Abstract
The oxygen sensing pathway modulates erythropoietin expression. In normal cells, intracellular oxygen tensions are directly sensed by prolyl hydroxylase domain (PHD)-containing proteins. PHD2 isozyme has a key role in tagging hypoxia-inducible factor (HIF)-α subunits for polyubiquitination and proteasomal degradation. Erythrocytosis-associated PHD2 mutations reduce hydroxylation of HIF-α. The investigation of 67 patients with isolated erythrocytosis, either sporadic or familial, allowed the identification of three novel mutations in the catalytic domain of the PHD2 protein. All new mutations are germ-line, heterozygous and missense, and code for a predicted full length mutant PHD2 protein. Identification of the disease-causing genes will be of critical importance for a better classification of familial and acquired erythrocytosis, offering additional insight into the erythropoietin regulating oxygen sensing pathway.
Keywords: isolated erythrocytosis, prolyl hydroxylase domain protein 2, oxygen sensing pathway, JAK2, Epo
Introduction
Isolated erythrocytosis (IE) defines a clinical phenotype characterized by a persistent increase of red cell mass and hematocrit without apparent cause in the absence of any Janus kinase 2 (JAK2) mutations. These patients typically present without splenomegaly and may show a wide range of serum erythropoietin (Epo) levels, reflecting the heterogeneous genetic origins of this disorder.1–3 In the presence of JAK2 mutations, these patients are usually classified as polycythemia vera (PV) according to the WHO 2008 classification.4
In IE without JAK2 mutations, the defect may be intrinsic to the erythroid progenitor cells. These cases are often familial, show a subnormal serum Epo level and are associated with mutations in the erythropoietin receptor (EPOR) gene.3 In other cases of sporadic or familial IE, the defects may be extrinsic to the erythroid progenitor cells and due to a defect in the oxygen sensing pathway.5,6 This pathway includes the prolyl hydroxylase domain protein 2 (PHD2),7 von Hippel-Lindau tumor suppressor (VHL)6 protein and α-subunit of the hypoxia-inducible factor (HIF-α)8 protein. The α subunit can be either HIF-1α or HIF-2α, while the aryl hydrocarbon nuclear translocator forms the β subunit. Epo levels in these patients may range from low to increased,9 reflecting the complexity and, maybe, the redundancy of the oxygen sensing system.
Under normal oxygen tension, PHD2, belonging to the Fe(II) and 2-oxoglutarate (2OG)-dependent oxygenase family, hydroxylates HIF-α allowing its ubiquitination by VHL, with consequent degradation of HIF-α in the proteasome and downregulation of the Epo production at physiological values.10 Under hypoxic conditions, the HIF-α subunit hydroxylation by PHD2 is hampered, thus allowing HIF-α to escape VHL-mediated degradation. HIF-α subunits, therefore, accumulate and dimerize with the HIF-β subunits, migrate into the nucleus and regulate the transcription of several target genes including those coding for Epo,10 causing an increased mRNA and protein production of this cytokine.
