Abstract
Background
Traditionally, single-unit red blood cell transfusions were believed to be insufficient to treat anemia, but recent data suggest that they may lead to a safe reduction of transfusion requirements. We tested this hypothesis by changing from a double- to a single-unit red blood cell transfusion policy.
Design and Methods
We performed a retrospective cohort study in patients with hematologic malignancies receiving intensive chemotherapy or hematopoietic stem cell transplantation. The major end-points were the reduction in the total number of red blood cell units per therapy cycle and per day of aplasia. The study comprised 139 patients who received 272 therapy cycles. Overall 2212 red blood cell units were administered in 1548 transfusions.
Results
During the periods of the double- and single-unit policies, one red blood cell unit was transfused in 25% and 84% of the cases and the median number of red blood cell units per transfusion was two and one, respectively. Single-unit transfusion led to a 25% reduction of red blood cell usage per therapy cycle and 24% per aplasia day, but was not associated with a higher out-patient transfusion frequency. In multivariate analysis, single-unit transfusion resulted in a reduction of 2.7 red blood cell units per treatment cycle (P=0.001). The pre-transfusion hemoglobin levels were lower during the single-unit period (median 61 g/L versus 64 g/L) and more transfusions were administered to patients with hemoglobin values of 60 gl/L or less (47% versus 26%). There was no evidence of more severe bleeding or more platelet transfusions during the single-unit period and the overall survival was similar in both cohorts.
Conclusions
Implementing a single-unit transfusion policy saves 25% of red blood cell units and, thereby, reduces the risks associated with allogeneic blood transfusions.
Keywords: red blood cells, transfusion, single-unit, acute leukemia, hematopoietic stem cell transplantation
Introduction
Single-unit red blood cell (RBC) transfusions were extensively criticized in the past. It was believed that RBC trans-fusions were useless if the transfusion requirements could be satisfied by infusion of one RBC unit and that patients were no more in need of the transfusion than their donors.1–3 However, data on the risk or benefit of a single-unit transfusion strategy are scarce and most evidence derives from studies analyzing peri-operative single-unit RBC transfusions in surgical and obstetric populations.3–7 The essential problem of all these studies is that they introduced a considerable selection bias by simply comparing the pre-transfusion hemoglobin levels and the transfusion requirements of patients having received one or two units of RBC. Not surprisingly, these studies showed that patients with single-unit transfusions had higher or even normal pre-transfusion hemoglobin levels when compared to those receiving double-unit transfusions, and a vast majority of those single-unit transfusions would not have been recommended at all, considering today’s guidelines. However, no study has evaluated the effect of double- or single-unit RBC transfusion in patients with hyporegenerative anemia with comparable pre-transfusion hemoglobin levels and there is no evidence supporting double-unit RBC transfusions in patients without active bleeding.
A restrictive transfusion policy with stringent transfusion triggers and a cautious use of blood products is the single most effective measure to reduce transfusion requirements and given the scarcity and inherent risks of allogeneic blood products a reduction of blood transfusions is of major health and economic interest. Reducing the volume per transfusion may save a considerable number of RBC units thereby reducing the patients’ exposure to allogeneic blood products.8 However, despite a lack of studies, most guidelines recommend double-unit transfusions, while only a few, more recent guidelines allow single-unit RBC transfusions in the absence of active bleeding.9–14 Nonetheless, surveys on transfusion practices have shown that more than 90% of physicians currently transfuse two RBC units simultaneously showing that they have not yet implemented this new transfusion policy.15–18
Since data suggest that single-unit transfusions may considerably reduce the total RBC requirements we changed our policy for hospitalized patients without active bleeding from double- to single-unit RBC transfusions. The current study analyzed the effect of the new RBC transfusion policy with regards to the overall RBC requirements and the transfusion efficiency, the adherence to the transfusion policy, as well as safety aspects including bleeding risk and the number of transfused platelets.
Design and Methods
Study population and protocol
This single-center study was performed in the leukemia and the hematopoietic stem cell transplantation (HSCT) units of the University Hospital Zurich. Patients were eligible if they were 16 years old or over, received intensive chemotherapy, underwent autologous or allogeneic HSCT for hematologic malignancies and were treated as inpatients. Patients with acute myeloid leukemia (AML) were treated according to the HOVON 42 protocol and patients with acute promyelocytic leukemia according to the APL 2000 study.19,20 Allogeneic HSCT was performed in a laminar flow unit using standard myeloablative- and non-myeloablative conditioning regimens. Patients receiving chemotherapy predominantly as outpatients were excluded from the study. The local ethical committee approved the study and waived the requirement for written informed consent given the retrospective nature of the study.
