical trial evaluation was governed by the Rapid Access to Intervention Development (RAID) program. Clinical grade antibody was manufactured by the Biologic Resource Branch (BRB) of the National Cancer Institute. Investigators from the NCI BRB, NCI Cancer Treatment Evaluation Program (CTEP) and Stanford worked with the FDA on the development, pre-clinical testing and clinical trial design for mAb216. Because this was the first natural occurring human mAb given to patients, the FDA only allowed two doses of mAb216 until in vivo toxicity could be evaluated. Rather than giving two doses of mAb216 alone, vincristine was added to the second dose of mAb216 because in vitro testing had shown enhancement of mAb216 cytoxicity in combination with vincristine, thereby providing a higher chance for response. The starting dose was 5% of the highest amount given to rabbits which did not cause toxicity.
The primary objective of this first-in-human phase I study was to determine the maximum-tolerated dose and dose-limiting toxicities of mAb216 as a single agent and in combination with vincristine in patients with relapsed or refractory B-cell ALL. The secondary objectives were to characterize the pharmacokinetic behavior of mAb216 and to preliminarily assess tumor targeting and clinical efficacy.
Design and Methods
Patient eligibility
Patients of any age with B-lineage ALL in first or later relapse or primary refractory disease occurring at any time after initial diagnosis were eligible provided their red blood cells did not bind mAb216. Due to a risk of hemolysis in the rare case of expression of the fetal i-antigen on adult red cells, patients’ RBCs were tested for mAb216 binding by flow cytometry. Additional eligibility requirements included a Karnofsky score of at least 50%, life expectancy of at least eight weeks, and adequate renal, hepatic and cardiac function. Patients could not have received myelosuppressive chemotherapy within one week of study entry or biological agents including monoclonal antibodies at least two weeks before. Patients with CNS leukemia or isolated extramedullary relapse were excluded. The study was approved by the Institutional Review Board of Stanford University. The trial IND number is 11887; the trial registration number is NCT-00313053/00313079. Informed consent was obtained from patients aged 18 years and older or from parents/legal guardians of children under the age of 18, with child assent when appropriate.
Dosage and drug administration
MAb216 was supplied by the Rapid Access to Intervention Development (RAID) program of the National Cancer Institute (NCI) as a sterile lyophilized powder that was reconstituted and diluted to a final concentration of 1 mg/mL. After pre-medication with acetaminophen and diphenhydramine, mAb216 was administered as a slow intravenous infusion starting at a rate of 1 mg/kg/h, with gradual incremental increases in the infusion rate to a maximum of 200 mg/h, as tolerated. Corticosteroids and meperidine could be administered for infusion reactions, but were otherwise not part of routine pre-medication.
Criteria for assessment of response
For the early response evaluation on days 4–7, good response was defined as less than 25% leukemic blasts detected on bone marrow examination or a more than 75% reduction in peripheral blood blast count. Patients not fulfilling criteria for good response were considered to have poor response. For all subsequent weekly response evaluations, residual disease was defined as more than 5% leukemic blasts detectable on bone marrow examination or the presence of blasts in the peripheral blood.
Complete remission (CR) was defined as attainment of M1 bone marrow (less than 5% blasts) with no evidence of circulating blasts or extramedullary disease and with recovery of peripheral counts (absolute neutrophil count above 1×109/L and platelet count above 100×109/L). Partial remission (PR) was defined as complete disappearance of circulating blasts, no evidence of extramedullary disease and achievement of M2 bone marrow status (equal or more than 5% but less than 25% blasts and adequate cellularity) with recovery of peripheral counts as above. Partial-remission cytolytic response (PRCR) was defined as complete disappearance of circulating blasts and achievement of at least 50% reduction from baseline in bone marrow blast count. Hematologic improvement (HI) was defined as a 75% or more decrease in the peripheral blast count when compared with baseline counts, regardless of bone marrow response. Progressive disease (PD) was defined as an increase of at least 25% in the absolute number of leukemic cells in the peripheral blood or bone marrow, or the development of extramedullary disease. Patients not fulfilling criteria for CR, PR, PRCL, HI or PD were considered to have stable disease (SD).
