Abstract
Background
The current gold-standard for diagnosing heparin-induced thrombocytopenia is the detection of platelet-activating antibodies by means of functional assays which, since they are time consuming and not widely available, are not suited to guiding acute treatment decisions. The objective of our study was to assess the ability of more rapid immunoassays to predict the presence of functionally relevant anti-platelet factor 4/heparin-antibodies.
Design and Methods
We analyzed 1,291 of 1,383 (93.4%) patients consecutively evaluated for suspected heparin-induced thrombocytopenia at our institution. Clinical pre-test probability was defined by the 4T-score. Anti-platelet factor 4/heparin-antibodies were measured with three immunoassays (ID-H/PF4-PaGIA, Asserachrom-HPIA, and GTI-PF4) and their functional relevance was assessed by a two-point heparin-induced platelet aggregation test. Performance of the immunoassays was evaluated by receiver operating characteristic analysis.
Results
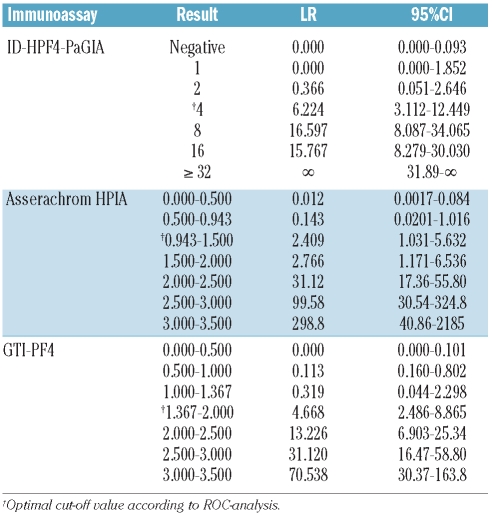
Among 1,291 patients, 96 (7.4%) had a positive heparin-induced platelet aggregation-test: 7 of 859 (0.8%) with a low, 50 of 358 (14.0%) with an intermediate, and 39 of 74 (52.7%) with a high 4T-score. Receiver operating characteristics analysis indicated that best immunoassay thresholds for predicting a positive platelet aggregation test were: Titer of 4 or more (ID-H/PF4-PaGIA), optical density more than 0.943 (Asserachrom-HPIA) and more than 1.367 (GTI-PF4). A 100% negative predictive value was observed at the following thresholds: Titer of 1 or under (ID-H/PF4-PaGIA), optical density less than 0.300 (Asserachrom-HPIA) and less than 0.870 (GTI-PF4). A 100% positive predictive value was reached only by ID-H/PF4-PaGIA, at titers of 32 or over. Positive and negative likelihood ratios were calculated for results between the thresholds with 100% negative or positive predictive value.
Conclusions
We show that: i) negative and weak positive results of immunoassays detecting anti-platelet factor 4/heparin-antibodies exclude heparin-induced thrombocytopenia; ii) anti-platelet factor 4/heparin-antibody titers of 32 or over (ID-H/PF4-PaGIA) have a 100% positive predictive value for functionally relevant antibodies; iii) combining the clinical pre-test probability with the likelihood ratio of intermediate immunoassay results allows assessment of post-test probability for heparin-induced thrombocytopenia in individual patients.
Keywords: heparin-induced thrombocytopenia, diagnosis, quantitative immunoassay, predictive value, likelihood ratio, Bayes’ theorem
Introduction
Heparin-induced thrombocytopenia (HIT) is a drug-induced, antibody-mediated condition characterized by a highly procoagulant state.1,2 HIT is usually caused by IgG antibodies directed against heparin-bound platelet factor 4 (PF4). Macromolecular ternary complexes (formed by HIT antibodies, PF4-tetramers and heparin chains) are able to activate platelets, endothelial cells and monocytes, leading to excessive in vivo thrombin generation.3 If unrecognized and left untreated, HIT can lead to severe venous and arterial thromboembolic complications threatening patients’ limbs and lives.
The diagnosis of HIT is based on clinical features, which can be employed to determine the 4T pre-test probability score,4–6 and laboratory documentation of heparin-dependent antibodies.7 Recent studies have shown that a low clinical probability assessed by the 4T scoring system has a high negative predictive value for the presence of HIT.6,8–12 However, these publications also indicate that a high 4T probability score is not strongly predictive for HIT and a relevant proportion of the investigated patients turn out to have an intermediate pre-test probability.8–12 These results support the concept that identification of patients with HIT cannot be made on a clinical basis only but requires laboratory demonstration of relevant HIT antibodies. The turn-around time of these assays has clinical implications because of the ensuing treatment decisions. In fact, continuing heparin, or even stopping it without starting an alternative anticoagulant drug in a patient with unrecognized HIT carries a high thrombotic risk;13 on the other hand, initiating danaparoid or a direct thrombin inhibitor (argatroban, lepirudin) in patients without HIT exposes them to an unnecessary high bleeding risk and is expensive.14,15 Therefore, a case can be made for the need for rapid laboratory HIT diagnosis to guide treatment decisions.16
Up to now, the laboratory gold-standard for the diagnosis of HIT is the demonstration of in vitro platelet-activating HIT antibodies.7 Unfortunately, these functional assays are time consuming and not widely available, making them unsuitable for helping clinicians dealing with a patient with suspected HIT.17 More rapid laboratory evidence of anti-PF4/heparin antibodies can be achieved by immunoassays, either enzyme-linked immunosorbent assays (ELISA)18,19 or particle-gel immune assays (PaGIA).20
The primary aim of the present work was to assess the ability of three commercial immunoassays for anti-PF4/heparin antibodies to predict the presence of HIT antibodies activating platelets in vitro. The second objective of our work was to evaluate the prevalence of functionally relevant antibodies according to the pre-test clinical probability for HIT, as assessed by the 4T score.4,5 We show that this information allows clinicians to apply Bayes’ theorem to HIT diagnostic workup of individual patients.
