Abstract
Ataxia telangiectasia patients, with constitutional bi-allelic ATM mutations, have a marked risk of lymphoid tumors and ATM mutation carriers have a smaller risk of cancer. Sporadic ATM mutations occur in 10–20% of chronic lymphocytic leukemia and are often associated with chromosome 11q deletions which cause loss of an ATM allele. The role of constitutional ATM mutations in the pathogenesis of chronic lymphocytic leukemia is unknown. Here we investigated the frequency of constitutional ATM mutations in either of two chronic lymphocytic leukemia cohorts, those with and without a chromosome 11q deletion. We found that in comparison to controls, constitutional pathogenic ATM mutations were increased in patients with chromosome 11q deletions (6 of 140 vs. 0 of 281, P=0.001) but not in those without 11q deletions (2 of 178 vs. 0 of 281, P=0.15). These results suggest that ATM germline heterozygosity does not play a role in chronic lymphocytic leukemia initiation but rather influences rapid disease progression through ATM loss.
Keywords: sickle cell disease, nephropathy, hemolysis, kidney
Introduction
Ataxia telangiectasia (AT) results from bi-allelic constitutional mutations in the Ataxia Telangiectasia Mutated (ATM) gene on chromosome 11q23. AT is characterized by a 200-fold increased risk of lymphoid tumors1 and carriers are also at an increased risk of cancer development albeit at a much lower level.2 Sporadic ATM mutations have been detected in tumor cells in patients with various lymphoid malignancies including B-cell chronic lymphocytic leukemia (CLL).3–5 The ATM protein signals cellular responses to DNA damage in the form of DNA double strand breaks (DSBs) and prevents accumulation of potentially tumorigenic cells with unrepaired DNA DSBs.1
The diagnosis of CLL requires a B-cell clone of more than 5×109 cells/L of blood with characteristic cell surface markers. Individuals with a CLL clone of less than 5×109/L and no additional pathological features are classified as having the pre-malignant condition monoclonal B-cell lymphocytosis (MBL).6,7 CLL shows marked clinical heterogeneity and prognostic markers associated with a poor outcome include deletions of chromosomes 17p and 11q, leading to loss of TP53 and ATM alleles, respectively.8 Mutations in ATM are linked to poor prognosis and are commonly, but not exclusively, associated with a chromosome 11q23 deletion. We found that 36% of CLLs with an 11q deletion carry a mutation in the remaining ATM allele resulting in bi-allelic ATM defects.3,4
Instances of familial occurrence are recognized in CLL9 and registry studies confirm that first degree relatives of CLL patients have a 7-fold increased risk of developing CLL.10 Relatives are also at an increased rate of MBL.11 Within CLL pedigrees there are no consistent patterns of leukemic development suggesting involvement of multiple low risk alleles rather than a single high-risk locus.9 Recent genome wide association studies provide evidence for low penetrance risk alleles that together may increase an individual’s risk of developing CLL.12,13
ATM mutations occur at different stages of CLL development and in occasional cases an ATM mutation is present in a patient’s germline.3,4,14 In one previous study, ATM mutations were not found to be responsible for CLL familial clustering;15 however, the role of constitutional pathogenic ATM mutations in the pathogenesis of CLL remains unknown. In this study, we have addressed the role of ATM carrier status in two CLL cohorts. We compared the frequency of constitutional ATM mutations in 140 CLL patients with an 11q deletion, where ATM mutation is a frequent genetic event,4 and 178 CLL patients with no 11q deletion, where ATM mutations are rare,3 with 281 healthy controls.
Design and Methods
CLL and control cohorts
CLL cohorts were taken from patients treated on the UK CLL4 Trial and at Birmingham, Bournemouth and Leicester hospitals. All were diagnosed according to standard criteria and had had fluorescence in situ hybridization (FISH) performed with the ATM (11q23) probe. Controls included a volunteer research subgroup (n=71) and anonymous blood donors (n=210). Ethical approval was obtained from South Birmingham Ethics Committee (ref. O4/Q2709/25).