Trial design
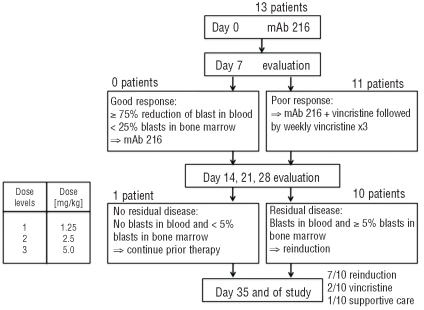
Patients received two treatment courses of mAb216, with the same dose of antibody administered on days 0 and 7. Early response evaluation was performed on days 4–7 (Figure 1). Patients who demonstrated a good response to the first dose of antibody received the second dose in identical fashion as on day 0. Patients who had a poor response received the second dose of mAb216 in conjunction with vincristine 1.5 mg/m2 up to 2 mg total dose followed by 3 weekly doses of vincristine. In the event that a patient clearly had a poor response to therapy by day 4, i.e. had an obvious rise in peripheral blast count, the patient could proceed with the second dose of mAb216 in conjunction with vincristine as early as day 4. The dose of mAb216 was escalated from 1.25 mg/kg to 5 mg/kg in three consecutive dose cohorts using a 3+2 dose escalation design. The quantity of material produced by the NCI did not permit dosing of 3 patients at 10 mg/kg as initially planned, therefore one extra patient was added to the 5mg/kg cohort.
Figure 1.
Trial schema.
Toxicities were graded according to the National Cancer Institute’s Common Terminology Criteria (version 3.0). Dose-limiting non-hematologic toxicity was defined as any grade 3 or 4 adverse event attributable to mAb216 with the specific exclusion of grade 3 nausea and vomiting. The toxicity profile for vincristine is well characterized, and should be distinguishable from toxicities associated with an immunotherapeutic agent such as mAb216.
Dose-limiting hematologic toxicity was defined as absence of peripheral blood count recovery (absolute neutrophil count above 0.5×109/L and platelet count above 20×109/L) of more than six weeks duration as documented by bone marrow aplasia, not marrow infiltration with leukemia cells. The highest dose level reached at which no more than one of 5 patients experienced a DLT was considered the maximum tolerated dose (MTD).
Response to mAb216 alone was determined on day 4–7 by peripheral blood count with differential or bone marrow aspirate as described above. Subsequent early response evaluations were performed weekly on days 14, 21 and 28 by bone marrow aspiration unless the patient’s peripheral blood blast count was clearly indicative of residual disease. Patients without residual disease continued on prior therapy, which consisted of mAb216 alone on days 0 and 7 of subsequent cycles in case of response to mAb216 single agent therapy or weekly vincristine alone for 3 doses starting on day 14 in case of response to mAb216 combined with vincristine. Patients with residual disease could discontinue the trial to begin reinduction with alternative chemotherapy regimens as early as day 14. At the end of the treatment block on day 35, response was again assessed by bone marrow morphology using standard criteria for assessment of response unless the patient’s peripheral blood blast count was clearly indicative of residual disease.
Plasma concentration studies
Blood samples for pharmacokinetic studies were collected before and at specific time points after completion of mAb216 infusion on days 0, 1, 2 and 3. Plasma concentrations of mAb216 were determined by enzyme-linked immunosorbent assay (ELISA) using the anti-idiotype rat mAb9G4 as the solid phase capture mAb. Since mAb216 is an IgM antibody with 10 binding sites, the anti-idiotype could be used for both capture and detection. Plasma was serially diluted (1:2) and binding detected with biotin-labeled 9G4 and horseradish peroxide-streptavidin (HRPO-SA). Pharmacokinetic values were determined from the ELISA data using Prism software (GraphPad Software).