Design and Methods
Patients
Between May 1995 and December 2009 we recorded 1,383 patients evaluated for clinically suspected HIT at our institution. After exclusion of patients with incomplete information concerning the 4T score or without the plasma samples needed to complete laboratory assays (see below), we were able to evaluate 1,291 (93.3%) patients. Clinical categories were as follows: 691 (53.5%) medical, 303 (23.5%) surgical, 259 (20.1%) intensive care, 15 (1.2%) obstetrics/gynecology, 7 (0.5%) pediatrics, and 16 (1.2%) unknown. Among the surgical patients, 154 (50.8%) were general or abdominal surgery, 95 (31.4%) cardio-vascular surgery, 43 (14.2%), orthopedics, and 11 (3.6%) neurosurgery. Overall median age was 67.9 years (range 1.5–106.4 years, interquartile range 56.7–76.2 years). There was no age difference between women (n=566, median 67.4 years) and men (n=725, median 68.3; P=0.7125). The study was performed in accordance with local regulations for diagnostic-laboratory studies (Kantonale Ethikkommission Bern; www.kek-bern.ch).
Historical note
The founding document of the Swiss Confederation, named Federal Charter or Letter of Alliance, was written in August 1291.
Pre-test clinical probability for HIT (4T score)
From January 2004, the clinical probability for HIT was routinely assessed according to the 4T score4,5 by the consulting hematologist (n=1,021). For 19 HIT patients treated with lepirudin between 2001 and 2003, the 4T score had been retrospectively calculated in the context of a previous study.21 The 4T score for the remaining 251 patients (19.4%) was retrospectively assessed by the first author in a blinded fashion and verified by the corresponding author.
Plasma samples
Blood was drawn into 10 ml plastic syringes (Monovette®, Sarstedt, Nümbrecht, Germany) containing 1 mL 0.106 mol/L trisodium citrate. Plasma was prepared by double centrifugation at 1,500 × g for 10 min each at room temperature. Plasma aliquots were stored in polypropylene tubes at −70ºC.
Detection of anti-PF4/heparin antibodies by immunoassays
Anti-PF4/heparin antibodies were detected by the ID-H/PF4-PaGIA (DiaMed SA, Cressier sur Morat, Switzerland).20 In detail, 10 μL of plasma were pipetted into the reaction chamber of the test ID-card followed by 50 μL of polymer particles (red high-density polystyrene beads coated with heparin/PF4 complexes). After incubation at room temperature for 5 min, the ID-card was centrifuged for 10 min in the dedicated ID-centrifuge (DiaMed SA). In the absence of a significant level of anti-PF4/heparin antibodies in the test sample, the particles sank to the bottom of the gel chamber. If anti-PF4/heparin antibodies were present, the red polymer particles were cross-linked and remained on the top of the gel chamber. In case of an indeterminate (defined as “Neither a clear agglutinate nor a full sedimentation of the particles”) or positive test with the undiluted sample, we repeated the assay with serially diluted plasma (up to 1:1024) until the result was negative. Thus, for a 1:2 dilution, 50 μL of plasma were mixed with 50 μL of Diluent II (DiaMed SA), and subsequently dilutions were obtained by pipetting 50 μL of the preceding dilution with 50 μL of Diluent II. The reported titer is the last positive detection followed by either indeterminate or negative results, as previously described.22 For the 130 patients evaluated before July 2001, the ID-H/PF4-PaGIA was performed in the context of our initial study.22 Since July 2001, we have routinely performed the ID-H/PF4-PaGIA at the moment of evaluation for suspected HIT. The ID-H/PF4-PaGIA results reported in this paper were obtained with carefully evaluated polymer lots, thus excluding false negative results.23
Anti-PF4/heparin antibodies were also detected with two commercially available enzyme-linked immunosorbent assays (ELISAs; GTI-PF4 Enhanced, Genetic Testing Institute, Waukesha, WI, USA and Asserachrom HPIA, Diagnostica Stago, Asnière sur Seine, France) and measured at 405 (GTI-PF4) or 450 (Asserachrom HPIA) nm with a microtiter plate reader (Anthos ht III, Hemotec, Gelterkinden, Switzerland). As previously published, we did not observe any difference in results when using serum instead of plasma.23
Heparin-induced Platelet Aggregation Test (PAT)
This assay was performed in a light transmission aggregometer (models PAP-4, Bio/Data, Hatboro, Pennsylvania, USA until 2005 and thereafter APACT 4A and APACT 4004 (LABiTec, Ahrensburg, Germany) as previously described.24 Briefly, 4 separate mixtures were prepared, each containing 100 μL patient’s platelet poor plasma and 100 μL platelet rich plasma from one of 4 different selected donors known to have platelets reactive to HIT antibodies. The samples were initially stirred for 4 min at 37º C in order to detect spontaneous heparin-independent aggregation. Thereafter, 10 μL of a solution of unfractionated heparin (Liquemin®) and, in a subsequent run, low molecular weight heparin (the preparation administered to the specific patient) are added and light transmission is recorded for up to 15 min. A positive test is defined by: i) at least 2 out of 4 samples reaching 50% or more aggregation with 0.5 U/mL heparin; and ii) abrogation of the reaction by 100 U/mL heparin.24 A two-point PAT with these two final heparin concentrations has been shown to reach a 100% specificity,24,25 with a sensitivity of approximately 80% if reactive platelets from selected donors were employed.25 As an internal control, we always test a plasma sample from a previously positive patient at the beginning and at the end of each PAT series.