In 2008 we changed our RBC transfusion policy by dispensing only one RBC unit at a time from the blood bank. The single-unit transfusion policy was established in all hospital wards with the exception of the intensive care units, the emergency wards and the operating rooms. More than one RBC unit was dispensed only if explicitly prescribed by the treating physician (e.g. in cases with active bleeding). To evaluate the effect of the new transfusion policy, we performed a retrospective single-center analysis from July 2007 to December 2009 comparing two cohorts of patients receiving either double- or single-unit RBC transfusions. We restricted the analysis to patients with hematologic malignancies receiving intensive chemotherapy or HSCT, as the transfusion requirements in these patients are high and the transfusion triggers predictable. In order to avoid a selection bias, the analysis also included all transfusions given in the intensive care units, emergency wards and operation rooms unless stated otherwise.
Transfusion policies
All patients had daily white blood cell and platelet counts, hemoglobin, and hematocrit determinations. The RBC transfusion trigger was a morning hemoglobin level of 60 g/L or the presence of symptoms of anemia such as fatigue, resting dyspnea, and dizziness. All RBC units were leukocyte-reduced by means of filtration before storage. The maximally tolerated leukocyte count after filtration was less than 1×106 leukocytes/unit. The volume of the RBC units ranged from 200 to 350 mL and the maximal age of the RBC was 42 days, in accordance with Swiss legislation. RBC units were generally not irradiated, but all patients with autologous or allogeneic HSCT or chemotherapies with purine analogs received exclusively cellular blood products that were irradiated with 25 Gy.
Platelets were transfused as previously described.21 All platelet units were leukocyte-reduced by means of filtration before storage and contained at least 2.5×1011 platelets. All patients with morning platelet counts of 5×109/L or less received prophylactic platelet transfusions irrespective of bleeding signs. In case of fever or during HSCT, the platelet transfusion trigger was 10×109/L. There was no change of the platelet transfusion policy in the two periods.
Measurements and definitions
We analyzed the reduction in the total number of RBC units per therapy cycle and per day of aplasia as well as determinants related to transfusion efficiency and safety, i.e. the number of platelet transfusions, bleeding incidence, outpatient RBC transfusions, the overall survival and adherence to transfusion policy.
The hemoglobin increment was determined by subtracting the morning hemoglobin value 1 day after the transfusion from the one directly before transfusion. Therapy cycles lasted from the first day of chemotherapy or the day of HSCT until neutrophil recovery [absolute neutrophil count (ANC) > 0.5×109/L for 3 consecutive days]. The time until RBC recovery was taken to be the time from the first day of chemotherapy or the day of HSCT until the reticulocytes increased to more than 1%. The duration of aplasia was from the first day that the ANC was less than 0.5×109/L until ANC recovery. Major bleeding episodes requiring RBC transfusions or non-elective interventions were analyzed from the patients’ charts. Given the retrospective assessment, minor bleeds could not be reliably assessed and were not evaluated in this study. The adherence to the transfusion policy was determined from the percentage of transfusions given as single units or double units in the respective transfusion periods. Risk assessment for AML was made according to the HOVON risk score and for patients with ALL according to the GRAALL 2005 study. Patients undergoing allogeneic transplantation other than in first complete remission or in first chronic phase for chronic myeloid leukemia were considered as poor risk as were all patients receiving HSCT for other malignancies.
Statistical analysis
The patients’ baseline characteristics are described by proportions or medians with interquartile ranges (IQR). Patients receiving double- or single-unit RBC transfusions were compared using the Mann-Whitney U test for continuous variables or the χ2 test for categorical data, as appropriate. To evaluate the independent association between the transfusion policy and the number of RBC units/therapy we applied multiple linear regression modeling. We controlled for the period of aplasia during a therapy cycle as an important determinant of the total requirement for RBC units and other potential confounders such as patients’ age at diagnosis, hemoglobin levels prior to transfusion and the proportion of irradiated RBC units. The model was further adjusted for the clustering of the data (i.e. repeated procedures in the same patients) by applying robust standard errors.22
The overall survival was calculated from the beginning of the chemotherapy or the day of HSCT until death or last follow-up. The survival observation of patients receiving more than one therapy cycle was censored at the beginning of the next cycle. Survival differences between the groups managed with the two transfusion policies were estimated with the method of Kaplan and Meier and compared by a log rank test as well as by using multivariate stepwise Cox regression analysis further controlling for potential confounders. All reported P values are two-sided and P values less than 0.05 were assumed to be statistically significant.
