β thalassemia intermedia (TI) is a clinical definition referring to a heterogeneous group of hereditary anemias resulting from defective β chain production, an α/β globin chain imbalance, and anemia that lie in severity between that of thalassemia minor and the transfusion-dependent thalassemia major (TM).1 The phenotype of TI may also result from the increased production of α globin chains by a triplicated or quadruplicated α genotype associated with β heterozygosity, also leading to α/β globin chain imbalance, and a presumably mild clinical phenotype.2–5 We report here 2 siblings with transfusion-independent TI who had immediate worsening of their clinical course and became transfusion-dependent after splenectomy, and discuss how the absolute excess of α globin chains in these 2 patients may explain this development.
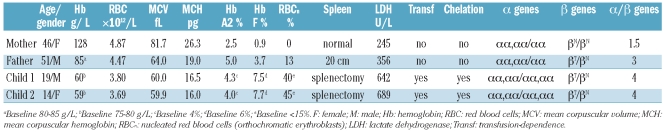
The father was diagnosed with TI at the age of 16 years (baseline hemoglobin level 80–85 g/L) and the mother had a normal hematologic profile. The 2 parents are not consanguineous at least as far back as four generations, although both are from a small village in the North East of Italy. The 2 children were diagnosed with TI at the ages of two and four years. The 2 children, who had never been transfused before (baseline hemoglobin levels 75–80 g/L), underwent splenectomy at the ages of ten and 12 years because of splenomegaly and secondary anemia. Five months following splenectomy, the clinical course of both patients worsened and anemia (hemoglobin level <70 g/L) necessitated initiating a transfusion regimen. Chelation therapy was initiated at the ages of 14 and 19 years. The younger child had thrombophlebitis at 17 years. The father, now aged 51 years, shows a well tolerated chronic hemolytic anemia without the need for transfusion (hemoglobin level maintained at 80–85 g/L), mild jaundice, splenomegaly and leg ulcers. The mother, still has a completely normal hematological profile and hemoglobin pattern.
The father and the 2 children were initially considered to have unusual TI because at molecular analysis they were carriers of a single β+ mutation (IVS1-110 G>A). After the unusual clinical outcome following splenectomy, all family members were referred to our center for molecular reevaluation. As according to our center’s ethical committee guidelines, all family members signed written informed consent to molecular studies for both diagnostic and research purposes. DNA analysis of β and α globin genes was performed. Mutation analysis of the β globin gene was established by direct DNA sequencing on the ABI Prism 310 genetic analyzer (PE Biosystems Foster City CA, USA). The α globin gene cluster was analyzed by Multiplex Ligation-dependent Probe Amplification (MLPA) according to Harteveld et al.4,6 RNA analysis was performed in triplicate on TaqMan 7500 (PE Biosystems Foster City, CA, USA) after DNAse treatment using specific gene assay (HBA1/2: Hs00361191_g1). The relative quantification (RQ) of alpha genes expression was achieved by normalization against two different house-keeping genes Beta-actine (ACTB:Hs99999903_m) and β2 microglobuline (β2M:Hs00187842-m1) using the 2−ΔΔct formula.
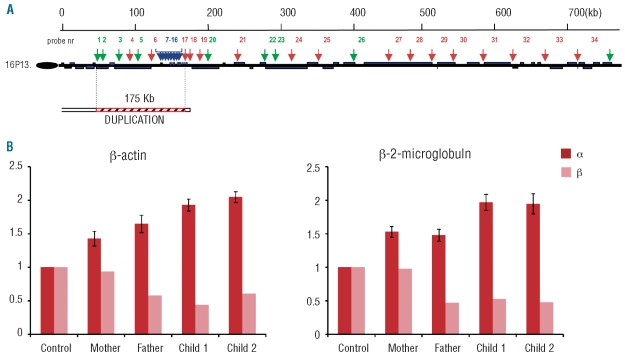
Molecular analysis confirmed that the father and the 2 children were heterozygotes for the β+ mutation IVSI-110 G>A. MLPA analysis disclosed a full duplication of the α globin locus spanning at least 175 kb, from the telomere to the 3’HVR downstream of the α globin gene, including the upstream regulatory element HS-40 (Figure 1A).4 This rearrangement increases the number of functional α globin genes in cis from 2 to 4 and was found in heterozygosis in both parents and in homozygosis in both children. The overproduction of alpha chains was confirmed by Q-PCR experiments with RQ increased in all members of the family (Figure 1B). As expected, the parents showed RQ values of about 1.5 while the children showed values of about 2. Hematologic and molecular data of the family are summarized in Table 1.
Figure 1.
(A) Schematic representation of the 750 Kb region from the telomere of the short arm of chromosome 16 analyzed by Multiplex Ligation-dependent Probe Amplification (MLPA). The position of the 35 MLPA-probe pairs along the region is indicated by green, red and blue arrows, referring to HEX, ROX and FAM labeling, respectively. The duplicated region is indicated as arched red bars and spans at least 175 Kb. (B) Real-time polymerase chain reaction experiments: the graphs show the mean and standard deviation of three different experiments. The expression of α and β globin genes is compared to β-actin and β-2 microglobulin house-keeping genes.