Statistics
Quantitative data were analyzed by SigmaStat software (version 3.5; Systat Software Inc., San Jose, CA, USA) and are expressed as median, range and interquartile range. The 95% confidence interval (95%CI) for the proportions (p) reported in Table 1 was calculated by the following equation: “p±1.96 × standard error (S.E.)”, where the S.E. was estimated by the quadratic square of [p(1-p)/n] (n = sample size). Receiver operating characteristics (ROC) analysis was performed with MedCalc software (version 11.1; Mariakerke, Belgium).26 Comparison of ROC curves obtained with the different immunoassays was performed by the non-parametric method of DeLong27 (MedCalc software). Significance was set at the 5% level. The interested reader is referred to the Online Supplementary Appendix “Brief tutorial on ROC analysis and clinical application of Bayes’ theorem”.
Table 1A.
Pre-test probability for in vitro platelet-activating HIT antibodies according to the 4T score.
Results
Prevalence of in vitro platelet-activating heparin-dependent antibodies in patients evaluated for suspected HIT
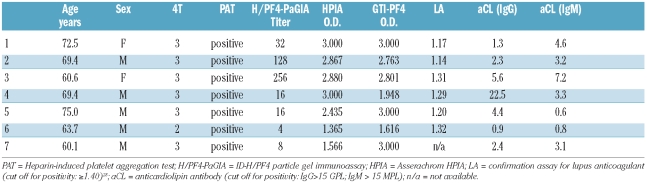
Among the 1,291 patients of our original Swiss cohort, 96 (7.4%) had a positive heparin-induced platelet aggregation test (PAT), demonstrating the presence of platelet-activating HIT antibodies. Table 1A shows that among the patients evaluated in Bern, 7 of 859 (0.8%) with a low 4T score (0–3),4,5 50 of 358 (14.0%) with an intermediate 4T score (4–5), and 39 of 74 (52.7%) with a high 4T score (6–8) had functionally relevant HIT antibodies. Laboratory data of the 7 patients with low 4T score and positive PAT are summarized in Table 1B. We consider that these 7 patients had bona fide heparin-dependent platelet-activating anti-PF4/heparin antibodies because: i) PAT excluded spontaneous platelet aggregation and demonstrated inhibition of aggregation with heparin excess (see Design and Methods section); ii) plasma samples contained high-titer anti-PF4/heparin antibodies (Table 1B); iii) the combination of a positive PAT with a positive ELISA has been shown to have a 100% positive predictive value for HIT;29 and iv) plasma samples did not test positive for antiphospholipid antibodies30 (Table 1B).
Table 1B.
Characteristics of the 7 patients with low 4T score and positive PAT (see Table 1A).
Performance of immunoassays in identifying patients with functionally relevant HIT antibodies
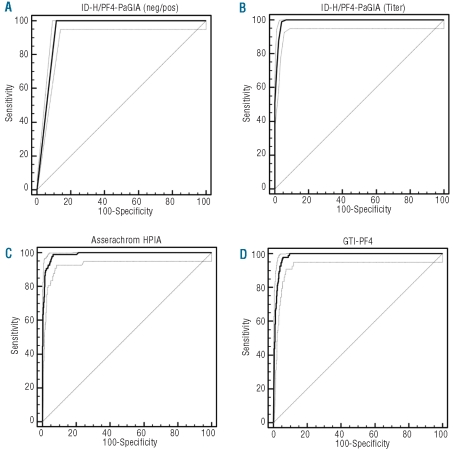
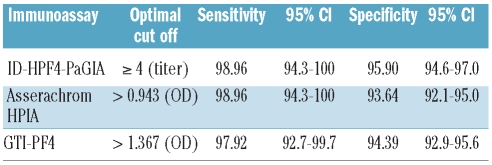
Figure 1 shows that the ID-H/PF4-PaGIA giving a negative/positive result according to the manufacturer’s instruction31 was significantly less informative than both ELISAs, with an area under the ROC curve (AUROC) of 0.943 compared to 0.990 for the Asserachrom HPIA (P<0.0001) and 0.985 for the GTI-PF4 (P<0.0001). However, when a positive result of the ID-H/PF4-PaGIA was semi-quantitated by titer3,22 the performance of this assay, with an AUROC of 0.992, was at least as good as that of both ELISAs (P=0.4683 vs. HPIA and P=0.0174 vs. GTI-PF4). According to ROC analysis, the cut offs with the best compromise between sensitivity and specificity for detecting functionally relevant HIT antibodies were a titer of 4 or more (ID-H/PF4-PaGIA), an optical density (OD) of more than 0.943 for the Asserachrom HPIA and an OD of more than 1.367 for the GTI-PF4 (Table 2).