Results
Baseline characteristics
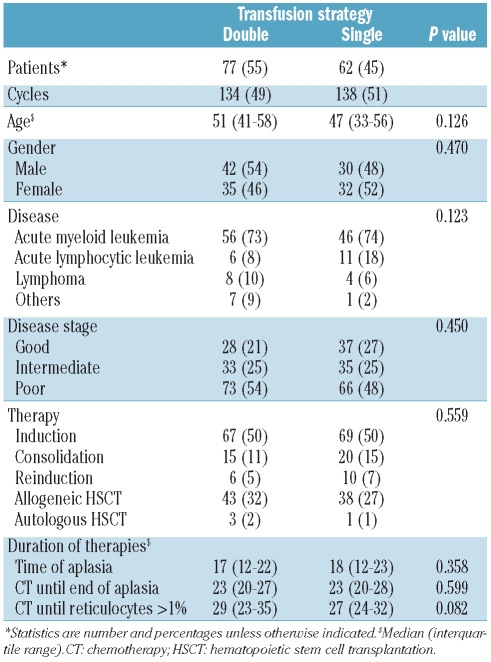
The study comprised 139 patients who received 272 therapy cycles. The patients’ baseline characteristics are shown in Table 1. The baseline characteristcs were distributed equally between the patients treated in the single- and double-unit periods. The median age of the study population was 49 years (IQR, 37–58); 72 (52%) of the patients were male and 67 (48%) were female. The majority of the patients were treated for AML (n=102, 73%), acute lymphoblastic leukemia (n=17, 12%) or Hodgkin’s and non-Hodgkin’s lymphoma (n=12; 9%). Intensive chemotherapy consisted of induction (n=136, 50%), consolidation (n=35, 13%) and re-induction (n=16, 6%). The remaining therapies were allogeneic (n=81, 30%) or autologous HSCT (n=4, 1%). The median time from the start of chemotherapy or from HSCT until neutrophil recovery was 23 days (IQR, 20–28) and the median time of aplasia was 17 days (IQR, 12–23). The median time until reticulocyte recovery was 27 days (IQR, 24–34).
Table 1.
Patients’ baseline characteristics.
Red blood cell transfusions
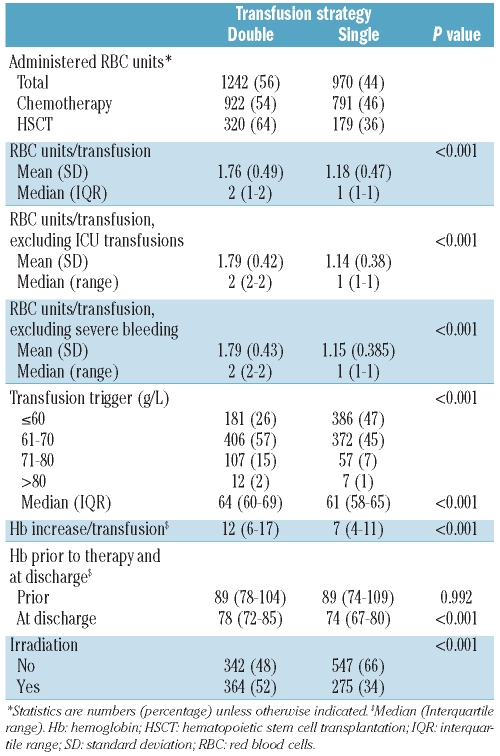
Table 2 presents the results of the RBC transfusions in more detail. A total of 2212 RBC units were given in 1548 transfusions. During the double-unit period 1242 (56%) RBC units were transfused in 134 (49%) therapy cycles and during the single-unit period 970 (44%) RBC units were transfused in 138 (51%) cycles. Ninety-six percent of the RBC transfusions were ABO-identical and 4% were ABO-compatible. During the study period, only one severe transfusion reaction was reported (transfusion-associated volume overload, 1/1548, 0.064%). The median number of RBC units transfused per therapy cycle was seven (IQR, 4–11) among the patients undergoing conventional intensive chemotherapy requiring significantly more RBC units (n=8; IQR, 5–12) as compared to HSCT (n=4; IQR, 2–8; P<0.001). However, there was no significant difference in the RBC transfusion requirements between patients treated with chemotherapy or HSCT when analyzed per day of aplasia (P=0.832). The median number of transfused RBC per day of aplasia was 0.38 units (IQR, 0.25–0.63).