This study aimed to identify PHD2 mutations in a cohort of 53 subjects with sporadic or familial IE. Fourteen additional cases presenting with an IE phenotype but with JAK2V617F mutation were also included. In all patients with PHD2 mutation, VHL and HIF2A-exon 12 were also investigated. DNA samples from all cases were screened for PHD2 mutations in the entire coding region and three new mutations were found. JAK2 V617F and exon 12 mutations were studied as previously described.11,12
Design and Methods
Patients
In 67 patients referred to our laboratory for investigation of increased hemoglobin/hematocrit level from 1980 to 2007, a clinical phenotype of IE was established by confirming an increased hematocrit over several years in the absence of any apparent cause. In addition, normal platelet and white blood cell counts, and absence of splenomegaly were required. Bone marrow biopsy showed erythroid hyperplasia without proliferation of megakaryocytic and myeloid lineages in all cases. Serum Epo level, when available, was consistently normal or low. In 12 cases, a familial history of erythrocytosis (almost one first-degree relative with the same diagnosis to the proband) was found, while 55 patients presented sporadic type. Sporadic forms included 14 cases positive for the JAK2V617F and 5 cases with JAK2-exon 12 mutation, thus requiring reclassification as PV according to the new WHO 2008 criteria.4
DNA extraction and amplification
Peripheral blood samples were collected at diagnosis from all patients and epithelial cells from the index cases. The study was approved by the local Ethical Committee and all subjects gave their informed consent. Genomic DNA was extracted with Blood Core Kit B (Qiagen, Hilton, Germany) according to the manufacturer’s instructions. Amplifications of the entire PHD2 coding region (exon 1 to 5) and of the intron/exon boundaries were performed under standard conditions by polymerase chain reaction (PCR) using FastStart Taq DNA Polymerase (Roche, New Jersey, USA), in a GeneAmp® PCR system 2700 (Applied Biosystems, Foster City, CA, USA). The three VHL exons and HIF2A-exon 12 were analyzed in the 3 PHD2 mutated patients, according to a similar procedure; details for PCR conditions are available upon request. Primer sequences (obtained from Primer3 software v4.0, http://frodo.wi.mit.edu/primer3/input.htm) and PCR fragment lengths are shown in Online Supplementary Table S1.
Sequencing analysis
The PCR products were purified. Sequencing reactions were carried out using Big Dye® sequencing kit (Applied Biosystems). Direct sequencing was performed in both directions for all samples on an automated sequencer ABI Prism® 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Electropherograms were compared with the PHD2 (NCBI GenBank accession n. NM_022051), VHL (NCBI GenBank accession n. NM_000551) and HIF2A (NCBI GenBank accession n. NC_000002) wild-type sequences. Mutations were named in accordance with the standard international nomenclature guidelines recommended by the Human Genome Variation Society (HGVS, http://www.hgvs.org/mutnomen/).
Characterization of the new PHD2 mutations
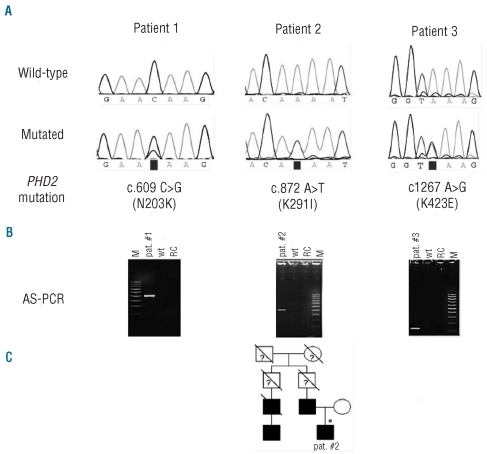
To confirm the identity of the newly identified mutations, allele-specific PCR (AS-PCR) was performed for each allelic variant (Figure 1B). Reaction mixtures were run on a GeneAmp® PCR System 2700 at standard conditions and PCR fragments were analyzed by agarose gel. Primer sequences and PCR fragment lengths are listed in Online Supplementary Table S1.
Figure 1.
Mutational analysis results and localization of the PHD2 mutations. (A) Sequencing results of wild-type and mutated alleles in 3 patients. Nucleotide positions (GenBank accession, NM 022051), nucleotide changes and corresponding amino acid changes are indicated below. (B) Confirmation of the new mutations by allele-specific PCR (AS-PCR) and agarose gel electrophoresis. AS-PCR fragment lengths agree with the respective primer positions (Online Supplementary Table S1). Lane M: molecular weight marker, lane pat #: patient’s DNA amplified with mutation specific primer, lane wt: control DNA amplified with mutation specific primer, lane RC: reaction control (no template). (C) Pedigree of the family with erythrocytosis. Squares represent males, circles females, affected individuals are indicated in black and slashes indicate deceased members. Genetically tested individual is indicated by an asterisk.
The germ-line origin of these new genetic lesions was confirmed by their identification on DNA obtained from epithelial cells (buccal cotton-swab sampling) of the probands.
DNA samples from peripheral blood mononuclear cells from 100 normal controls with the same ethnic background were also screened for the novel mutations, by means of AS-PCR. Control DNA was obtained from the DNA samples stored in a biobank of healthy subjects at San Bortolo Hospital. Informed consent from the donors to the biobank had already been obtained for research purposes.
