Abstract
Early mortality in acute promyelocytic leukemia has been reported to occur in less than 10% of patients treated in clinical trials. This study reports the incidence and clinical features of acute promyelocytic leukemia patients treated at Stanford Hospital, CA, USA since March 1997, focusing on early mortality. We show that the risk of early death in acute promyelocytic leukemia patients is higher than previously reported. In a cohort of 70 patients who received induction therapy at Stanford Hospital, 19% and 26% died within seven and 30 days of admission, respectively. High early mortality was not limited to our institution as evaluation of the Surveillance, Epidemiology and End Results Database demonstrated that 30-day mortality for acute promyelocytic leukemia averaged 20% from 1977–2007 and did not improve significantly over this interval. Our findings show that early death is now the greatest contributor to treatment failure in this otherwise highly curable form of leukemia.
Keywords: acute promyelocytic leukemia, early death, treatment, incidence, predictors
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myelogenous leukemia (AML), accounting for 5% of AML cases in the US.1 Historically, APL was considered one of the most rapidly lethal forms of AML.2 More recently, the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide has revolutionized the treatment of APL. APL is now considered a highly curable disease, with 2-year event-free survival rates of 75–84%.3–6
Early mortality is common in APL and is frequently related to hemorrhagic complications.7–8 Prior to ATRA therapy, early death (ED) related to hemorrhage occurred in up to 26% of cases.9–11 However, most clinical trials involving ATRA report ED rates (defined variously as death during or within seven or 30 days from the start of induction chemotherapy, or as death during induction therapy) of less than 10%.4,5,7,10,12,13 These improved results have been attributed to amelioration of APL-induced coagulopathy by prompt ATRA therapy. However, these results may represent either an underestimation of early mortality, as these patients tend to be excluded from clinical trials, or improvements in supportive care compared to historical cohorts. In fact, certain trials have found that the addition of ATRA to combination chemotherapy did not impact ED rates.4 Enrollment in clinical trials for APL may be difficult in specific clinical situations, including after-hours admissions and/or cases requiring emergent therapy. Additionally, patients with significant co-morbid conditions, up to 30%, may be deemed unfit for study enrollment.14 Therefore, published trials may underestimate the true mortality associated with APL, particularly if a significant portion of deaths occur within days of diagnosis. Finally, some trials have excluded patients from analysis if they die prior to receiving treatment.3 We, therefore, sought to determine the incidence of ED in consecutive APL patients managed at Stanford Hospital since 1997, and to identify clinical characteristics of APL predictive of ED. Unexpectedly, we found that almost one in 5 patients treated for APL at Stanford Hospital died within seven days of admission to the hospital, and more than a quarter of patients died within 30 days. Next, we used the Surveillance, Epidemiology and End Results (SEER) Database to determine ED rates from 1976 until 2005 to compare our results to a larger cohort of APL patients. Surprisingly, while 3-year survival has improved steadily over this time period within SEER registries, mortality at 30 days has remained constant.
Design and Methods
Seventy consecutive APL patients who were treated at Stanford Hospital between March 1997 (electronic records before this time are unavailable) and July 2009 were identified. Diagnosis of APL with t(15;17)(q22;q12); PML-RARA according to the WHO classification system15 was confirmed in all cases. Clinical features assessed include age, gender, white blood cell count, platelet count, fibrinogen, PTT, INR, BUN, creatinine, and treatment protocol. Additionally, we determined the day of the week each patient was admitted to Stanford Hospital and the time from admission to the initiation of ATRA. ED was defined as death within seven days of presentation to Stanford Hospital. Univariate analysis was performed by Mann-Whitney U test, Fisher’s exact test, and Cox’s regression models. Overall survival was assessed by Kaplan-Meier analysis. Statistical testing was performed using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA), MedCalc version 11.5.0.0 (MedCalc Software, Mariakerke, Belgium), and R version 2.92. This study was approved by the Stanford University School of Medicine’s Institutional Review Board (Stanford IRB ID # 16312, approved March 19, 2009).
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database is a compilation of cancer registries that covered more than a quarter of the U.S. population.16 Using the SEER*Stat 6.6.2 software package and the SEER 17 database, 30-day death rates and 3-year survival data were obtained for APL patients aged 15 years and older. Relapse cases and cases diagnosed only at autopsy were excluded.