Table 2.
Red blood cell transfusions.
During one transfusion the median number of RBC units administered was two (IQR: 1–2) in the double unit and one (IQR: 1–1) in the single-unit period (P<0.001). To avoid a selection bias, this analysis also included all transfusions given to patients in the intensive care unit and the operating theater (n=133, 9%), while there were no transfusions in the emergency ward. However, as shown in Table 2, exclusion of RBC units given in the intensive care unit setting did not significantly change the results.
During 20 therapy cycles no RBC units were transfused at all. Eight cycles without RBC transfusion support were administered during the double-unit period and 12 during the single-unit period. Fifteen of these therapy cycles were for allogeneic HSCT with reduced-intensity conditioning (n=7) or myeloablative conditioning (n=8). The median aplasia time in this group was 8 days (IQR, 5–12). The remaining five therapy cycles were consolidation chemotherapy for AML with a median aplasia time of 9 days (IQR, 7–12). The aplasia time was significantly shorter in therapy cycles without transfusions than in those with transfusions (median 8 versus 20 days, P<0.001).
The effect of the single-unit red blood cell transfusion policy
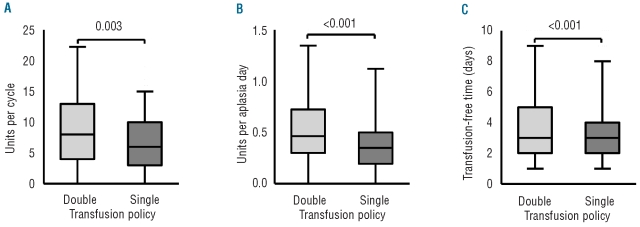
As shown in Figure 1, the change of transfusion policy led to a 25% reduction of transfused RBC units per therapy cycle (double-unit: median 8, IQR 4–13; single-unit: median 6, IQR 3–10; P=0.003). Normalization of the transfusion requirements to one aplasia day resulted in a 24% reduction of the RBC transfusions in the single-unit period. During the double-unit period, a median of 0.46 RBC (IQR, 0.30–0.72) units were transfused per aplasia day, while a median of 0.35 (IQR, 0.20–0.50) RBC units were transfused during the single-unit period (P<0.001).
Figure 1.
Reduction of RBC units per therapy and transfusion-free time. The box plots display medians, interquartile ranges, and 95% confidence intervals. The double RBC-unit period is represented in light gray and the single-unit period in dark gray. (A) Changing the transfusion policy led to a 25% reduction of the transfused RBC units per therapy cycle (P=0.003). (B) Normalization to one aplasia day resulted in a 24% reduction of the RBC transfusions in the single-unit period (P<0.001). (C) The mean time between two transfusions was 20% longer in the double-unit period (P<0.001).
Even though the median RBC transfusions per aplasia day were less in the single-unit period, the time between transfusions was also shorter in the single-unit period. Although there was no difference in the median number of days between the two groups, the mean time between transfusions was significantly shorter in the single-unit period (3.25 versus 4.05 days, P<0.001). The difference between the two groups was approximately 20% indicating that the change to a single-unit transfusion policy moderately increases the workload for the hospital health care employees.
Patients transfused during the single-unit period had slightly lower hemoglobin levels at the time of discharge (74 g/L versus 78 g/L, P<0.001), while there was no difference at the beginning of the therapy (89 versus 89 g/L). However, the lower hemoglobin levels at the time of discharge did not translate into higher RBC transfusion requirements as outpatients [double-unit: median 0 (IQR: 0–1, range: 0–21) RBC units; single-unit: median 0 (0–0, range: 0–45) RBC units; P=0.819]. Likewise, the time until RBC recovery was similar in the two groups.
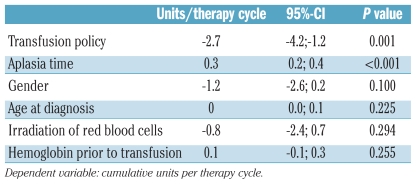
The effect of the transfusion policy was further confirmed in a linear regression model adjusting for confounding factors and clustering of multiple transfusions in the same patient. The change from a double- to single-unit transfusion policy remained independently associated with a significant reduction of 2.7 units (95%-CI −4.3;−1.1, P=0.001) per therapy cycle (Table 3). As expected, increasing aplasia time was associated with higher RBC transfusion requirements, while the gender and age of the recipients, irradiation of the RBC as well as the hemoglobin levels prior to transfusion did not influence the transfusion requirements.