Flow cytometry assessment of mAb216 binding to patient RBCs and blasts
Bone marrow aspirate or peripheral blood samples were obtained before therapy to determine binding of mAb216 to leukemic blasts and red blood cells by flow cytometry. Buffy coat and red cells were separated by centrifugation and analyzed independently. Leukemic blasts were gated using a combination of fluorescence labeled antibodies against CD45, CD19 (Caltag/Invitrogen, Carlsbad, CA and BD Bioscience, San Jose, CA, USA) and side scatter signal. Mean channel fluorescence of mAb216 biotin/streptavidin PE on blasts and RBCs was quantified. Human IgM mAbMS2B6-biotin21 was used as an isotype control to determine background fluorescence. Similarly, binding of mAb216 biotin/streptavidin PE to red blood cells was measured. Control RBCs obtained from normal healthy subjects were run in parallel. For a positive control, the pre-B cell line Nalm-6 was stained with mAb216 biotin or mAbMS2B6-biotin, followed by streptavidin PE in each experiment. Samples were analyzed on Facscan or LSRII flow cytometer (Becton Dickinson) using Cell Quest (BD) for data interface and Flojo (Treestar) software for analysis. Ratio of mean channel fluorescence of mAb216 to iso-type control mAb was used to compare binding among patients.
Peripheral blood samples taken pre-infusion, end of infusion, one hour, 4 h and 24 h post infusion were stained with fluorescent labeled anti-CD antibodies and anti-idiotypic antibody 9G4-biotin in various combinations and analyzed as described above. Percentage of blasts at each time point was determined by electronic gating for CD45 low, side scatter low cells. Mean channel fluorescence of anti-idiotypic Ab 9G4-biotin/SA-PE on CD45 low, side scatter low blasts was determined to assess presence of mAb216 on blasts. Complete blood count (CBC) and serum chemistries were performed in the Stanford Clinical Laboratories.
Formation of human anti-mAb216 antibodies and immune complex formation
Patient IgG mediated immune response to mAb216 was measured on days 0, 7, 14 and 35 using an ELISA. Purified mAb216 and control human IgM mAbMS2B6 was bound to the solid phase. Patient sera were serially diluted and IgG antibody binding detected using HRPO-labeled anti-human IgG (Caltag/Invitrogen). Binding of post-infusion mAb216 sera was compared to pre-infusion sera and IgG binding to mAb216 compared to the control human IgM mAbMS2B6. Blood was assayed for detection of circulating immune complexes on days 0 and 7 using the C1Q assay performed at a reference laboratory.
Results