Mutational analysis of the ATM gene
Genomic DNA was extracted from mononuclear cells, as previously described.3,4 Screening for ATM sequence changes was performed using denaturing high-performance liquid chromatography (DHPLC).3,4 DHPLC is able to detect mutations present in just 5–10% of the total cell population.16 The PCR products were sequenced whenever variant chromatogram patterns were detected.3,4 Sequence changes were compared to published results17,18 and ATM databases (http://chromium.liacs.nl/LOVD2/home.php). To exclude consideration of rare polymorphisms, sequence changes were classified as pathogenic mutations only if they were previously reported as causative mutations in AT patients or were predicted to cause protein truncation. When an ATM mutation was detected in the leukemic cells from a CLL case, subsequent analysis of constitutional DNA was performed on either buccal cells or peripheral blood (PB) granulocytes. When PB granulocytes were used, we adopted a strategy to ensure changes were genuine germline mutations and not due to contamination with leukemia cells. Mutations were classified as acquired if the heteroduplex peak area representing mutation in the CLL DNA was either absent from the granulocyte DNA or represented less than 40% of the peak area observed in the corresponding tumor DNA. Mutations were classified as germline if the peak area in the granulocyte DNA represented more than 90% of that seen in the CLL DNA. To validate this approach, we amplified clonal VDJ recombinations from representative paired samples (Online Supplementary Figure S1A and B).
Analysis of immunoglobulin gene usage and class switch recombination (CSR)
Immunoglobulin heavy chain (IGH) VDJ rearrangements were amplified using primers corresponding to the consensus sequences of the framework 1 (FR1) and joining (J6) region. Nucleotide sequences were analyzed as previously described.19 IGHV genes were classified as unmutated if there was 98% or more homology with the parent sequence. Analysis of CSR involved amplification of the Sμ–Sα and Sμ–Sγ switch joints, as previously described.20 The amplified sequences were aligned using Basic Local Alignment Search Tool database (http://www.ncbi.nlm.nih.gov/BLAST/).
Statistical analysis
Fisher’s exact t-test was used for comparisons of parameters and the Kaplan-Meier method was used to determine survival outcomes.
Results and Discussion
ATM sequence changes in CLL patients and controls
We analyzed the ATM coding region in 318 CLL patients (140 with a chromosome 11q deletion and 178 with no 11q deletion) and 281 controls. Altogether, 85 unique sequence changes were detected in CLL patients, which included 19 changes that were also found in control individuals and 66 changes that were only observed among CLL patients. Sixty of these CLL-specific changes were identified in just one CLL patient, and 6 were identified in more than one CLL case. Thirty-one changes were classified as pathogenic ATM mutation using our stringent criteria. The remaining changes represented 27 known polymorphisms and 27 missense ‘sequence variants’, where the pathogenecity of the specific sequence change has not been proven. In total, 35 unique sequence changes were detected in controls including the 19 changes that were common to both CLLs and controls. Twenty-nine changes were known polymorphic variants, 6 were classified as sequence variants and no changes fulfilled our criteria for a pathogenic ATM mutation (Online Supplementary Tables S1 and S2).