Table 3.
Multivariate linear regression analysis.
Adherence to the single-unit red blood cell transfusion policy
Adherence to the assigned RBC transfusion strategy was analyzed by calculating the percentage of correctly administered RBC transfusions in the two study periods (Figure 2). Single units were transfused in 25% of the cases during the double-unit period and in 84% during the single-unit period. In 130 transfusions (16%) during the single-unit period, two or more RBC units were administered consecutively. The reason for the non-adherence to the single-unit transfusion policy was evaluable in 63% of the cases. Lack of knowledge of the new transfusion policy (21%), hospitalization in the ICU (15%), bleeding events (13%), low hemoglobin values (11%), or transfusions before discharge (3%) were the major reasons for breaking the transfusion policy. Lack of knowledge was primarily seen within the first 4 months after changing the transfusion policy or if new physicians prescribed RBC transfusions. In the remaining 37% of the transfusions there was no clear indication for the non-adherence of the transfusion policy.
Figure 2.
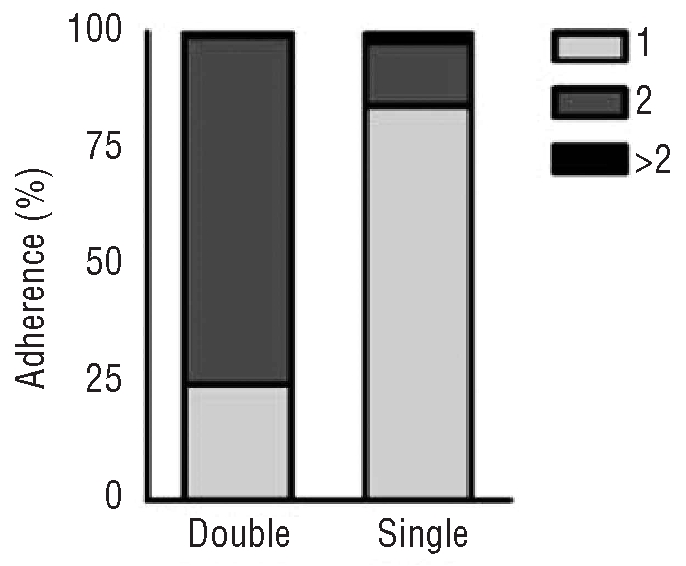
Adherence to the transfusion policy. Adherence to the assigned RBC transfusion strategy was analyzed by calculating the percentage of correctly administered RBC transfusions in the two study periods. Light gray indicates one RBC unit, dark gray two, and black more than two RBC units per transfusion. Single units were transfused in 25% of the cases during the double-unit period and in 84% during the single-unit period.
Red blood cell transfusion triggers
The RBC transfusion trigger in hospitalized patients was a hemoglobin of 60 g/L or less in the absence of symptoms of anemia during both periods. A total of 567 (37%) RBC transfusions were given to patients with morning hemoglobin levels lower or equal to 60 g/L, 778 (51%) to patients with levels of 61–70 g/L, 164 (11%) to patients with levels of 71–80 g/L, and 19 (1%) to patients with hemoglobin levels higher than 80 g/L. During the single-unit period, significantly more transfusions were administered in patients with hemoglobin values of 60 gl/L or less (26% versus 47%, P<0.001), while more patients received RBC transfusions with hemoglobin levels between 61–80 g/L during the double-unit period (74% versus 53%). This resulted in a significantly lower hemoglobin level at the time of RBC transfusions during the single-unit period [median 61 (IQR, 58–65) g/L as compared to the double-unit period: median 64, (IQR: 60–69) g/L (P<0.001) ].
Safety of single-unit red blood cell transfusions
Lower hemoglobin levels may result in a higher risk of bleeding because of the altered rheological properties in severely anemic patients. To exclude this, we analyzed the bleeding episodes in the two cohorts and the total number of transfused platelets. Severe bleeding occurred in 18 therapy cycles. During these cycles 213 RBC units were administered, which is 14% of the total RBC units. There was no significant difference in the number of therapy cycles with severe bleeding episodes between the double-unit period (n=7, 5.2%) and the single-unit period (n=11, 8.0%, P=0.362) and the median number of platelets transfused per therapy cycle was five (2–9) and five (3–9) in the double- and single-unit periods, respectively (P=0.896).