Patients’ characteristics
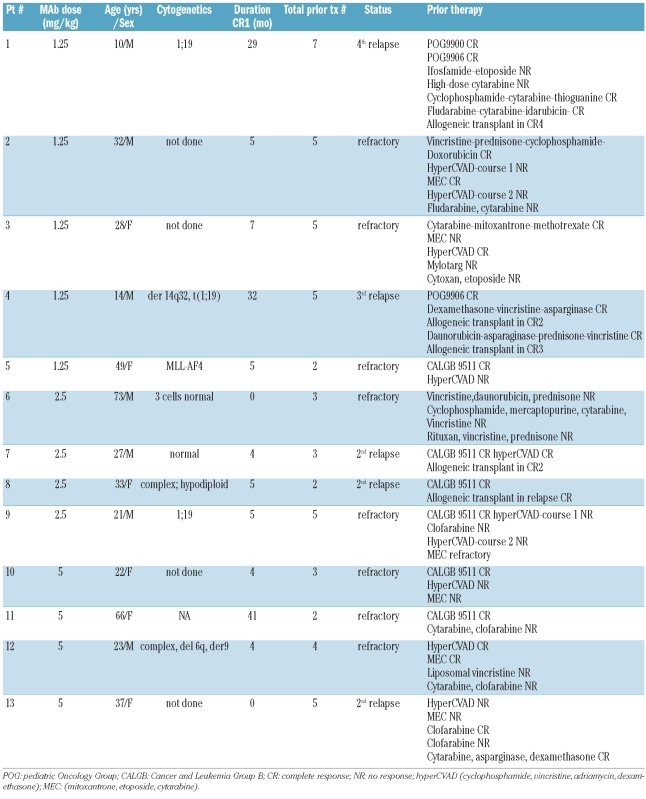
From April 2005 to November 2008, 14 patients were screened for participation in the study. All screened patients showed binding of mAb216 to blasts, and none of the screened patients showed binding of mAb216 to red cells. One patient declined the trial. Accordingly, 13 patients, all Philadelphia chromosome translocation negative, were enrolled in the study. Twelve of these 13 were on study and fully assessable for toxicity with follow up of at least 14 days. One patient received 2.5 mg/kg mAb216 on day 0 and died on day 5 due to sepsis not attributed to mAb216. The median age of the reported cohort was 33 years (range 10–73 years). Eight patients had refractory disease, and 5 patients were in second or greater relapse. Most patients had received three or more prior lines of treatment including allogeneic stem cell transplantation in 4 cases (Table 1).
Table 1.
Patients’ characteristics.
Toxicity
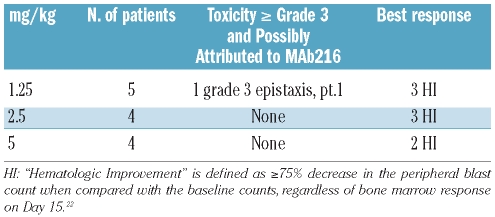
Overall, mAb216 was well tolerated with acceptable toxicity when given alone or in combination with vincristine. Grade 1 or 2 infusion reactions, characterized by urticaria and pruritus, were observed in 3 of 13 patients. These reactions resolved after the infusions were temporarily stopped and diphenhydramine and hydrocortisone were administered. All patients were able to resume and complete the infusions. One patient experienced a dose limiting-toxicity. The patient developed grade 3 epistaxis three days after the first infusion of 1.25 mg/kg of mAb216, and left the protocol. While this is a common event in a patient population with thrombocytopenia and can be easily managed, it was cautiously decided to categorize it as a DLT given that the patient was a child and it was the very first dose of mAb216 given. The first dose cohort was, therefore, expanded to 5 patients. There were no other grade 3 or greater toxicities (Table 2). At a dose of 2.5 mg/kg, one patient developed sepsis and died on day 5 prior to receiving the second dose of therapy. The patient had entered the study in second relapse following allogeneic stem cell transplantation. At the time of death, the patient had residual leukemia as demonstrated by the presence of peripheral blood blasts. The event was not attributed to mAb216. Because the patient was not fully assessable for toxicity, one additional patient was added to the 2.5 mg/kg dose cohort.
Table 2.
Toxicity possibly or probably related to mAb216 and response to mAb216 and vincristine by dose level.