Results and Discussion
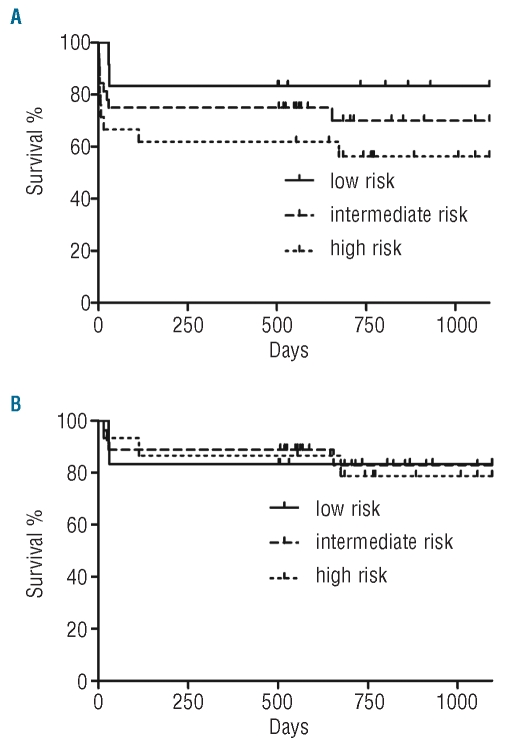
We identified 70 consecutive APL patients who underwent initial treatment at Stanford Hospital. In this cohort, the median age at diagnosis was 50 years (range 19–93) and 44 (63%) were females. Using the PETHEMA relapse risk criteria,17 there were 24 (35%), 33 (47%), and 13 (19%) patients with high-, intermediate- and low-risk disease, respectively. With a median follow-up time of 654 (1–3,991) days for the entire cohort, 24 (34%) have died. Nineteen and 26% of these patients died within seven and 30 days of presentation, respectively. PETHEMA risk stratification was predictive of death by Day 7 (P=0.04) but not death by Day 30 (P=0.44). The seven-/30-day mortality rates in the high-, intermediate-, and low-risk groups were 33%/38%, 15%/24%, and 0%/15%, respectively. Risk group stratification was not predictive of OS at three or five years, consistent with prior studies6 (P>0.1 for all comparisons). Three-year OS in the high-, intermediate-, and low-risk patients was 56%, 70%, and 83%, respectively (Figure 1A). Excluding patients who suffered ED, 3-year OS was greater than 78%, regardless of PETHEMA risk category (Figure 1B).
Figure 1.
Three-year overall survival of the 70 patient cohort treated at Stanford Hospital. (A) All patients. (B) Patients with no early death.
The median age of patients who suffered ED was 57 years as compared to 47 years in non-ED patients (P=0.06). WBC count at presentation to Stanford Hospital was higher in ED patients (median 26.5×109/L) as compared to non-ED patients (2.7×109/L, P=0.01). Using receiver operating characteristic curve analysis, we determined that a WBC count greater than 17×109/L optimally discriminated patients based on risk of ED (relative risk of 3.8). The sensitivity and specificity of this cut-off point for ED was 62% and 84%, respectively. INR at presentation was also higher in patients who experienced ED (median 1.6 vs. 1.3 for non-ED patients, P<0.01) Renal function, platelet count, aPTT, and fibrinogen concentration parameters at presentation were not predictive of ED.
Current recommendations for supportive care of APL patients during induction therapy include maintenance of fibrinogen levels above 100mg/dL and platelet counts above 30-50×109/L.18,19 For 63 of 70 patients in this cohort, we were able to obtain data regarding INR, fibrinogen levels, and platelet counts during the first seven days of initial admission to Stanford Hospital. There was no significant difference in the percentage of hospital days in which the platelet count was below a goal of 30×109 cells/L between ED and non-ED patients. However, similar to previous data,19 we found that within the initial seven days of hospitalization, the percentage of days spent with fibrinogen levels less than 100mg/dL was greater in patients who suffered ED (mean of 33% vs. 6%, P<0.05). Likewise, within the first seven days from presentation, the percentage of days in which the INR was greater than 1.5 was greater in patients suffering ED (mean of 54% vs. 11%, P<0.01).