Figure 1.
ROC curves of three immunoassays for anti-PF4/heparin antibodies. The results of the immunoassays were defined to be true positive or negative depending on whether the respective plasma sample was able to activate donor platelets in a heparin-induced platelet aggregation test (PAT). (A) ID-H/PF4-PaGIA with qualitative result. (B) ID-H/PF4-PaGIA with quantitative result. (C) Asserachrom HPIA. (D) GTI-PF4. The dotted lines represent the 95%CI of the ROC curve. Note the significantly lower performance of the ID-H/PF4-PaGIA with a categorical (negative/positive) result (A) as compared to a semi-quantitated result by titer (B). See text and Online Supplementary Appendix for details.
Table 2.
Sensitivity and specificity for optimal cut offs of immunoassays for in vitro platelet-activating HIT antibodies
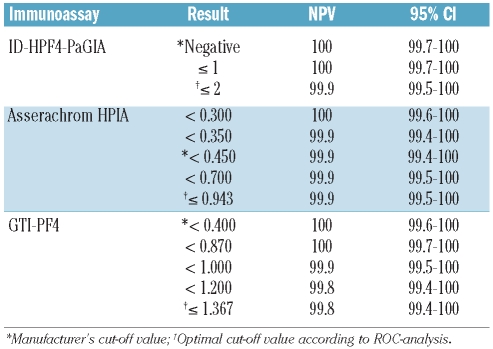
Negative predictive values (NPV) and negative likelihood ratios (LR –) of immunoassays for functionally relevant HIT antibodies
From a clinical point of view, the most useful characteristic of a diagnostic test result is its ability to exclude or confirm a given disease. This information is conveyed by the predictive value and likelihood ratio. Specifically, the negative predictive value (NPV) represents the proportion of individuals with a negative test result who do not have the disease and excludes a disease when it reaches a value of 100%. Table 3 shows that all three assays were able to exclude the presence of functionally relevant HIT antibodies at the following thresholds: titer 1 or under (ID-H/PF4-PaGIA), OD less than 0.300 (Asserachrom HPIA), OD less than 0.870 (GTI-PF4). Of note, these thresholds differ from those suggested by the manufacturers, being a negative result for the ID-H/PF4-PaGIA, an OD of about 0.450 for the Asserachrom HPIA and an OD of 0.400 for the GTI-PF4. For immunoassay results above the threshold with a 100% NPV but below the cut-off values with the best compromise between sensitivity and specificity, and, therefore, with a NPV less than 100%, it is possible to calculate the negative likelihood ratio (LR –). This ratio represents the probability of an individual with the disease having a negative test divided by the probability of an individual without the disease having a negative test result, and can be used to modify the individual clinical pre-test probability for a given disease (see below). Negative likelihood ratios (i.e. < 1.0) of the three immunoassays are shown in Table 4.
Table 3.
Cut-off negative predictive values (NPV) of different quantitative immunoassays for in vitro platelet-activating HIT antibodies.
Table 4.
Interval likelihood ratios (LR) of quantitative immunoassays for in vitro platelet-activating HIT antibodies.
Positive predictive values (PPV) and positive likelihood ratios (LR +) of immunoassays for functionally relevant HIT antibodies
Similarly to the ability of a negative or low quantitative assay result to exclude a disease, the magnitude of a positive test result can be used to confirm the presence of a given disease. This is expressed by the positive predictive value (PPV), which represents the proportion of individuals with a positive test result who do have the disease. Among the three immunoassays investigated, only the ID-H/PF4-PaGIA reached (at titers of 32 or over) a 100% PPV for a positive PAT (Table 5), and is, therefore, able to predict with certainty the presence of functionally relevant HIT antibodies. Of note, in our cohort, 49 of the 96 (51%) patients with a positive PAT had an anti-PF4/heparin antibody titer of 32 or over. For test results lying above the cut-off value with the best compromise between sensitivity and specificity, but lower than the threshold with a 100% PPV, it is possible to calculate the positive likelihood ratio (LR +). This ratio expresses the probability of an individual with the disease having a positive test divided by the probability of an individual without the disease having a positive test result. Positive likelihood ratios (i.e. > 1.0) of the three immunoassays are shown in Table 4.
Table 5.
Cut-off positive predictive values (PPV) of different quantitative immunoassays for in vitro platelet-activating HIT antibodies.