Results and Discussion
In this study, three different new germ-line heterozygous mutations of PHD2 were found in a cohort of 67 patients with IE, including 14 and 5 cases subsequently reclassified as PV on the basis of JAK2 V617F or exon 12 mutations. None of the PHD2 mutated subjects was JAK2V617F positive while one co-harbored JAK2-exon 12 mutation. Main clinical and laboratory features of mutated patients are listed in Table 1.
Table 1.
Clinical and laboratory features of the 3 patients carrying PHD2 mutation.
In Patient 1, molecular studies revealed a C>G missense mutation at coding nucleotide position c.609 resulting in a N203K replacement in the putative amino acid sequence (Figure 1A). This genetic variation occurred in a male aged 80 years referred 19 years before for erythrocytosis. A large increase was seen in hemoglobin and hematocrit values. The patient resulted positive for the JAK2547insL+I540−F547dup8 mutation13 in exon 12, and his serum Epo level was low (less than 5 mUI/mL, normal range 5–25 mUI/mL). The same N203K PHD2 mutation was detected on DNA extracted from four single Epo-independent endogenous erythroid colonies obtained from the patient.13 Low serum Epo level presented by Patient 1 typically characterizes JAK2-exon 12 mutated subjects suggesting that, in this patient, clinical manifestations are mainly driven by his acquired JAK2 lesion.
Patient 2 harbored PHD2 mutation consisting of an A>T substitution at position c.872 leading to K291I replacement (Figure 1A). He was referred at age 29 for a familial history of erythrocytosis observed in his father, uncle and first cousin (Figure 1C).
The last PHD2 mutation was found in Patient 3, a male aged 60 years at diagnosis with sporadic IE consisting in an A>G substitution at coding position c.1267, resulting in a K423E replacement (Figure 1A).
To exclude the possibility that single-nucleotide polymorphisms (SNPs), increasingly reported during the last two years, could be interpreted as mutations, a control panel of 100 DNA samples from healthy subjects was screened by AS-PCR and found negative. All the three new erythrocytosis-associated PHD2 mutations here reported involve the catalytic domain of the protein and add to those already known (Figure 2A–B).16–19 We previously described as mutation a germ-line missense substitution,20 Q157H, found in association with JAK2V617F in a propositus with polycythemia vera and found in an isolated form also in his son, presenting only with mild erythrocytosis. Q157H has been reported also as SNP (NCBI entry, rs61750991) with a frequency of around 2% in normal subjects but with a much higher frequency in cancer patients.21 Whether this substitution contributes to the phenotypic expression of myeloproliferative neoplasms or erythrocytosis must be determined with more extensive studies. The most extensively studied and biochemically characterized PHD2 mutations are P317R16 and R371H.17 P317 and R371 are close to the active site amino acids that directly interact with the Fe(II) (H313 and H374, respectively) (Figure 2C) and are likely to contribute to an HIF-α substrate binding groove. Three additional heterozygous PHD2 mutations have been identified, producing truncated proteins lacking the catalytic domain (M202IfsX71, R281TfsX3)18 or removing the last C-terminal amino acids tail (Q377X).18 Finally, the H374R variation has been recently identified in a patient with erythrocytosis and recurrent paraganglioma,19 suggesting that PHD2 can also act as a tumor-suppressor gene. Our series of newly identified mutations are all germ-line and missense type, coding predicted mutant full length PHD2 proteins of 426 amino acids.
Figure 2.
(A) Schematic diagram representing the human PHD2 protein. Znf_MYND, Zinc finger MYND-like domain, 2OG_FeII_oxy, 2-oxoglutarate and Fe(II)-dependent oxygenase-type domain. Diamonds indicate the location of the mutations, the novel genetic variations described in this study are underscored. Numbers indicate amino acid residue positions. (B) Three dimensional ribbon representation of the catalytic domain of the human PHD2, highlighting the locations of the mutated residues. The structure was generated using Swiss-PdbViewer v3.7 software14 from Protein Data Bank coordinates of the X-ray structure, 2G1M.15 Note that the C-terminal K423 is not visible as it falls outside the available PHD2 X-ray maps (residues from 188 to 403). (C) Active site contacts. P317 and R371 are close to the iron-chelating residues H313 and H374, respectively, both in the active site. Hydrogen bonds are shown as dashed lines.