Response
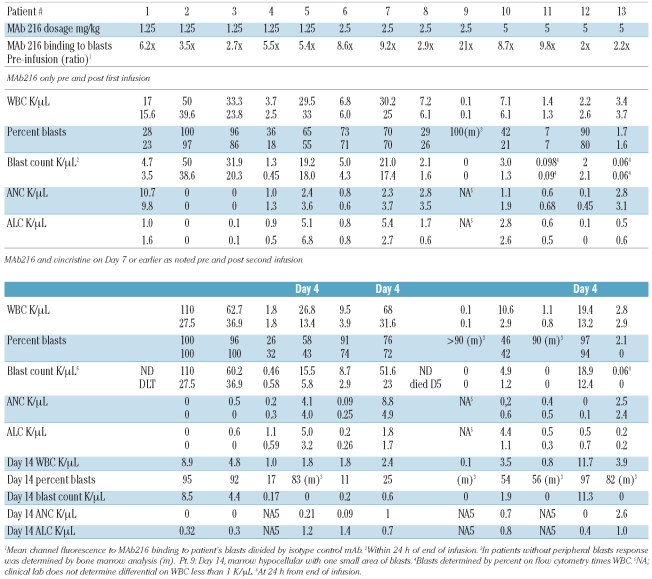
In 10 patients with circulating blasts, the median absolute blast count in the peripheral blood decreased from 4,850/μL (range 1,300–50,000/μL) at study entry to 3,900/μL (range 450–38,600/μL) within 24 h after completion of the first infusion of mAb216 resulting in a median blast count reduction of 23.5% (range 0–65%) (Table 3). At the time of planned response assessment on days 4–7, all 13 patients had a poor response to the initial dose of mAb216 including 3 patients who had a rising blast count. Two patients received only one dose of mAb216; one patient due to grade 3 epistaxis constituting a DLT and one patient due to death related to sepsis. Owing to poor response to mAb216 alone, the remaining 11 patients received their second dose of mAb216 in conjunction with vincristine. Three of the 11 patients had a rise in their peripheral blast count after the initial mAb216 infusion and received mAb216 and vincristine on day 4, the other 8 patients received the combination therapy on day 7. In 8 patients with circulating blasts, the absolute blood blast count decreased from a median of 17,200/μL (range 460–110,000/μL) to 1,250/μL (range 0–11,300/μL) by day 14 of mAb216 and vincristine resulting in a median blast count reduction of 92.5% (range 40–100%) (Table 3). Two patients did not have peripheral blasts and one patient’s peripheral blasts were only detectable by flow cytometry and remained unchanged. Response to chemoimmunotherapy with mAb216 and vincristine was determined weekly starting on day 14 up to the end of the treatment block on day 35. For patients with residual disease on day 14 or thereafter, alternative reinduction chemotherapy could be given. By day 14, none of the 11 patients had achieved a CR or PR. However, 8 of 11 patients were in the response category of “Hematological Improvement” (HI), used by Kantarjian et al.22 (Table 2). One of these patients achieved a partial remission cytolytic response with complete absence of blasts in a hypoplastic bone marrow. While the protocol specified continuing treatment with vincristine only, the FDA allowed the patient to receive one additional dose of mAb216 in combination with vincristine. Unfortunately he subsequently relapsed after two weeks and died without receiving further therapy. One additional patient chose not to undergo further therapy. The remaining 9 patients received reinduction with alternative chemotherapy regimens (n=7) or palliative chemotherapy (n=2) with weekly vincristine on day 15 or thereafter.
Table 3.
Blood counts before and after infusion of mAb216 and mAb216/vincristine.
Plasma concentrations and half-life of mAb216
Plasma mAb216 concentrations measured at the end of the initial infusion by ELISA increased in a dose-dependent manner with median values of 2.3 μg/mL (range 1.1–3.6 μg/mL), 3.95 μg/mL (range 0.8–5.9 μg/mL), and 11.2 μg/ml (range 9.8–39 μg/mL) for mAb216 dose cohorts of 1.25 mg/kg, 2.5 mg/kg and 5 mg/kg, respectively. The median half-life of mAb216 was 1.99 h (range 0.78–9.36 h).
Formation of human anti-mAb216 antibodies and immune complex formation
None of the patients developed anti-mAb216 IgG anti-bodies on days 0, 7, 14 or 35 as determined by ELISA or showed any evidence of immune complex formation when measured on days 0 and 7 by C1Q assay.