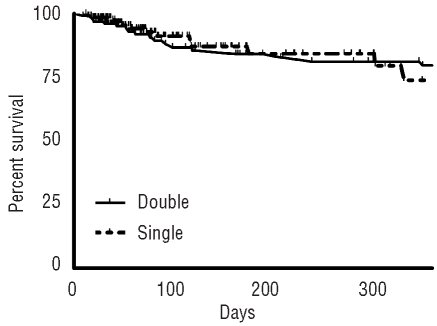
Finally, as shown in Figure 3, we evaluated the overall survival as a measure of the safety after chemotherapy and HSCT. Patients who received more than one therapy cycle were censored at the time of the next cycle. The median observation time was 78 (9–1087) days. The 30-and 100-day survival probabilities were 98% (95%-confidence interval 96–99%) and 89% (84–94%) without differences between the two groups (P=0.893) indicating that the transfusion policy had no influence on the overall survival.
Figure 3.
Overall survival according to the RBC transfusion policy. Kaplan-Meier survival estimates in patients during the double- and single-unit RBC periods. The 30- and 100-day survival probabilities were 98% (95%-confidence interval 96–99%) and 89% (84–94%) without differences between the two groups (P=0.893) indicating that the transfusion policy had no influence on overall survival.
Discussion
Our retrospective cohort study demonstrates for the first time that a change from a double- to single-unit RBC transfusion policy is safe and associated with a reduction of 25% of RBC transfusion requirements in a population with hemato-oncological disorders. This finding demonstrates that the long-standing dogma that two RBC units are necessary for an adequate hemoglobin increase must be critically revised.
Although each year over 75 million units of blood are transfused worldwide, both the optimal number of RBC units per transfusion and the best RBC transfusion trigger remain controversial.11 As a consequence, physicians have to rely primarily on clinical experience rather than published data for their decision-making. In the last decades, the general recommendation was to give two RBC units simultaneously, while single-unit RBC transfusions were discredited as useless1–3 and some authors even suggested critical revision of the local transfusion program if more than 50% of the RBC transfusions were given as single units.23
However, this was mainly based on few studies specifically analyzing the effect of single-unit transfusions. The main findings of these studies were that in surgical or obstetric units approximately 25% of all transfusions were single units. However, over 50% of all patients received single-unit transfusions at hemoglobin levels above 100 g/L and approximately 80% of all transfusions were judged to be questionable or not indicated.5–7 The studies share several limitations: (i) they were performed several decades ago, when the transfusion practices as well as the blood products differed considerably from today’s; (ii) all studies analyzed single-unit RBC transfusions by comparing patients having received one or two RBC units without clear transfusion guidelines. In the vast majority of patients receiving only one RBC unit the transfusions were indeed not indicated because the pre-transfusion hemoglobin values were close to normal; and (iii) none of the studies analyzed single-unit RBC transfusions in a non-surgical population.
In contrast, a single-center analysis found that almost 50% of all transfusions were given as single units, 62% of which were indicated.24 The authors of the study concluded that it would be an error to give two units, if one unit is sufficient to correct anemia. One more recent study theoretically analyzed the effect of transfusing only one RBC unit at a time, concluding that a single-unit RBC transfusion strategy has considerable potential to save RBC units and that this is more pronounced when applying lower transfusion triggers.8 Our current study analyzed for the first time two cohorts of patients who were subjected to either transfusion policy, thus avoiding the selection bias of earlier studies. The limitations of the study are its retrospective, single-center nature, the lack of standardized bleeding assessments and the lack of a quality of life assessment of the patients during the therapy.
Some studies have suggested that a lower hematocrit in the peripheral blood is associated with poorer marginalization of the circulating platelets and consequently with an increased bleeding risk.15 Thus, one major concern at the time of changing the transfusion policy in our institution was an increased risk of major bleeding and higher platelet transfusion requirements. Our data did not, however, show any evidence of a higher bleeding rate or higher platelet requirement in the single-unit transfusion group.
Other concerns are related to a higher workload for the health care professionals due to more frequent transport of RBC units from the local blood bank. It is difficult to assess the exact costs of this blood transport. Indeed, the time between two RBC transfusions was approximately 20% shorter during the single-unit period, leading to a higher transfusion frequency and, potentially, to a higher workload of the health care professionals. However, it is not clear whether the reduced workload due to fewer transfusions outweighs the workload of the blood transport. Even if the workload is moderately increased, we believe that the higher workload is justified, given the inherent risk of each blood transfusion, and that the hospital logistics for blood supply should be improved rather than giving unnecessary blood transfusions to patients.