Applying Bayes’ theorem to HIT diagnostic workup
PPV and NPV strongly depend on the disease prevalence in the patients being tested and can be misleading. Likelihood ratios are clinically more useful, because they provide information on how many times more (or less) likely patients with the disease are to have a particular test result than patients without the disease. Therefore, likelihood ratios can be used to calculate the probability of disease for individual patients.32,33 More specifically, the LR of a quantitative test result can be combined with the clinical pre-test probability for a given disease in order to reach a higher or lower post-test probability. This can be performed either by a mathematical calculation (see Online Supplementary Appendix) or visually with the aid of Fagan’s nomogram.33,34
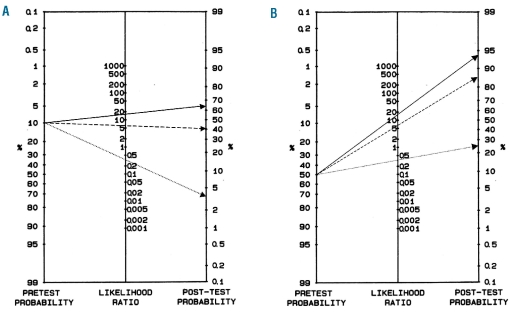
The 4T scoring system allows pre-test probability for the presence of functionally relevant HIT antibodies to be defined. Table 1A summarizes the published results of several groups around the world and shows that for patients with a low 4T score (0–3) the probability of having a positive functional assay for HIT antibodies is less than 1% (0.5%; 95%CI: 0.2–0.9). For patients with an intermediate 4T score (4–5) this probability is about 10% (10.8; 95%CI: 8.8–12.8) and for patients with a high 4T score (6–8) it is about 50% (47.8%; 95%CI: 41.1–54.7). Figure 2A illustrates the application of Bayes’ theorem to HIT diagnosis for a hypothetical patient with an intermediate 4T score. For this patient, the pre-test clinical probability of having in vitro platelet-activating HIT antibodies is about 10% (Table 1A), corresponding to a pre-test odds ratio of 0.11 (see Online Supplementary Appendix).
Figure 2.
Applying Bayes’ theorem to HIT diagnostic work-up. (A) Fagan’s nomogram32,33 illustrating how a titer of 2 (dotted line), 4 (interrupted line) or 8 (straight line) modifies post-test probability for HIT, as defined by a positive heparin-induced platelet aggregation test (PAT), in a patient with an intermediate 4T score. (B) Fagan’s nomogram32,33 illustrating how a titer of 2 (dotted line), 4 (interrupted line) or 8 (straight line) modifies post-test probability for HIT, as defined by a positive heparin-induced platelet aggregation test (PAT), in a patient with a high 4T score. See text for details.
In case of a positive ID-H/PF4-PaGIA with a titer of 2, which is below the optimal cut off but does not have a 100% NPV (in our cohort one of 35 patients with a titer of 2 had a positive PAT), we can use its LR – (0.366; Table 4) to decrease the post-test probability of HIT. By plotting both variables on the Fagan’s nomogram (Figure 2A) we derive a probability for functionally relevant HIT antibodies of about 4% (by mathematical calculation: post-test odds = 0.11×0.366 = 0.04026, corresponding to a post-test probability of 3.9%). This probability argues against the presence of HIT. On the other hand, in case of a positive ID-H/PF4-PaGIA with a titer of 4, which is above the optimal cut off without reaching a 100% PPV, we can use its LR + (6.224; Table 4) in order to estimate post-test probability of HIT. With Fagan’s nomogram (Figure 2A) we derive a probability for a positive PAT of about 40% (post-test odds = 0.11×6.224 = 0.68464, corresponding to a post-test probability of 40.6%). Finally, the same patient with a titer of 8 (LR + 16.597; Table 4) reaches a post test-probability for HIT of about 65% (post-test odds = 0.11×16.597= 1.82567, corresponding to a post-test probability of 64.6%). Both these probabilities argue in favor of HIT and are high enough to justify acute treatment as potential HIT, until further testing can confirm the diagnosis.
Similarly, for an imaginary patient with a high 4T score (Figure 2B) and, therefore, a clinical pre-test probability for a positive PAT of about 50% (Table 1A), the same test results would either decrease post-test probability to about 25% (titer of 2: post-test odds = 1.0×0.366= 0.366, i.e. post-test probability 26.8%) or increase it to about 85% (titer of 4: post-test odds = 1.0×6.224= 6.224, i.e. post-test probability 86.2%) and about 95% (titer of 8: post-test odds = 1.0×16.597 = 16.597, i.e. post-test probability 94.3%).
It is important to underline that the diverging clinical implications of a titer of 4 compared to a titer of 2 derive from the fact that these values lie at opposite sides of the optimal cut offs (Table 2). As a consequence (and similarly to categorical yes/no cut offs) quantitative immunoassay results close to the cut-off threshold, must be interpreted with care, taking into account the coefficient of variation of the assay employed.
Discussion
In our Swiss cohort of 1,291 patients evaluated for suspected HIT, we found a prevalence of platelet-activating antibodies of 0.8% among those with a low 4T score,4,5 14.4% among those with an intermediate 4T score, and 52.7% among patients with a high 4T score. These numbers are consistent with the prevalence published by various groups around the world (Table 1A). Overall, the diagnosis of HIT, as defined by a positive functional assay for heparin-dependent antibodies, could be confirmed in a small proportion (less than 10%) of patients and, therefore, the suggestion of switching to an alternative anticoagulant drug as soon as HIT is suspected is questionable. In order to guide treatment decisions, it would be very helpful to achieve rapid laboratory diagnosis of HIT.16 The aim of the present work was to evaluate the ability of immunoassays to predict the presence of platelet-activating HIT antibodies which are usually assessed by more time-consuming functional assays.