N203K, found in Patient 1, flanks the M202 residue in the helice α1 (Figure 2B), previously found to be mutated in IE,18 and introduces a positive charged amino acid with a long side chain in place of a polar asparagine. The patient with this new genetic variation is also affected by a complex mutation in the exon 12 of JAK2.13 The mutation presented by Patient 2, K291I, lies at the beginning of the strand β4 of the hydroxylase domain (Figure 2B) and, in contrast to the other heterozygous PHD2 mutations, this novel mutation involves a codon that was neither conserved in other species, nor in the human isoforms PHD1 and PHD3. N203 and K291 map in the region responsible for the differential target preference of PHD2,22 corresponding to residues 201–296 (N1 region, Figure 2A). It is worthy of note that this distinct domain is relatively far from the catalytic site; it contains the highest density of dissimilar residues between PHD2 and PHD3 and it was previously suggested to have a role on substrate discrimination.22 The K291I genetic lesion points to haploinsufficiency, leading to a decreased prolyl hydroxylase activity disturbing the oxygen sensing pathway, as the cause of erythrocytosis in Patient 2. K423E substitution, harbored by Patient 3, causes the loss of a positive charged side chain with the acquisition of a complementary charged glutamate, three coding residues from the end of the enzyme. Lys423 is an evolutionary conserved residue in ortholog forms of PHD2 (Mus musculus and Rattus norvegicus, http://toolkit.tuebingen.mpg.de/prot_blast) but it was absent in the two closely related human isozymes, PHD1 and PHD3, consisting of 407 and 239 amino acids, respectively. Mutation analyses of the catalytic domains of the PHDs as shown by X-ray crystallography for PHD2,15 and results for other 2OG oxygenases,23,24 suggested that removal of the C-terminus of the enzyme actually promotes uncoupled turnover of 2OG. However, due to the lack of the experimental solution of the C-terminal PHD2 structure,15 it is not possible to anticipate the K423E mutation effects on protein stability and function.
A better identification of the disease-causing genes will improve classification of familial and acquired IE, in many cases still not characterized at a molecular level. Furthermore, the study of IE can give additional insight into the human oxygen sensing pathway that regulates Epo.
In conclusion, our findings emphasize the wide spectrum of genetic defects in IE. Furthermore, the co-presence of PHD2 and JAK2 mutations in one of the studied patients highlights the difficulties in clearly differentiating intrinsic (primary) from extrinsic (secondary) forms and suggests that a positivity for JAK2 mutation should not prevent a more extensive investigation of other genes involved in the homeostatic system regulating erythrocyte production in sporadic or familial cases with erythrocytosis. Further studies will be required to assess the functional consequences on the substrate binding affinity and the differential effect of these new mutations on HIF-α hydroxylation.