As for the workload, it is difficult to provide exact data on the real costs of transfusions of blood products. A recently published study meticulously analyzed the real costs of RBC transfusion in four hospitals.25 In this study, an activity-based costing model was constructed taking into considerations tasks and resource consumption (materials, labor, third-party services, capital) related to blood administration. The median cost of one RBC transfusion in surgical patients was $760.82±293.74. In the current study, each patient received a median of 15 RBC units during the whole treatment resulting in a total cost of $11412 for RBC transfusions. A 25% reduction implies a saving of $2853 per patient.
The best transfusion trigger is a long-standing matter of debate. It is generally acknowledged that patients should receive RBC transfusions if the hemoglobin is lower or equal to 60 g/L.26,27 A number of studies have analyzed transfusion triggers in the ICU and, recently, in cardiac surgery patients showing that a restrictive transfusion policy resulted in a significant reduction of the transfusion requirements with comparable or even superior mortality rates.28–33 A meta-analysis indicated that the use of a restrictive transfusion trigger resulted in an average saving of 0.93 units of red cells per transfused patient.26 In patients with hemato-oncological disorders only limited data exist regarding the optimal transfusion threshold.34 In patients receiving intensive chemotherapy for AML the requirements differed considerably between centers, but a more restrictive transfusion threshold seems to be feasible in these patients.35,36
An interesting finding of our study is that the hemoglobin levels immediately before transfusion of RBC were slightly lower during the single-unit period despite similar transfusion guidelines in the two periods. Likewise, the hemoglobin levels at the time of discharge were slightly lower, but this difference did not translate into a higher transfusion rate as outpatients. These findings may indicate that anemia during transfusion dependency is better tolerated in the absence of large fluctuations between the peak and trough hemoglobin caused by the administration of two RBC units. In the situation of fewer clinical symptoms the patients may also tolerate the lower transfusion threshold better. It seems rather unlikely that more patients suffered from fatigue and anemia symptoms during the single-unit period, since these symptoms were considered as transfusion triggers throughout the study duration. However, we did not perform a proper assessment of fatigue and quality of life and cannot, therefore, draw definite conclusions.
In conclusion, this is the first study indicating that a change to a single-unit transfusion policy can safely reduce the RBC transfusion requirements by approximately 25% without changing transfusion triggers. Our data suggest that a single-unit RBC transfusion policy is effective and cost-saving and is not associated with an increased risk for the patients, but with a moderately elevated workload for the health care professionals. Given the scarcity of allogeneic blood products and the inherent risk of all blood transfusions, these results may have a major impact on transfusion strategies for patients with hyporegenerative anemias. These data need to be confirmed in prospective randomized trials.
Acknowledgments
We would like to thank Prof. Kurt Leibundgut for critical review of the manuscript and helpful comments.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Graham-Stewart C, Lond MB. A clinical survey of blood-transfusion. Lancet. 1960;2(7147):421–4. doi: 10.1016/s0140-6736(60)92860-9. [DOI] [PubMed] [Google Scholar]
- 2.Alsever JB. The blood bank and homologous serum jaundice: a review of medicolegal considerations. N Engl J Med. 1959;261:383–6. doi: 10.1056/NEJM195908202610805. [DOI] [PubMed] [Google Scholar]
- 3.Morton JH. Surgical transfusion practices, 1967. Surgery. 1969;65(3):407–16. [PubMed] [Google Scholar]
- 4.Crispen JF. The single-unit transfusion. A continuing problem. Pa Med. 1966;69(1):44–8. [PubMed] [Google Scholar]
- 5.Reece RL, Beckett RS. Epidemiology of single-unit transfusion. A one-year experience in a community hospital. JAMA. 1966;195(10):801–16. doi: 10.1001/jama.1966.03100100053014. [DOI] [PubMed] [Google Scholar]
- 6.Fadell EJ. Utilization of blood: a single-unit blood transfusion study. J Ky Med Assoc. 1967;65(6):573–5. [PubMed] [Google Scholar]
- 7.Domen RE. The single-unit transfusion. J Fla Med Assoc. 1986;73(11):855–7. [PubMed] [Google Scholar]
- 8.Ma M, Eckert K, Ralley F, Chin-Yee I. A retrospective study evaluating single-unit red blood cell transfusions in reducing allogeneic blood exposure. Transfus Med (Oxford, England) 2005;15(4):307–12. doi: 10.1111/j.0958-7578.2005.00592.x. [DOI] [PubMed] [Google Scholar]