Table 1.
Hematologic and molecular data of the family at the time of study.
In the father, coinheritance of a β globin mutation with 6 α globin genes led to a moderate-severe, well tolerated TI phenotype. The 2 children had 8 functional α globin genes (since the regulatory element HS-40 was also duplicated) producing a large amount of α chains (twice than normal) that, associated with reduced β chain production, causes a severe α/β globin imbalance.
In transfusion-independent TI, erythropoietic stress, ineffective erythropoiesis, and chronic intravascular hemolysis are highly variable. An increased number of erythroblasts, reticulocytes, and damaged erythrocytes are seen in the peripheral blood. The excess free α chains precipitate within the erythroid precursors, form hemi-chromes, and alter the membrane cytoskeletal proteins by clustering band 3 and enhancing deposition of opsonin autologous immunogobulins and C3 fragments.7–8 About 80% of senescent or altered erythrocytes are removed extravascularly by macrophages present mainly in the spleen.7 Thus, removal of the spleen in patients with an absolute excess of α chains, as in our patients, may lead to more severe hemolysis and persistence of damaged erythroblasts and erythrocytes in the blood stream compared to patients with defective β chain production and a relative excess of α chains. These persistent abnormal erythroid cells may justify the high rate of thromboembolism in splenectomized TI patients,9–10 including the one reported herein. They acquire procoagulant properties from increased exposure of phosphatidylserine instead of phosphatidyletanolamine on their outer surface, by an altered flip flop mechanism mediated by band 3 clusters.11 Thus, in the 2 siblings, splenectomy was associated with an increase in the number of nucleated red blood cells (as documented in the blood smear), increased peripheral hemolysis (worsening anemia), and increased risk of thrombotic events (in one of the children).
In the light of several reports linking splenectomy to adverse long-term outcomes, it was recently recommended that the decision to perform a splenectomy in TI patients should be taken with caution and the procedure should be avoided or delayed unless absolutely necessary.1 Given our experience, we agree with this recommendation, especially in TI patients characterized by a severe imbalance between α/non-α globin chains due to an absolute excess of α globin chains. We also recommend including α globin gene testing in the molecular workup of patients with TI where the β gene defect does not completely explain the observed phenotype.
References
- 1.Taher AT, Musallam KM, Cappellini MD, Weatherall DJ. Optimal management of beta thalassaemia intermedia. Br J Haematol. 2011;152(5):512–23. doi: 10.1111/j.1365-2141.2010.08486.x. [DOI] [PubMed] [Google Scholar]
- 2.Camaschella C, Kattamis AC, Petroni D, Roetto A, Sivera P, Sbaiz L, et al. Different hematological phenotypes caused by the interaction of triplicated alpha-globin genes and heterozygous beta-thalassemia. Am J Hematol. 1997;55(2):83–8. doi: 10.1002/(sici)1096-8652(199706)55:2<83::aid-ajh6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Sampietro M, Cazzola M, Cappellini MD, Fiorelli G. The triplicated alpha-gene locus and heterozygous beta thalassaemia: a case of thalassaemia intermedia. Br J Haematol. 1983;55(4):709–10. doi: 10.1111/j.1365-2141.1983.tb02854.x. [DOI] [PubMed] [Google Scholar]
- 4.Harteveld CL, Refaldi C, Cassinerio E, Cappellini MD, Giordano PC. Segmental duplications involving the alpha-globin gene cluster are causing beta-thalassemia intermedia phenotypes in beta-thalassemia heterozygous patients. Blood Cells Mol Dis. 2008;40(3):312–6. doi: 10.1016/j.bcmd.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Sollaino MC, Paglietti ME, Perseu L, Giagu N, Loi D, Galanello R. Association of alpha globin gene quadruplication and heterozygous beta thalassemia in patients with thalassemia intermedia. Haematologica. 2009;94(10):1445–8. doi: 10.3324/haematol.2009.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harteveld CL, Voskamp A, Phylipsen M, Akkermans N, den Dunnen JT, White SJ, et al. Nine unknown rearrangements in 16p13.3 and 11p15.4 causing alpha- and beta-thalassaemia characterised by high resolution multiplex ligation-dependent probe amplification. J Med Genet. 2005;42(12):922–31. doi: 10.1136/jmg.2005.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannu F, Arese P, Cappellini MD, Fiorelli G, Cappadoro M, Giribaldi G, et al. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood. 1995;86(5):2014–20. [PubMed] [Google Scholar]
- 8.Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia. Semin Hematol. 1990;27(1):70–82. [PubMed] [Google Scholar]
- 9.Taher AT, Musallam KM, Karimi M, El-Beshlawy A, Belhoul K, Daar S, et al. Splenectomy and thrombosis: the case of thalassemia intermedia. J Thromb Haemost. 2010;8(10):2152–8. doi: 10.1111/j.1538-7836.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 10.Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli G, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111(2):467–73. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- 11.Cappellini MD, Motta I, Musallam KM, Taher AT. Redefining thalassemia as a hypercoagulable state. Ann NY Acad Sci. 2010;1202:231–6. doi: 10.1111/j.1749-6632.2010.05548.x. [DOI] [PubMed] [Google Scholar]