We compared the operating characteristics of three commercial immunoassays for anti-PF4/heparin antibodies: Asserachrom HPIA, GTI-PF4 and ID-H/PF4-PaGIA. Figure 1 shows that the ID-H/PF4-PaGIA giving a categorical (negative/positive) result according to the manufacturer’s instructions31 was significantly less informative than either of the ELISAs. However, when a positive result was semi-quantitated by titer,22 the ID-H/PF4-PaGIA performed at least as well as both quantitative ELISAs (Figure 1). Of note, the optimal cut-off value of 1.367 identified by ROC analysis for the GTI-PF4 ELISA (Table 2) is well in line with threshold OD values published by other groups: 1.400 described by the group of Warkentin35,36 and 1.185 described by Bakchoul et al.12 ROC analysis also confirms an ideal cut-off of or over 4 for the ID-H/PF4-PaGIA (Table 2), as previously published.3,22 Besides identifying optimal cut-off thresholds for the three immunoassays, ROC analysis allows definition of likelihood ratios for all possible results (Table 4). This information is clinically very useful because, applying Bayes’ theorem, it allows us to transform pre-test clinical probability for HIT into a post-test probability when evaluating individual patients.32,33
Applying Bayes’ theorem to HIT diagnostic work up
In centers where a low 4T score has been shown to exclude HIT with a 100% NPV (Table 1A), one may rely on clinical judgment. In our cohort, we found 7 of 859 (0.8%) patients with a low 4T score and bona fide platelet activating anti-PF4/heparin antibodies (Table 1B). Therefore, we still prefer to employ the ID-H/PF4-PaGIA in order to safely exclude HIT.16,37 This concept has been confirmed by four groups.9–12 For instance, Pouplard et al., also employing a Bayesian approach, have demonstrated that in patients with an intermediate 4T score, a negative ID-H/PF4-PaGIA significantly decreased the probability of HIT from 10.9% to 0.6%.9 Of note, in that study a negative PaGIA did not reach a 100% NPV for platelet-activating HIT antibodies because of one out of 22 false negative result,9 probably due to a faulty polymer lot.23 The present work, indicating negative likelihood ratios for different values results below the optimal cut offs according to ROC analysis (Table 4), expands this diagnostic concept and allows also low positive results to be used in order to exclude HIT. In fact, as stated by Warkentin et al.,36 a weak positive immunoassay for anti-PF4/heparin antibodies is paradoxically a strong argument against the presence of HIT. According to our data, OD values of 0.943 or below with the Asserachrom HPIA, of 1.367 or below with the GTI-PF4, and a titer less than 4 with the ID-H/PF4-PaGIA significantly decrease the post-test probability for HIT. In our experience, this approach can also be used for patients with an intermediate or even high pre-test clinical probability for HIT.
The present work supports the possibility of rapidly confirming HIT using the quantitative result of an immunoassay for anti-PF4/heparin antibodies. Several groups have suggested that the magnitude of a positive ELISA is clinically relevant,35,38–41 and concerning the ID-H/PF4-PaGIA, we had already demonstrated the clinical utility of this approach.3,22 In the present study, ROC analysis confirms a cut-off titer of 4 or over3,22 as providing the best compromise between sensitivity and specificity, and shows that titers of 32 or over have a PPV of 100% for the presence of in vitro platelet-activating HIT antibodies. Additionally, we show that the magnitude of a quantitative immunoassay result above the ideal cut off as indicated by ROC analysis, can be employed to modify the clinical pre-test probability of HIT in individual patients (Figure 2).
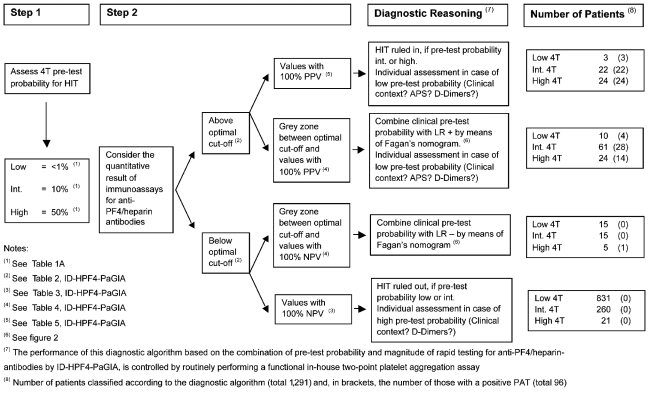
In conclusion, we suggest that combining pre-test clinical probability for HIT as assessed by the 4T score with the quantitative result of an immunoassay for antibodies directed against the PF4/heparin complex can be used not only for excluding but also for confirming HIT. On one hand, we show that a negative and a weak positive immunoassay result can be used to exclude HIT. On the other hand, we demonstrate that a quantitative positive result above the ideal cut-off value (as defined by ROC analysis) increases the post-test probability for HIT as a function of its magnitude. Of particular note, a positive ID-H/PF4-PaGIA with a titer of 32 or more has a PPV of 100% for functionally relevant HIT antibodies. Figure 3 shows the diagnostic algorithm used at our institution.
Figure 3.