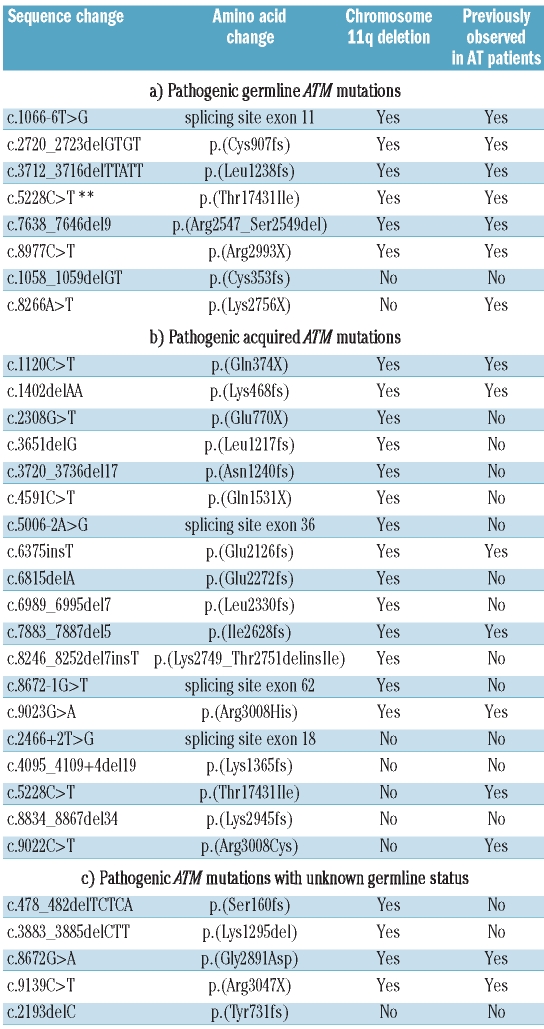
Pathogenic constitutional ATM mutations
The pathogenic ATM mutations occurred in 24 CLLs with an 11q deletion and 8 CLLs without an 11q deletion (Table 1). Amongst CLLs with an 11q deletion, 6 mutations were of germline origin, 14 were acquired in leukemic DNA and 4 remained of unknown origin due to lack of germline material. Amongst CLLs without a chromosome 11q deletion, 2 mutations were constitutional, 5 were acquired and one of unknown origin. In cases with an 11q deletion, 3 germline mutations were predicted to lead to a truncated protein (c.2720_2723delGTGT, c.3712_3716delTTATT, c.8977C>T), one to an amino acid substitution (c.5228C>T [p.T1743I]), one to an in-frame deletion (c.7638_7646del9 [p.R2547_S2549del]) and one to altered splicing (skipping of exon 11) (c.1066-6T>G). In non-11q deleted CLLs, both constitutional mutations (c.1058_1059delGT, c.8266A>T) were predicted to lead to premature termination of the protein. Seven out of 8 of the constitutional mutations have been previously reported in AT families.17,18 Consideration of the two separate CLL cohorts demonstrated that in comparison to controls, constitutional pathogenic ATM mutations are significantly more common in CLL patients who develop a chromosome 11q deletion (6 of 140 vs. 0 of 281, P=0.001) but not in those who do not subsequently acquire this deletion in their leukemic clone (2 of 178 vs. 0 of 281, P=0.15).
Table 1.
Pathogenic ATM mutations within the CLL patients.
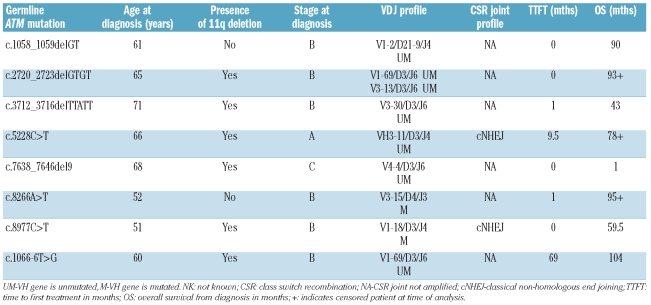
Characteristics of the pathogenic ATM mutation carriers
We compared patients’ characteristics between those with constitutional ATM mutations, acquired ATM mutations, ATM sequence variants (with no additional ATM mutation) and those with ATM wild-type or known ATM polymorphisms (Table 2, Online Supplementary Table S3). Overall there was no difference in stage at diagnosis or VH mutations status between the 4 categories, but notably amongst patients with constitutional ATM mutations, 7 of 8 were advanced stage at diagnosis and 6 of 8 had unmutated VH genes. There was a significant difference in overall survival between these groups (P=0.005). Patients with constitutional and acquired ATM pathogenic mutations and those with ATM sequence variants all had inferior survival compared to those with wild-type ATM.
Table 2.
Clinical and biological characteristics of CLL in ATM germline mutation carriers.
The ATM protein ensures fidelity of CSR and prevents use of non-classical non-homologous end joining (NHEJ).21 We investigated CSR and VDJ profiles in CLL cells of ATM mutant carriers.22 Both CLLs analyzed were found to have normal CSR joints. Two patients had a CLL clone carrying the V1-69/D3/J6 segment pattern22 and the remaining cases showed a range of V segments (Table 2).