Of the 70 patients in this cohort, 63 were treated with a regimen containing both ATRA and an anthracycline. Of the remaining 7 patients, 3 received ATRA alone, 2 chose to pursue supportive care alone, and 2 patients died before definitive therapy could be instituted. Ten of 13 patients who experienced ED received ATRA therapy. Ten of 70 patients were treated on clinical trials; all of these trials included induction with ATRA. Treatment delay upon presentation to Stanford Hospital did not appear to be a contributor to ED, as there was no difference in time to start of initial APL therapy (relative to hospital admission) between patients who suffered ED (mean one day) and those who did not suffer ED (mean 1.25 days, P>0.1). Patients admitted at the weekend (Friday, Saturday, and Sunday) were no more likely to suffer ED than those admitted on weekdays (P>0.1). The most common cause of ED was intracranial hemorrhage, occurring in 7 of 13 cases (54%). Age was not associated with cause of ED. Other causes of ED were respiratory failure (n=2), diffuse alveolar hemorrhage (n=1), cardiopulmonary arrest (n=1), pulmonary embolism (n=1), and undetermined (n=1). While APL differentiation syndrome was not implicated in the medical record as a cause of death in any patients in the ED group, we cannot rule out the possibility that this syndrome was unrecognized and contributed to ED.
We considered the possibility that patients suffering ED presented later in the course of their illness, and we therefore reviewed hospital admission records for the 70 patients in this cohort. The timing of initial symptoms was not listed for 4 patients, and one patient was asymptomatic, with abnormalities being noted initially on a routine CBC. For the remaining 65 patients, the time from the first reported symptom(s) to the initiation of APL therapy at Stanford Hospital was not predictive of ED. This was true whether antecedent symptomatic period was considered in a dichotomous fashion or as a continuous variable (P>0.05 in each case). Median symptomatic time was seven days and 14 days prior to start of therapy for the ED and non-ED groups, respectively. Patients admitted to an outside hospital prior to transfer were not more likely to suffer ED than patients admitted directly.
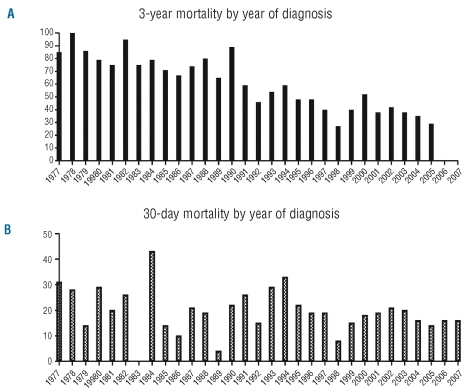
The ED rate for our APL patients was considerably higher than that of previously reported clinical trials, and we therefore sought to determine whether this was unique to our institution. Since 1973, the SEER Database has used a distinct code for APL that distinguishes these cases from other forms of AML.20 We determined the 3-year and 30-day mortality for APL (Figure 2A and B, respectively) across the SEER database regions starting in 1977 (7-day mortality rates are not recorded in the SEER database). We excluded the period between 1973 and 1976 as fewer than 10 cases of APL were recorded during each of these years. Logistical regression was then used to determine whether year of diagnosis was significantly associated with 30-day and 3-year survival. As expected, 3-year overall survival improved significantly from 1977 until 2007. During this period, the risk of dying within three years from diagnosis decreased at a rate of 61% per decade (P<0.001). In contrast, the year of diagnosis was not associated with risk of death within 30 days from diagnosis. Thus, in spite of the implementation of urgent ATRA treatment in newly diagnosed APL, 30-day mortality did not improve from 1977–2007. The average 30-day mortality from 1977–2007 was 20%.
Figure 2.
(A) Three-year and (B) 30-day mortality of APL patients in the SEER databse, 1977–2007.
While therapy for APL has improved dramatically over the past three decades, our results demonstrate that ED remains a significant problem. Surprisingly, analysis of the SEER database indicates that the rate of 30-day mortality has remained static over the same period despite a significant increase in 3-year overall survival. Although ED rates are reported to be less than 10% in modern clinical trials, our data and the work of others14,21,22 suggest a much higher incidence of ED, mostly due to hemorrhagic complications. Furthermore, the difference between our findings and previous studies reflect, in part, the exclusion of a significant proportion of patients from clinical trial participation, due to multiple medical, logistical, or social issues.23
Although prompt initiation of ATRA-based therapy is important to improving outcome,18 our analysis of ED does not suggest that treatment delay was a major contributor to ED. Furthermore, analysis of SEER data presented here indicates that the ED rate has not significantly decreased since the introduction of ATRA in the early 1990s. Finally, our analysis did not find evidence of a lengthier antecedent symptomatic period contributing to ED. This suggests that patients suffering ED were not simply presenting to medical attention later in the course of their disease, though analysis of a larger cohort would be required to definitively address this.