MAb216 binding to patient RBC and blasts
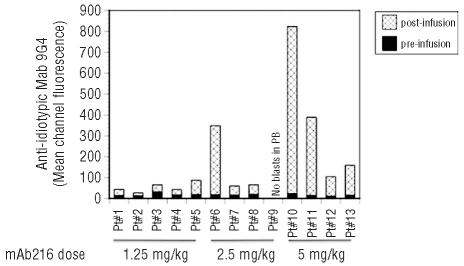
Prior to enrollment in the trial, patient RBC and blasts were tested for mAb216 binding in vitro. None of the patients showed binding of mAb216 to red blood cells. Binding of mAb216 to blasts, measured as a ratio of mean channel fluorescence of biotin-labeled mAb216 to isotype control, ranged from low (1.3) to high (31) (median 4.3). The efficiency of blast targeting in vivo by mAb216 was determined by measuring binding of anti-idiotypic antibody 9G4 to blast cells post mAb216 infusion. Presence of mAb216 was detected in all 13 patients (Figure 2). There was no significant correlation between degree of mAb216 binding in vitro prior to treatment using biotin-labeled mAb216 and response.
Figure 2.
MAb216 binds to blasts in vivo. Mean channel fluorescence of blasts pre [black] and post [stippled] infusion of mAb216. Peripheral blood was stained with CD19APC, CD45FL and mAb 9G4 biotin followed by Streptavidin PE. Blasts were gated and the mean channel fluorescence of mAb 9G4 bound to mAb216 was calculated. MAb216 bound to blasts in vivo in all patients.
Discussion
Despite improvements in initial therapy for ALL, results in relapsed patients remain significantly inferior for both children and adults, as patients frequently do not tolerate or respond to reinduction therapy.3,4,23 This is a first-in-human study of the human monoclonal IgM antibody 216 alone and in combination with vincristine for pediatric and adult patients with relapsed or refractory B-cell ALL. MAb216 administration was well tolerated, both as a single agent and when combined with vincristine. The most common single-agent toxicities observed were grade 1 and 2 infusion reactions that responded to administration of diphenhydramine and/or hydrocortisone and slowing of the infusion rate. In all cases, the full dose of mAb216 could be administered. Given the high-risk status of the population treated in phase I trials, the single death observed secondary to sepsis was not unexpected.22,24–26
Although efficacy was not a primary endpoint of this phase I study, the preliminary results obtained, including clearance of peripheral blood blasts with mAb216 and vincristine in 3 patients, and a hypoplastic marrow without evidence of leukemia in one patient are encouraging. Because this was the first clinical trial with a human derived monoclonal antibody, the FDA only permitted short-term administration and initial low dosage. Most clinical trials with mAbs use repeated weekly infusions.27,28 With repeat dosing the efficacy of mAb216 is expected to increase. As is frequently seen in phase I trials, the degree of response to mAb216 did not correlate with dose level,29,30 but the ability to assess a dose-response relationship was limited as only three dose cohorts were tested.
All patients enrolled in this trial were heavily pre-treated; the majority of patients had received at least three prior regimens and 4 had undergone allogeneic stem cell transplantation. Although we cannot entirely exclude the possibility that in some patients the decrease in blasts observed after the infusion of mAb216 and vincristine was due to vincristine, this appears unlikely because all patients had been previously treated with vincristine-containing regimens and the majority had been refractory.