Acquired defects in ATM are common in CLL and their impact on cellular phenotype and clinical prognosis has already been established.3,4 Constitutional ATM mutations have rarely been identified in CLL patients but their contribution to the pathogenesis of CLL is unknown.18,19 Here we show that, in comparison to controls, a significantly increased frequency of constitutional pathogenic ATM mutant alleles occurs specifically within the subset of CLL patients who subsequently develop a deletion of chromosome 11q in their leukemic cells.
In this study, we used highly stringent criteria to classify pathogenic ATM mutations. Classification of missense changes is difficult and although features such as occurrence within functional domains and acquisition within cancer cells suggest pathogenecity, the only way to prove functional consequences is by mutation modeling. Given our focus in this study on constitutional changes, where classification can be more difficult, we used more stringent criteria than in previous publications. Consequently, certain changes previously classified as mutations were here labeled as sequence variants. The poor survival outcome observed in the ‘sequence variant’ group is interesting and suggests many of these changes may indeed be exerting functional consequences.
Control and CLL cohorts were not age matched but there was no reason to indicate imposed bias. If controls were younger than patients, then some individuals could develop CLL in later life. Given an incidence of CLL of 4 per 100,000, this would be a rare event.6 It is possible that the number of pathogenic germ-line ATM mutations was higher among CLL patients than we were able to demonstrate. In 4 patients with and one without a chromosome 11q deletion, pathogenic ATM mutations were identified but constitutional material was not available. Thus, the frequency of ATM mutation carriers in CLL patients could have been underestimated; but even if all of these 5 cases carried constitutional ATM mutations it would not alter the significance of our findings.
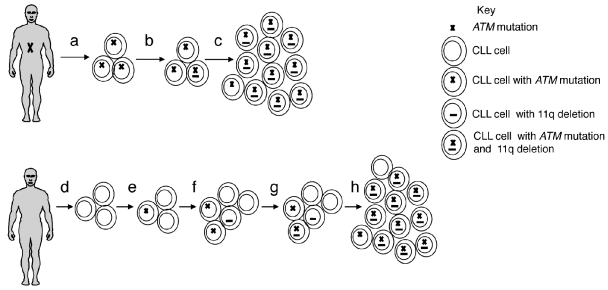
The finding of a significantly increased frequency of ATM carriers only amongst the 11q deleted CLL subgroup suggested that the principal effect of a germline ATM mutation might be on progression rather than leukemia initiation. Several lines of evidence support this interpretation. First, there is no evidence of an increased frequency of CLL among AT family members.2 Second, chromosome 11q deletion in leukemic cells is generally considered a late event in CLL pathogenesis suggesting that complete loss of ATM function occurs after initial clonal proliferation.23 Finally, here we observed no evidence for the effect of ATM loss on cellular processes, such as CSR and VDJ segment usage, that might be associated with CLL initiation.24 Thus, we propose a model whereby ATM germline mutations do not contribute to CLL initiation but rather predispose to rapid disease progression through acquired chromosome 11q deletions, loss of ATM activity and clonal expansion (Figure 1). In keeping with this model, we found that most ATM mutant carriers were already high stage at diagnosis. Furthermore, although our entire CLL cohort was biased for patients with advanced disease, via selection for 11q deletion and inclusion of CLL4 trial patients, carriers of ATM mutations still demonstrated short overall survival.
Figure 1.
Model for CLL development in ATM mutation carriers. The upper panel represents a model for CLL pathogenesis in an ATM germline mutation carrier. CLL clonal initiation occurs independently of the germline ATM mutation but all cells within the clone will carry the ATM mutation because this is present in all body cells (a). Subsequent loss of chromosome 11q in any CLL cell (b) will lead to loss of ATM function resulting in apoptotic resistance and a selective pressure for rapid clonal expansion and disease progression (c). By comparison, the lower panel represents a model of CLL pathogenesis with wild-type germline ATM. Following clonal initiation (d), subsequent development of an acquired ATM mutation or chromosome 11q deletion might be expected to initially exert limited selective pressure (e,f). Only if both defects are acquired within a cell does loss of ATM function occur and rapid clonal expansion (g,h). Loss of ATM function will occur less frequently and over a more prolonged time course in this model than in the germline ATM mutation model and here development of alternative genetic events may influence disease progress rather than ATM/11q defects (not illustrated).