Diagnostic algorithm for patients with suspected HIT at our institution. We first asses pre-test probability for a positive PAT calculating the 4T score. We then consider the magnitude of the immunoassay for anti-PF4/heparin antibodies (ID-HPF4-PaGIA) and combine both as detailed under “Diagnostic Reasoning”. Immunoassay results close to the optimal cut-off value should be interpreted with cautioun, considering the in house coefficient of variation of the assay employed. APS = Look for clinical or laboratory clues for an antiphospsholipid syndrome.29 D-dimers = Low D-dimers represent a strong argument against the presence of HIT.3
We think that our results should be clinically relevant and widely applicable because: i) we present data on 1,291 of 1,383 (93.4%) patients evaluated for suspected HIT over a period of 15 years; ii) the patient population is mixed (53.5% medical, 23.5% surgical, 20.5% intensive care) representing the real life context of a general hospital; iii) the majority of the 4T scores (1,021 of 1,291) were prospectively assessed by the consulting hematologist; iv) the majority of assays for HIT-antibodies were also prospectively performed at the time of diagnostic work up (see Design and Methods section). Moreover, using a two-point PAT as gold-standard for platelet activating HIT anti-bodies, which has been shown to have a high specificity and an acceptable sensitivity,25 we avoid the risk of overestimating the performance of immunoassays.
This is the first report showing, on a large cohort of over 1,200 patients, that a quantitative result of an immunoassay above a given threshold is equivalent to a positive functional assay for HIT antibodies. In addition, we show that applying Bayes’ theorem to HIT diagnostic work-up allows the rapid exclusion or confirmation of diagnosis within a few hours after HIT has been suspected, thus enhancing the rationale for individualized treatment decisions.
Acknowledgment
We would like to thank all the physicians of our Department of Hematology who were involved in the care of HIT patients over the years and the technicians of the Central Hematology Laboratory who perform routine diagnostic HIT assays. We also thank Therese Jost for her efficient technical assistance.
Footnotes
Funding: Lorenzo Alberio is supported by a grant from the Swiss National Science Foundation (Grant Nr. 3200-065337.01)
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Keeling D, Davidson S, Watson H. The management of heparin-induced thrombocytopenia. Br J Haematol. 2006;133(3):259–69. doi: 10.1111/j.1365-2141.2006.06018.x. [DOI] [PubMed] [Google Scholar]
- 2.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):340S–80S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 3.Chilver-Stainer L, Lämmle B, Alberio L. Titre of anti-heparin/PF4-antibodies and extent of in vivo activation of the coagulation and fibrinolytic systems. Thromb Haemost. 2004;91(2):276–82. doi: 10.1160/TH03-07-0454. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Heddle NM. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr Hematol Rep. 2003;2(2):148–57. [PubMed] [Google Scholar]
- 5.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003;121(4):535–55. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 6.Crowther MA, Cook DJ, Albert M, Williamson D, Meade M, Granton J, et al. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. J Crit Care. 2010;25(2):287–93. doi: 10.1016/j.jcrc.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(Suppl 1):9–12. doi: 10.1111/j.1538-7836.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 8.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759–65. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 9.Pouplard C, Gueret P, Fouassier M, Ternisien C, Trossaert M, Regina S, et al. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific to heparin/PF4 for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2007;5(7):1373–9. doi: 10.1111/j.1538-7836.2007.02524.x. [DOI] [PubMed] [Google Scholar]
- 10.Denys B, Stove V, Philippe J, Devreese K. A clinical-laboratory approach contributing to a rapid and reliable diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2008;123(1):137–45. doi: 10.1016/j.thromres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Bryant A, Low J, Austin S, Joseph JE. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T’s score and particle gel immunoassay. Br J Haematol. 2008;143(5):721–6. doi: 10.1111/j.1365-2141.2008.07401.x. [DOI] [PubMed] [Google Scholar]
- 12.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(8):1260–5. doi: 10.1111/j.1538-7836.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallis DE, Workman DL, Lewis BE, Steen L, Pifarre R, Moran JF. Failure of early heparin cessation as treatment for heparin-induced thrombocytopenia. Am J Med. 1999;106(6):629–35. doi: 10.1016/s0002-9343(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 14.Lewis BE, Wallis DE, Berkowitz SD, Matthai WH, Fareed J, Walenga JM, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103(14):1838–43. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 15.Lubenow N, Eichler P, Lietz T, Greinacher A. Lepirudin in patients with heparin-induced thrombocytopenia - results of the third prospective study (HAT-3) and a combined analysis of HAT-1, HAT-2, and HAT-3. J Thromb Haemost. 2005;3(11):2428–36. doi: 10.1111/j.1538-7836.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 16.Alberio L. Heparin-induced thrombocytopenia: some working hypotheses on pathogenesis, diagnostic strategies and treatment. Curr Opin Hematol. 2008;15(5):456–64. doi: 10.1097/MOH.0b013e32830b84a2. [DOI] [PubMed] [Google Scholar]