- 9.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 10.Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives. Transfus Med Hemother. 2009;36(6):351–482. doi: 10.1159/000293349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 12.Consensus conference. Perioperative red blood cell transfusion. JAMA. 1988;260(18):2700–3. [PubMed] [Google Scholar]
- 13.Haematology BCfSi. Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135(4):450–74. doi: 10.1111/j.1365-2141.2006.06314.x. [DOI] [PubMed] [Google Scholar]
- 14.Force SoTSBCGT. Ferraris VA, Ferraris SP, Saha SP, Hessel EA, Haan CK, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 15.Feagan BG, Wong CJ, Lau CY, Wheeler SL, Sue-A-Quan G, Kirkley A. Transfusion practice in elective orthopaedic surgery. Transfus Med (Oxford, England) 2001;11(2):87–95. doi: 10.1046/j.1365-3148.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 16.Hébert PC, Wells G, Martin C, Tweeddale M, Marshall J, Blajchman M, et al. A Canadian survey of transfusion practices in critically ill patients. Transfusion Requirements in Critical Care Investigators and the Canadian Critical Care Trials Group. Crit Care Med. 1998;26(3):482–7. doi: 10.1097/00003246-199803000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Chohan SS, Mcardle F, McClelland DBL, Mackenzie SJ, Walsh TS. Red cell transfusion practice following the transfusion requirements in critical care (TRICC) study: prospective observational cohort study in a large UK intensive care unit. Vox Sang. 2003;84(3):211–8. doi: 10.1046/j.1423-0410.2003.00284.x. [DOI] [PubMed] [Google Scholar]
- 18.Boralessa H, Goldhill DR, Tucker K, Mortimer AJ, Grant-Casey J. National comparative audit of blood use in elective primary unilateral total hip replacement surgery in the UK. Ann R Coll Surg Engl. 2009;91(7):599–605. doi: 10.1308/003588409X432464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adès L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10. doi: 10.1200/JCO.2006.08.1596. [DOI] [PubMed] [Google Scholar]
- 20.Löwenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–52. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 21.Gmür J, Burger J, Schanz U, Fehr J, Schaffner A. Safety of stringent prophylactic platelet transfusion policy for patients with acute leukaemia. Lancet. 1991;338(8777):1223–6. doi: 10.1016/0140-6736(91)92098-m. [DOI] [PubMed] [Google Scholar]
- 22.Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd. Blackwell Science Ltd; 2003. p. 501. [Google Scholar]
- 23.Special Communication. Joint Blood Council Transfusion Review Programm. JAMA. 1962:1–2. [Google Scholar]
- 24.Micolonghi T, Simon S, Paull A, Healey PJ. The single-unit transfusion in a community hospital–a critical evaluation. Improved blood transfusion practices may result in increased incidence of single-unit transfusions. R I Med J. 1966;49(9):533–6. [PubMed] [Google Scholar]
- 25.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 26.Carson JL, Hill S, Carless P, Hébert P, Henry D. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16(3):187–99. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- 27.Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2002;(2):CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 28.Hajjar LA, Vincent J-L, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA: the journal of the American Medical Association. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 29.Hébert PC, Fergusson DA. Red blood cell transfusions in critically ill patients. JAMA. 2002;288(12):1525–6. doi: 10.1001/jama.288.12.1525. [DOI] [PubMed] [Google Scholar]
- 30.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 31.Carson JL, Terrin ML, Magaziner J, Chaitman BR, Apple FS, Heck DA, et al. Transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (FOCUS) Transfusion. 2006;46(12):2192–206. doi: 10.1111/j.1537-2995.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 32.Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39(10):1070–7. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 33.Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. Am J Surg. 1997;174(2):143–8. doi: 10.1016/s0002-9610(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 34.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 35.Jansen AJG, Caljouw MAA, Hop WCJ, van Rhenen DJ, Schipperus MR. Feasibility of a restrictive red-cell transfusion policy for patients treated with intensive chemotherapy for acute myeloid leukaemia. Transfus Med (Oxford, England) 2004;14(1):33–8. doi: 10.1111/j.0958-7578.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 36.Favre G, Fopp M, Gmür J, Tichelli A, Fey MF, Tobler A, et al. Factors associated with transfusion requirements during treatment for acute myelogenous leukemia. Ann Hematol. 1993;67(4):153–60. doi: 10.1007/BF01695861. [DOI] [PubMed] [Google Scholar]