Many cases of CLL are preceded by an asymptomatic MBL and studies suggest a prevalence of MBL of 3.5% and a transformation rate to CLL of 1% per annum.7 Given this prevalence, it is likely that occasional ATM mutation carriers will develop MBL by chance during their lifetime and in such individuals there will be selective pressure for the loss of a second ATM allele by 11q deletion leading to an aggressive leukemia phenotype characterized by impaired DNA damage induced apoptosis and genomic instability.1,4 This explanation is in keeping with our finding of an excess of ATM carriers amongst the CLL cohort who had a chromosome 11q deletion.
Acknowledgments
We thank Maria Podinovskaya, Clemency Hawksley and Ian Edwards for technical support.
Footnotes
Funding: this work was supported by the Leukaemia Lymphoma Research UK and Cancer Research UK.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Taylor AM, Byrd P. Molecular pathology of ataxia telangiectasia. J Clin Pathol. 2005;58(10):1009–15. doi: 10.1136/jcp.2005.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, et al. Cancer risks and mortality in heterozygous ATM mutations carriers. J Natl Cancer Inst. 2005;97(11):813–22. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 3.Austen B, Powell JE, Alvi A, Edwards I, Hooper L, Starczynski J, et al. Mutations in the ATM gene lead to impaired overall and treatment free survival of B-CLL patients that is independent of IGVH mutation status. Blood. 2005;106(9):3175–82. doi: 10.1182/blood-2004-11-4516. [DOI] [PubMed] [Google Scholar]
- 4.Austen B, Skowronska A, Baker C, Powell JE, Gardiner A, Oscier D, et al. The mutation status of the residual ATM allele is a determinant of in vitro cellular responses, clone expansion and survival in CLL patients with chromosome 11q deletion. J Clin Onc. 2007;25(34):5448–57. doi: 10.1200/JCO.2007.11.2649. [DOI] [PubMed] [Google Scholar]
- 5.Schaffner C, Idler I, Stilgenbauer S, Dohner H, Lichter P. Mantle cell lymphoma is characterized by inactivation of the ATM gene. Proc Natl Acad Sci. 2000;97(6):2773–78. doi: 10.1073/pnas.050400997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oscier D, Fegan C, Hillmen P, Illidge T, Johnson S, Maguire P, et al. Guidelines Working Group of the UK CLL Forum. British Committee for Standards in Haematology. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol. 2004;125(3):294–317. doi: 10.1111/j.1365-2141.2004.04898.x. [DOI] [PubMed] [Google Scholar]
- 7.Rawstron AC, Bennett FL, O'Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–83. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 8.Stilgenbauer S, Bullinger L, Lichter P, Dohner H German CLL Study Group (GCLLSG) Chronic Lymphocytic Leukaemia. Genetics of chronic lymphocytic leukaemia: genomic aberrations and VH gene mutation status in pathogenesis and clinical course. Leukaemia. 2002;16(6):993–1007. doi: 10.1038/sj.leu.2402537. [DOI] [PubMed] [Google Scholar]
- 9.Goldin LR, Slager SL. Familial CLL. Genes and Environment. Hematology. 2007:339–45. doi: 10.1182/asheducation-2007.1.339. [DOI] [PubMed] [Google Scholar]
- 10.Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumours in families of patients with chronic lymphocytic leukaemia: results from the Swedish Family-Cancer Database. Blood. 2004;104(6):1850–4. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 11.De Tute R, Yuille M, Catovsky D, Houlston RS, Hillmen P, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL) in CLL families:substantial increase in relative risk in young adults. Leukaemia. 2006;20(4):728–39. doi: 10.1038/sj.leu.2404116. [DOI] [PubMed] [Google Scholar]