Taken together, the above findings emphasize the need for a better understanding of the pathogenesis of hemorrhagic complications of APL and risk factors associated with ED in APL patients. The utilization of more aggressive supportive measures in patients with a high risk of ED needs to be tested prospectively. Additionally, it is still unclear whether further refinements of APL therapy (e.g. the addition of arsenic trioxide to the induction regimen24 will decrease ED rates in this otherwise highly curable disease. Finally, the results of our retrospective SEER analysis suggest that death within 30 days of diagnosis may become synonymous to overall mortality in APL, as OS and ED curves are converging and are anticipated to intersect by 2015. Improving early death rates may become the greatest challenge for the future treatment of APL.
Acknowledgments
The authors would like to thank all clinicians and staff involved in the care of these patients.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Yamamoto J, Goodman M. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes and Control. 2008;19(4):379–90. doi: 10.1007/s10552-007-9097-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z-Y, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505–15. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 3.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–8. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 4.Mandelli F, Diverio D, Avvisati G, Luciano A, Barbui T, Bernasconi C, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 1997;90(3):1014–21. [PubMed] [Google Scholar]
- 5.Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. Blood. 1999;94(4):1192–200. [PubMed] [Google Scholar]
- 6.Sanz MA, Montesinos P, Vellenga E, Rayón C, de la Serna J, Parody R, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHE-MA Group. Blood. 2008;112(8):3130–4. doi: 10.1182/blood-2008-05-159632. [DOI] [PubMed] [Google Scholar]
- 7.Yanada M, Matsushita T, Asou N, Kishimoto Y, Tsuzuki M, Maeda Y, et al. Severe hemorrhagic complications during remission induction therapy for acute promyelocytic leukemia: incidence, risk factors, and influence on outcome. Eur J Haematol. 2007;78(3):213–9. doi: 10.1111/j.1600-0609.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacomo RH, Melo RAM, Souto FR, de Mattos ER, de Oliveira CT, Fagundes EM, et al. Clinical features and outcomes of 134 Brazilians with acute promyelocytic leukemia who received ATRA and anthracyclines. Haematologica. 2007;92(10):1431–2. doi: 10.3324/haematol.10874. [DOI] [PubMed] [Google Scholar]
- 9.Cordonnier C, Vernant JP, Brun B, Heilmann MG, Kuentz M, Bierling P, et al. Acute promyelocytic leukemia in 57 previously untreated patients. Cancer. 1985;55(1):18–25. doi: 10.1002/1097-0142(19850101)55:1<18::aid-cncr2820550104>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Di Bona E, Avvisati G, Castaman G, Luce Vegna M, De Sanctis V, Rodeghiero F, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. 2000;108(4):689–95. doi: 10.1046/j.1365-2141.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 11.Tallman MS, Kwaan HC. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood. 1992;79(3):543–53. [PubMed] [Google Scholar]
- 12.Sanz MA, Martín G, Rayón C, Esteve J, González M, Díaz-Mediavilla J, et al. A modified AIDA protocol with anthracy-cline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–21. [PubMed] [Google Scholar]
- 13.Rodeghiero F, Avvisati G, Castaman G, Barbui T, Mandelli F. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75(11):2112–7. [PubMed] [Google Scholar]
- 14.Micol J-B, Raffoux E, Boissel N, Lengline E, Canet E, Leblanc T, et al. Do early events excluding patients with acute promyelocytic leukemia (APL) from trial enrollment modify treatment result evaluation? Real-Life Management of 100 Patients Referred to the University Hospital Saint-Louis Between 2000 and 2010. ASH Annual Meeting Abstracts. 2010;116(21):1083. [Google Scholar]
- 15.Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al., editors. The WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue (IARC WHO Classification of Tumours) 14th Edition. Lyon, France: IARC Press; 2008. [Google Scholar]
- 16.National Cancer Institute. Surveillance, Epidemiology, and EndResults (SEER) Program. http://seer.cancer.gov/
- 17.Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53. [PubMed] [Google Scholar]
- 18.Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114(25):5126–35. doi: 10.1182/blood-2009-07-216457. [DOI] [PubMed] [Google Scholar]
- 19.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 20.Douer D. The epidemiology of acute promyelocytic leukaemia. Best Practice & Research Clinical Haematology. 2003;16(3):357–67. doi: 10.1016/s1521-6926(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Möllgård L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25(7):1128–34. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111(7):3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 24.Tsimberidou A-M, Kantarjian H, O’Brien S, Garcia-Manero G, Koller C, Jones D, et al. All-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) combination therapy induces high rates of durable molecular remission in newly diagnosed acute promyelocytic leukemia (APL) ASH Annual Meeting Abstracts. 2007;110(11):1834. [Google Scholar]