Abstract
Background
Numerous studies report that bariatric surgery patients report more physical activity (PA) after surgery than before, but the quality of PA assessment has been questionable.
Methods
The Longitudinal Assessment of Bariatric Surgery-2 is a 10-center longitudinal study of adults undergoing bariatric surgery. Of 2458 participants, 455 were given an activity monitor, which records steps/minute, and an exercise diary before and 1 year after surgery. Mean step/day, active minutes/day, and high-cadence minutes/week were calculated for 310 participants who wore the monitor at least 10 hours/day for at least 3 days at both time points. Pre- and post-surgery PA were compared for differences using the Wilcoxon signed-rank test. Generalized Estimating Equations identified independent pre-operative predictors of post-operative PA.
Results
PA increased significantly (p<.0001) pre- to post-operative for all PA measures. Median values pre- and post-operative were: 7563 and 8788 steps/day; 309 and 340 active minutes/day; and 72 and 112 high-cadence minutes/week, respectively. However, depending on the PA measure, 24–29% of participants were at least 5% less active post-operative than pre-operative. Controlling for surgical procedure, sex, age and BMI, higher PA preoperative independently predicted higher PA post-operative (p<.0001, all PA measures). Less pain, not having asthma and self-report of increasing PA as a weight loss strategy pre-operative also independently predicted more high-cadence minutes/week post-operative (p<.05).
Conclusion
The majority of adults increase their PA level following bariatric surgery. However, most remain insufficiently active and some become less active. Increasing PA, addressing pain and treating asthma prior to surgery may have a positive impact on post-operative PA.
Keywords: Physical activity, exercise, activity monitor, bariatric surgery, severe obesity
INTRODUCTION
Observational studies suggest that more pre- and post-operative physical activity (PA) is related to improved surgical outcomes, including weight loss(1;2). Studies of non-surgical weight loss suggest that PA may also be an important contributor to long-term weight loss maintenance following surgery(3–5). Furthermore, participating in regular PA improves flexibility, strength and balance, helps build and maintain healthy bones, reduces risk of cardiovascular disease, stroke, diabetes mellitus type 2, breast cancer and colon cancer, improves immunity, promotes psychological well being, improves or maintains some aspects of cognitive function, enhances quality of sleep, and delays all-cause mortality(6).
While there is a wide range of PA levels among bariatric surgery candidates, the majority are insufficiently active to reap the benefits of regular PA(7;8). Studies of self-reported PA have consistently shown that patients are more active following surgery(1). However, it is unclear whether these increases are at least partially explained by misperception (i.e., improvements in functional capacity following surgery may cause patients to feel more active) or reporting bias (i.e., knowing that they are expected to be more active, patients may feel more pressure to over-report their PA following surgery). In a recent study utilizing both a PA survey and an accelerometer in 20 Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric band (LAGB) patients, self-reported moderate-vigorous intensity PA (MVPA) significantly increased more than four-fold from pre-operative to six months post-operative while there was not a significant change in MVPA (or low intensity PA) measured by accelerometer, suggesting patients over-reported post-operative PA(9). Only two other studies(10;11) have reported pre- to post-operative change in objectively-measured PA and both were limited by small sample size, potential selection bias, and use of pedometers known to perform less accurately at slow walking speeds and with abnormal gaits(12;13), which are more common with severe obesity(14;15).
In addition to determining mean change in PA following surgery it is important to consider the variability in change. Using a cut point of at least 200 minutes/week of self-reported walking and MVPA to define “active,” Bond and colleagues classified 199 RYGB and LAGB patients into groups according to their pre- and 1-year post-operative PA level(16). While most remained in the same category, either active (42%; n=83) or inactive (20%; n=39) at both time points, roughly one-third (34%; n=68) went from inactive to active, and 5% (n=9) went from active to inactive, with a mean decrease of 407 minutes/week, suggesting that not only do some patients fail to increase their PA level 1-year following surgery, some experience a clinically significant decrease in PA. However, to date, no other studies have characterized the variability in change in pre- to post-operative PA. In addition, little effort has gone into determining whether there are pre-operative patient characteristics that can be used to identify patients at highest risk for insufficient post-operative PA.
To address shortcomings in the literature this study aimed to 1) characterize change in PA pre- to 1 year post-operative in a large sample of bariatric surgery patients using a high-quality PA monitor, and 2) identify pre-operative patient characteristics that predict change in PA from pre- to post-operative and PA level at 1 year.