- 12.Rudd MF, Sellick GS, Webb EL, Catovsky D, Houlston RS. Variants in the ATM-BRCA2-CHEK2 axis predispose to chronic lymphocytic leukaemia. Blood. 2006;108(2):638–44. doi: 10.1182/blood-2005-12-5022. [DOI] [PubMed] [Google Scholar]
- 13.Di Bernardo MC, Crowther-Swanepoel D, Broderick P, Webb E, Sellick G, Wild R, et al. A genome-wide association study identifies six susceptibility loci for chronic lymphocytic leukaemia. Nat Genet. 2008;40(10):1204–10. doi: 10.1038/ng.219. [DOI] [PubMed] [Google Scholar]
- 14.Bullrich F, Rasio D, Kitada S, Starostik P, Kipps T, Keating M, et al. ATM mutations in B-cell chronic lymphocytic leukemia. Cancer Res. 1999;59(1):24–7. [PubMed] [Google Scholar]
- 15.Yuille MR, Condie A, Hudson CD, Bradshaw PS, Stone EM, Matutes E, et al. ATM mutations are rare in familial chronic lymphocytic leukemia. Blood. 2002;100(2):603–9. doi: 10.1182/blood.v100.2.603. [DOI] [PubMed] [Google Scholar]
- 16.Xiao W, Oefner PJ. Denaturing high-performance liquid chromatograph: A review. Hum Mutat. 2001;17(6):439–74. doi: 10.1002/humu.1130. [DOI] [PubMed] [Google Scholar]
- 17.Stankovic T, Kidd AMJ, Sutcliffe A, McGuire GM, Robinson P, Weber P, et al. ATM mutations and phenotypes in A-T families in the British Isles: Expression of mutant ATM and the risk of leukaemia, lymphoma and breast cancer. Am J Hum Genet. 1998;62(2):334–45. doi: 10.1086/301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandoval N, Platzer M, Rosenthal A, Dörk T, Bendix R, Skawran B, et al. Characterisation of ATM gene mutations in 66 ataxia telangiectasia families. Hum Mol Genet. 1999;8(1):69–79. doi: 10.1093/hmg/8.1.69. [DOI] [PubMed] [Google Scholar]
- 19.Stankovic T, Stewart G, Fegan C, Biggs P, Last J, Byrd PJ, et al. Ataxia Telangiectasia mutated-deficient B-cell chronic lymphocytic leukaemia occurs in pregerminal center cells and results in defective damage response and unrepaired chromosome damage. Blood. 2002;99(1):300–9. doi: 10.1182/blood.v99.1.300. [DOI] [PubMed] [Google Scholar]
- 20.Stewart GS, Stankovic T, Byrd PJ, Wechsler T, Miller ES, Huissoon A, et al. RIDDLE immunodeficiency syndrome is linked to defects in 53BP1-mediated DNA damage signaling. Proc Natl Acad Sci USA. 2007;104(43):16910–5. doi: 10.1073/pnas.0708408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan-Hammarström Q, Lähdesmäki A, Zhao Y, Du L, Zhao Z, Wen S, et al. Disparate roles of ATR and ATM in immunoglobulin class switch recombination and somatic hypermutation. J Exp Med. 2006;203(1):99–110. doi: 10.1084/jem.20050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, et al. Over 20% of patients with chronic lymphocytic leukaemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 2007;109(1):259–70. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 23.Cuneo A, Bigoni R, Rigolin GM, Roberti MG, Bardi A, Cavazzini F, et al. Late appearance of the 11q22.3-23.1 deletion involving the ATM locus in B-cell chronic lymphocytic leukemia and related disorders. Clinicobiological significance. Haematologica. 2002;87(1):44–51. [PubMed] [Google Scholar]
- 24.Oppezzo P, Dighiero G. What do somatic hypermutation and class switch recombination teach us about chronic lymphocytic leukaemia pathogenesis? Curr Top Microbiol Immunol. 2005;294:71–89. doi: 10.1007/3-540-29933-5_5. [DOI] [PubMed] [Google Scholar]