None of the patients screened showed binding of mAb216 to red blood cells, and accordingly no cases of hemolysis were observed. As expected, the plasma level of mAb216 increased with increasing dose levels. The median half-life was 1.99 h, indicating that mAb216 was immediately bound to leukemic blasts. None of the patients developed anti-mAb216 IgG antibodies or showed any evidence of immune complex formation during the study. Prior to treatment, binding of mAb216 to leukemic blasts in vitro was confirmed for all patients using biotin-labeled mAb216. After the administration of mAb216 alone, anti-idiotypic mAb 9G4 was used to confirm MAb216 binding to circulating blasts using flow cytometry. While there was no significant correlation between the degree of pre-treatment mAb216 binding to leukemic blasts in vitro and response, a trend suggesting more blast killing with higher mAb216 binding was observed. Additional factors potentially determining response include disease burden and mAb216 dose. In vitro, the ability of mAb216 to lyse B-cell ALL cell lines depends on the expression of the lactosamine antigen and the antibody concentration.14,20
In conclusion, this first-in-human trial with the naturally occurring human mAb216 in children and adults with relapsed or refractory B-cell ALL demonstrated that mAb216 is well tolerated as a single agent and in combination with vincristine. At the dosage of mAb given (mAb216 at 5mg/kg and less) the decrease in peripheral blasts indicates biological activity. The encouraging early responses seen after administration of mAb216 with vincristine suggest that the antibody may enhance the effect of cytotoxic chemotherapy. Given the limitations of using monoclonal antibodies as single agents in rapidly proliferative diseases, the goal of future trials is to use mAb216 in combination with chemotherapy to exploit synergy as demonstrated for rituximab and chemotherapy in B-lymphoblastic leukemia and Burkitt’s leukemia.
MAb216 for this study was produced by the RAID program of the NCI, and the trial was performed under an investigator IND.
Footnotes
Funding: this work was supported by grant NCI RO3 CA85199 (NT), grant NSC 711294 from the NCI RAID program (NT), the ASCO foundation (ML), National Institutes of Health grant K08 CA120349 (ML) and the Stephanie Chancellor Research fund (SC).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Larson RA. The U.S. trials in adult acute lymphoblastic leukemia. Ann Hematol. 2004;83(Suppl 1):S127–8. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 3.Saarinen-Pihkala UM, Heilmann C, Winiarski J, Glomstein A, Abrahamsson J, Arvidson J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24(36):5750–62. doi: 10.1200/JCO.2006.07.1225. [DOI] [PubMed] [Google Scholar]
- 4.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol. 2008;26(22):3756–62. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DA, O’Brien S, Kantarjian HM. Monoclonal antibody therapy with rituximab for acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):949–71. v. doi: 10.1016/j.hoc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwaan CM, Reinhardt D, Jurgens H, Huismans DR, Hahlen K, Smith OP, et al. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: first clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia. 2003;17(2):468–70. doi: 10.1038/sj.leu.2402749. [DOI] [PubMed] [Google Scholar]
- 10.Stock W, Yu D, Sanford B, et al. Incorporation of alemtuzumab into front-line therapy of adult acute lymphoblastic leukemia (ALL) is feasible: A Phase I/II Study from the Cancer and Leukemia Group B (CALGB 10102) ASH Annual Meeting Abstracts. Blood. 106:145. [Google Scholar]
- 11.Roelcke D. Cold agglutination. Transfus Med Rev. 1989;3(2):140–66. doi: 10.1016/s0887-7963(89)70075-4. [DOI] [PubMed] [Google Scholar]
- 12.Silberstein LE, Jefferies LC, Goldman J, Friedman D, Moore JS, Nowell PC, et al. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood. 1991;78(9):2372–86. [PubMed] [Google Scholar]
- 13.Grillot-Courvalin C, Brouet JC, Piller F, Rassenti LZ, Labaume S, Silverman GJ, et al. An anti-B cell autoantibody from Wiskott-Aldrich syndrome which recognizes i blood group specificity on normal human B cells. Eur J Immunol. 1992;22(7):1781–8. doi: 10.1002/eji.1830220717. [DOI] [PubMed] [Google Scholar]