METHODS
Participants
The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) is an observational study designed to assess the risks and benefits of bariatric surgery(17). Between March 2006 and April 2009 2458 participants at least 18 years old who had not previously had bariatric surgery underwent bariatric surgery at one of ten participating locations throughout the United States. A research visit was scheduled within 30 days prior to scheduled surgery. Participants returned for a 1 year post-operative follow-up visit between 90 days before and 180 days after their 1 year surgery anniversary date. All participating centers had institutional review board approval and all participants provided informed consent.
Measures
Physical activity
The StepWatch™ 3 Activity Monitor (SAM, OrthoCare Innovations, Washington, D.C.) is a small (75×50×20 mm) lightweight (38g) microprocessor-controlled biaxial activity monitor, worn above the ankle, that combines acceleration, position, and timing information to count strides (i.e. single leg-steps) per time interval. It is accurate in lean and obese individuals at “slow” (i.e., 1.6 km/h) and “purposeful walking” (i.e., 3.2–5.6 km/h) paces and with a variety of gait styles, with accuracy typically exceeding 98%(18). Of 2458 participants in LABS-2, 455 were given a SAM at both the pre- and 1 year post-operative research visit. Almost 70% (n=1707) of LABS-2 participants were not given a SAM at one or both visits due to lack of available monitors; 12% (n=296) were not given a SAM at one or both visits for other reasons (pre-operative research visit was less than 3 days before surgery (n=71), walking limitation not related to obesity (n=8), wheel chair use (n=8), refused to wear a monitor (n=121), did not attend the 1 year research visit (n=87), reason unknown (n=1)). Trained research staff programmed the SAM to double count all strides in 1 minute intervals with sensitivity settings appropriate to a participant’s height, gait, and cadence using accompanying software. Participants were asked to wear the SAM at least during waking hours for the seven consecutive days following their research visit, with an option to wear the monitor continuously. Participants were also asked to complete a PA diary, which assessed activities done specifically for exercise and the duration of each activity, for this seven day period. Data from the manufacturer software were exported to a SAS version 9.1 dataset (SAS Institute Inc, Cary, NC). Step counts at the minute level were screened for signs of monitor malfunction. Non-wear periods were identified by intervals of at least 120 minutes with no activity(19). Daily wear time was calculated as 24 hours minus the duration of non-wear periods. For data to be valid, participants had to have at least 10 hours of monitor wear/day for at least 3 days at both the pre- and 1 year post-operative assessments. These criteria were met by 310 (68%) of the 455 participants who were given a SAM at both time points. Step counts at the minute level were used to calculate mean daily values for each participant for the following PA parameters: steps; minutes of activity, i.e., the number of minutes with step count greater than 0; high cadence minutes, i.e., the number of minutes with more than 80 steps. For comparison with U.S. physical activity recommendations, the number of high cadence minutes occurring in bouts of at least 10 minutes was also computed, allowing interruptions of 1 or 2 minutes below the threshold(20).
As proxy measures of weekly minutes of MVPA, high-cadence minutes (accumulated individually and in bouts) and self-reported exercise were standardized to 1 week periods by multiplying mean daily values by seven. Participants were categorized as “active” if they accumulated at least 150 minutes/week(6). In addition, participants were categorized as “active” if they accumulated at least 10000 steps/day(21). Changes in PA parameters were calculated as the post-operative value minus the pre-operative value so that positive values indicate an increase in PA. Participants were categorized as having a decrease in PA of at least 5% if the post-operative value divided by pre-operative value was less than 95%.
Weight loss strategies related to PA
Participants completed a questionnaire modeled after a questionnaire used in the Look AHEAD trial(22), which assessed use of specific weight loss strategies related to PA (i.e., increased exercise level, used home exercise equipment, recorded exercise daily, participated in group exercise, seen a personal trainer) in the prior 6 months.
Anthropometrics, sociodemographics and physical illness
Anthropometric measurements were made by trained personnel using standardized protocols. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Percentage weight loss was calculated as (pre-operative weight minus 1 year post-operative weight) divided by pre-operative weight. Age, sex, race, ethnicity, marital status, education, employment status, household income, smoking status, and walking limitations (i.e., inability to walk 200 feet unassisted and walking aid use) were assessed with a study-specific questionnaire. Comorbidities were determined with a combination of self-report, clinical assessment and medical chart review. An index of comorbidities was created as the number of the following 10 comorbidities (range 0–10): diabetes, hypertension, ischemic heart disease, congestive heart failure, history of stroke, sleep apnea, pulmonary hypertension, asthma, history of deep vein thrombosis or pulmonary embolism (DVT/PE), and venous edema with ulcerations.