Footnotes
Funding: this work was supported by the Associazione Vicentina per le Leucemie, i Linfomi e il Mieloma (AViLL-AIL). EA and MB were recipients of a grant from the “Fondazione Progetto Ematologia”, Vicenza.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Patnaik MM, Tefferi A. The complete evaluation of erythrocytosis: congenital and acquired. Leukemia. 2009;23(5):834–44. doi: 10.1038/leu.2009.54. [DOI] [PubMed] [Google Scholar]
- 2.McMullin MF. Idiopathic erythrocytosis: a disappearing entity. Hematology Am Soc Hematol Educ Program. 2009:629–35. doi: 10.1182/asheducation-2009.1.629. Review. [DOI] [PubMed] [Google Scholar]
- 3.Percy MJ. Genetically heterogeneous origins of idiopathic erythrocytosis. Hematology. 2007;12(2):131–9. doi: 10.1080/10245330601111979. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood Rev. 2008;22(6):321–32. doi: 10.1016/j.blre.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114(10):2015–9. doi: 10.1182/blood-2009-05-189985. [DOI] [PubMed] [Google Scholar]
- 7.Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun. 2005;338(1):617–26. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- 8.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordeuk VR, Stockton DW, Prchal JT. Congenital polycythemias/erythrocytoses. Haematologica. 2005;90(1):109–16. [PubMed] [Google Scholar]
- 10.Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun. 2005;338(1):610–6. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 11.Albiero E, Bernardi M, Madeo D, Ruggeri M, Rodeghiero F. A new TMHA-DHPLC assay for the rapid mutation screening of JAK2 exon 14 in myeloproliferative disorders. Am J Hematol. 2008;83(7):603–4. doi: 10.1002/ajh.21116. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi M, Ruggeri M, Albiero E, Madeo D, Rodeghiero F. Isolated erythrocytosis in V617F negative patients with JAK2 exon 12 mutations: report of a new mutation. Am J Hematol. 2009;84(4):258–60. doi: 10.1002/ajh.21357. [DOI] [PubMed] [Google Scholar]
- 13.Albiero E, Madeo D, Ruggeri M, Bernardi M, Giorgetti A, Rodeghiero F. Loss of the JAK2 intramolecular auto-inhibition mechanism is predicted by structural modelling of a novel exon 12 insertion mutation in a case of idiopathic erythrocytosis. Br J Haematol. 2008;142(6):986–90. doi: 10.1111/j.1365-2141.2008.07180.x. [DOI] [PubMed] [Google Scholar]
- 14.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 15.McDonough MA, Li V, Flashman E, Chowdhury R, Mohr C, Liénard BM, et al. Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc Natl Acad Sci USA. 2006;103(26):9814–9. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci USA. 2006;103(3):654–9. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percy MJ, Furlow PW, Beer PA, Lappin TR, McMullin MF, Lee FS. A novel erythrocytosis-associated PHD2 mutation suggests the location of a HIF binding groove. Blood. 2007;110(6):2193–6. doi: 10.1182/blood-2007-04-084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Sheikh M, Moradkhani K, Lopez M, Wajcman H, Préhu C. Disturbance in the HIF-1alpha pathway associated with erythrocytosis: further evidences brought by frameshift and nonsense mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Blood Cells Mol Dis. 2008;40(2):160–5. doi: 10.1016/j.bcmd.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359(25):2685–92. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- 20.Albiero E, Ruggeri M, Fortuna S, Bernardi M, Finotto S, Madeo D, et al. Analysis of the oxygen sensing pathway genes in familial chronic myeloproliferative neoplasms and identification of a novel EGLN1 germ-line mutation. Br J Haematol. 2011;153(3):405–8. doi: 10.1111/j.1365-2141.2010.08551.x. [DOI] [PubMed] [Google Scholar]
- 21.Astuti D, Ricketts CJ, Chowdhury R, McDonough MA, Gentle D, Kirby G, et al. Mutation analysis of HIF prolyl hydroxylases (PHD/EGLN) in individuals with features of phaeochromocytoma and renal cell carcinoma susceptibility. Endocr Relat Cancer. 2011;18(1):73–83. doi: 10.1677/ERC-10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villar D, Vara-Vega A, Landázuri MO, Del Peso L. Identification of a region on hypoxia-inducible-factor prolyl 4-hydroxylases that determines their specificity for the oxygen degradation domains. Biochem J. 2007;408(2):231–40. doi: 10.1042/BJ20071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flashman E, Bagg EA, Chowdhury R, Mecinovi J, Loenarz C, McDonough MA, et al. Kinetic rationale for selectivity toward N- and C-terminal oxygen-dependent degradation domain substrates mediated by a loop region of hypoxia-inducible factor prolyl hydroxylases. J Biol Chem. 2008;283(7):3808–15. doi: 10.1074/jbc.M707411200. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Lloyd MD, Harlos K, Clifton IJ, Baldwin JE, Schofield CJ. Kinetic and crystallographic studies on deacetoxy-cephalosporin C synthase (DAOCS) J Mol Biol. 2001;308(5):937–48. doi: 10.1006/jmbi.2001.4649. [DOI] [PubMed] [Google Scholar]