- 17.Otis SA, Zehnder JL. Heparin-induced thrombocytopenia: current status and diagnostic challenges. Am J Hematol. 2010;85(9):700–6. doi: 10.1002/ajh.21770. [DOI] [PubMed] [Google Scholar]
- 18.Amiral J, Bridey F, Dreyfus M, Vissoc AM, Fressinaud E, Wolf M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68(1):95–6. [PubMed] [Google Scholar]
- 19.Visentin GP, Moghaddam M, Beery SE, McFarland JG, Aster RH. Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med. 2001;138(1):22–31. doi: 10.1067/mlc.2001.115525. [DOI] [PubMed] [Google Scholar]
- 20.Meyer O, Salama A, Pittet N, Schwind P. Rapid detection of heparin-induced platelet antibodies with particle gel immunoassay (ID-HPF4) Lancet. 1999;354(9189):1525–6. doi: 10.1016/S0140-6736(99)03625-9. [DOI] [PubMed] [Google Scholar]
- 21.Tschudi M, Lämmle B, Alberio L. Dosing lepirudin in patients with heparin-induced thrombocytopenia and normal or impaired renal function: a single-center experience with 68 patients. Blood. 2009;113(11):2402–9. doi: 10.1182/blood-2008-07-162271. [DOI] [PubMed] [Google Scholar]
- 22.Alberio L, Kimmerle S, Baumann A, Taleghani BM, Biasiutti FD, Lämmle B. Rapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopenia. Am J Med. 2003;114(7):528–36. doi: 10.1016/s0002-9343(03)00080-9. [DOI] [PubMed] [Google Scholar]
- 23.Schneiter S, Colucci G, Sulzer I, Barizzi G, Lämmle B, Alberio L. Variability of anti-PF4/heparin antibody results obtained by the rapid testing system ID-H/PF4-PaGIA. J Thromb Haemost. 2009;7(10):1649–55. doi: 10.1111/j.1538-7836.2009.03507.x. [DOI] [PubMed] [Google Scholar]
- 24.Stricker H, Lämmle B, Furlan M, Sulzer I. Heparin-dependent in vitro aggregation of normal platelets by plasma of a patient with heparin-induced skin necrosis: specific diagnostic test for a rare side effect. Am J Med. 1988;85(5):721–4. doi: 10.1016/s0002-9343(88)80250-x. [DOI] [PubMed] [Google Scholar]
- 25.Chong BH, Burgess J, Ismail F. The clinical usefulness of the platelet aggregation test for the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost. 1993;69(4):344–50. [PubMed] [Google Scholar]
- 26.Stephan C, Wesseling S, Schink T, Jung K. Comparison of eight computer programs for receiver-operating characteristic analysis. Clin Chem. 2003;49(3):433–9. doi: 10.1373/49.3.433. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 28.Luginbühl R, Barizzi G, Sulzer I, Lämmle B, Alberio L. Screening for lupus anticoagulant: improving the performance of the lupus-sensitive PTT-LA. Int J Lab Hematol. 2011;33(2):168–75. doi: 10.1111/j.1751-553X.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- 29.Pouplard C, Amiral J, Borg JY, Laporte-Simitsidis S, Delahousse B, Gruel Y. Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1999;111(5):700–6. doi: 10.1093/ajcp/111.5.700. [DOI] [PubMed] [Google Scholar]
- 30.Pauzner R, Greinacher A, Selleng K, Althaus K, Shenkman B, Seligsohn U. False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Thromb Haemost. 2009;7(7):1070–4. doi: 10.1111/j.1538-7836.2009.03335.x. [DOI] [PubMed] [Google Scholar]
- 31.Köchli A. Variability of anti-PF4/heparin antibody results obtained by the rapid testing system ID-H/PF4-PaGIA: a rebuttal. J Thromb Haemost. 2009;7(10):1753–5. doi: 10.1111/j.1538-7836.2009.03516.x. [DOI] [PubMed] [Google Scholar]
- 32.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329(7458):168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sackett DL. Interpretation of diagnostic data: 5. How to do it with simple maths. Can Med Assoc J. 1983;129(9):947–54. [PMC free article] [PubMed] [Google Scholar]
- 34.Fagan TJ. Letter: Nomogram for Bayes theorem. N Engl J Med. 1975;293(5):257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 35.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol. 2007;82(12):1037–43. doi: 10.1002/ajh.21032. [DOI] [PubMed] [Google Scholar]
- 36.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304–12. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 37.Rodgers GM. Improving the laboratory diagnosis of heparin-induced thrombocytopenia. Am J Med. 2003;114(7):609–10. doi: 10.1016/s0002-9343(03)00138-4. [DOI] [PubMed] [Google Scholar]
- 38.Warkentin TE, Sheppard JA, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146(6):341–6. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2(12):2133–7. doi: 10.1111/j.1538-7836.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 40.Janatpour KA, Gosselin RC, Dager WE, Lee A, Owings JT, Zhou J, et al. Usefulness of optical density values from heparin-platelet factor 4 antibody testing and probability scoring models to diagnose heparin-induced thrombocytopenia. Am J Clin Pathol. 2007;127(3):429–33. doi: 10.1309/RPE753J4PMG9773Q. [DOI] [PubMed] [Google Scholar]
- 41.Schenk S, El-Banayosy A, Morshuis M, Arusoglu L, Eichler P, Lubenow N, et al. IgG classification of anti-PF4/heparin antibodies to identify patients with heparin-induced thrombocytopenia during mechanical circulatory support. J Thromb Haemost. 2007;5(2):235–41. doi: 10.1111/j.1538-7836.2007.02295.x. [DOI] [PubMed] [Google Scholar]
- 42.Altuntas F, Matevosyan K, Burner J, Shen YM, Sarode R. Higher optical density of an antigen assay predicts thrombosis in patients with heparin-induced thrombocytopenia. Eur J Haematol. 2008;80(5):429–35. doi: 10.1111/j.1600-0609.2008.01035.x. [DOI] [PubMed] [Google Scholar]