- 14.Bhat NM, Bieber MM, Stevenson FK, Teng NN. Rapid cytotoxicity of human B lymphocytes induced by VH4-34 (VH4.21) gene-encoded monoclonal antibodies. Clin Exp Immunol. 1996;105(1):183–90. doi: 10.1046/j.1365-2249.1996.d01-733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr TB, Johnson TA, Silberstein LE, Kipps TJ. Anti-B cell autoantibodies encoded by VH 4-21 genes in human fetal spleen do not require in vivo somatic selection. Eur J Immunol. 1994;24(12):2941–9. doi: 10.1002/eji.1830241204. [DOI] [PubMed] [Google Scholar]
- 16.Inaba N, Hiruma T, Togayachi A, Iwasaki H, Wang XH, Furukawa Y, et al. A novel I-branching beta-1,6-N-acetylglucosaminyl-transferase involved in human blood group I antigen expression. Blood. 2003;101(7):2870–6. doi: 10.1182/blood-2002-09-2838. [DOI] [PubMed] [Google Scholar]
- 17.Yu LC, Twu YC, Chang CY, Lin M. Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood. 2001;98(13):3840–5. doi: 10.1182/blood.v98.13.3840. [DOI] [PubMed] [Google Scholar]
- 18.Bhat NM, Bieber MM, Hsu FJ, Chapman CJ, Spellerberg M, Stevenson FK, et al. Rapid cytotoxicity of human B lymphocytes induced by VH4-34 (VH4.21) gene-encoded monoclonal antibodies, II. Clin Exp Immunol. 1997;108(1):151–9. doi: 10.1046/j.1365-2249.1997.d01-976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat NM, Bieber MM, Young LW, Teng NN. Susceptibility of B-cell lymphoma to human antibodies encoded by the V4-34 gene. Crit Rev Oncol Hematol. 2001;39(1–2):59–68. doi: 10.1016/s1040-8428(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 20.Bieber MM, Twist CJ, Bhat NM, Teng NN. Effects of human monoclonal antibody 216 on B-progenitor acute lymphoblastic leukemia in vitro. Pediatr Blood Cancer. 2007;48(4):380–6. doi: 10.1002/pbc.20770. [DOI] [PubMed] [Google Scholar]
- 21.Glasky MS, Yin A, Smith LH, Bieber M, Teng NN. Adenocarcinoma-reactive human monoclonal antibody MS2B6 defines an antigen in simple glandular epithelium. Hum Antibodies Hybridomas. 1992;3(3):114–22. [PubMed] [Google Scholar]
- 22.Kantarjian HM, Gandhi V, Kozuch P, Faderl S, Giles F, Cortes J, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21(6):1167–73. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23(31):7942–50. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 24.Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG, et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31(12):936–41. doi: 10.1097/MPH.0b013e3181bdf211. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien MM, Lacayo NJ, Lum BL, Kshirsagar S, Buck S, Ravindranath Y, et al. Phase I study of valspodar (PSC-833) with mitoxantrone and etoposide in refractory and relapsed pediatric acute leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;54(5):694–702. doi: 10.1002/pbc.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas DA, Kantarjian HM, Stock W, Heffner LT, Faderl S, Garcia-Manero G, et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer. 2009;115(23):5490–8. doi: 10.1002/cncr.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd JC, O’Brien S, Flinn IW, Kipps TJ, Weiss M, Rai K, et al. Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2007;13(15):4448–55. doi: 10.1158/1078-0432.CCR-06-1463. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo AL, Yu AL, Reaman G, Ingle AM, Secola R, Adamson PC. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children’s Oncology Group report. Pediatr Blood Cancer. 2009;53(6):978–83. doi: 10.1002/pbc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irie RF, Ollila DW, O’Day S, Morton DL. Phase I pilot clinical trial of human IgM monoclonal antibody to ganglioside GM3 in patients with metastatic melanoma. Cancer Immunol Immunother. 2004;53(2):110–7. doi: 10.1007/s00262-003-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin TS, Stock W, Xu H, Phelps MA, Lucas MS, Guster SK, et al. A phase I/II dose escalation study of apolizumab (Hu1D10) using a stepped-up dosing schedule in patients with chronic lymphocytic leukemia and acute leukemia. Leuk Lymphoma. 2009;50(12):1958–63. doi: 10.3109/10428190903186486. [DOI] [PMC free article] [PubMed] [Google Scholar]