Physical and mental health
Self-reported functional health and well-being were assessed with the Medical Outcomes Study 36-item Short-Form Health Survey, a widely used instrument with well-established validity and reliability(23). Responses to the SF-36 are used to generate a score in four physical (physical functioning, role limitations due to physical problems, bodily pain, general health) and four mental (vitality, social functioning, role limitations due to emotional problems, and mental health) scales and two summary scores (Physical Component Summary [PCS] and Mental Component Summary [MCS]). Transformed scores have a mean of 50 and standard deviation of 10 in the general U.S. population. A higher score implies better functioning.
Symptoms of depression
Past week depressive symptomatology was assessed with the Beck Depression Inventory (BDI), version 1(24). The 21-item test is scored by assigning a value of 0 to 3 to each response. However, no points were assigned to the BDI item assessing weight loss for participants reporting that they had purposefully tried to lose weight, which was assessed with an additional study-specific item, resulting in a maximum score of either 60 or 63, with higher scores indicating greater depressive symptomatology. Those with a score of 10 or higher were categorized as having depressive symptoms.
Public distress and self-esteem
The Impact of Weight on Quality of Life-Lite (IWQOL-Lite) is a 31-item questionnaire measuring the impact of weight on quality of life(25). Participants rate items according to the extent to which they perceive their weight adversely affects various activities, cognitions, and feelings, using a five-point scale, ranging from "never true" to "always true." This analysis utilized two of the five summary scores: public distress and self-esteem. Scores were transformed to range from 0 to 100. A higher score implies better functioning.
Analysis
Potential selection bias was examined by comparing select pre-surgical characteristics of LABS-2 participants in the analysis sample (n=310) to those excluded (n=2148) using Pearson’s chi-square tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. The Wilcoxon matched pairs signed-rank test was used to test whether there was a significant change in PA parameters. McNemar’s test was used to test for an association between PA categories and time point. Logistic regression analysis was used to determine pre-operative predictors of a decrease in steps of at least 5% from pre- to post-operative, as well as accumulating at least 10,000 steps/day and, in a separate analysis, at least 150 high-cadence minutes/week post-operative, with site and surgeon treated as random effects. Generalized Estimating Equations (GEE) were used to determine pre-operative predictors of steps, active minutes, and high-cadence minutes post-operative with site and surgeon treated as a cluster. GEE were also used to determine factors associated with change in continuous measures of PA. In addition to examining pre-operative factors, changes in measures from pre-operative to post-operative (i.e., number of comorbidities, SF-36 scores, BDI score, IWQOL-lite public distress and self-esteem scores, and percentage weight lost) and 1 year post-operative measures were considered. In all multivariable analyses, BMI, age, sex, and surgical procedure (RYGB, LAGB, other) were included as independent variables. The following variables were retained in final models if they had a significant (p<.05) independent relationship with the outcome: height, race, ethnicity, education, employment status, household income, marital status, smoking status, comorbidity index/individual comorbidities, walking aid use, SF-36 scores, IWQOL-lite public distress and self-esteem scores, depressive symptoms, weight loss strategies related to PA used in the 6 months prior to surgery, and pre-operative steps, active minutes, and high-cadence minutes.
RESULTS
Participants
The only significant differences in pre-surgical characteristics between the 310 LABS-2 participants included in this analysis and the 2148 not included (Table 1) are that those with SAM data at both time points were better educated (44.6% vs. 35.2% had a college degree; p<.01), more likely to be employed full time (75.4% vs. 67.7%; p<.01), and less likely to have walking limitations (e.g. 12.2% vs. 17.0% used a walking aid; p=.04). In addition, more participants in the current analysis underwent either a Biliopancreatic Diversion with Duodenal Switch, Sleeve Gastrectomy, or Banded Gastric Bypass (9.4% vs. 3.8%; p<.01).
Table 1.
Pre-operative characteristics of LABS-2 participants included and excluded from analysis. N (%) unless otherwise noted.
| Included (n=310) | Excluded (n=2148) | p-value | |
|---|---|---|---|
| Male | 69 (22.3) | 458 (21.3) | 0.71 |
| Age, range | 19–76 | 18–78 | 0.75 |
| median (Q1, Q3) | 46 (37, 55) | 46 (37, 54) | |
| Race (missing=13) | 0.53 | ||
| White | 267 (86.7) | 1833 (85.8) | |
| Black | 33 (10.7) | 221 (10.3) | |
| Other | 8 (2.6) | 83 (3.9) | |
| Hispanic ethnicity (missing=1) | 22 (7.1) | 98 (4.6) | 0.053 |
| Education (missing=192) | <0.01 | ||
| High school or less | 51 (16.8) | 470 (23.9) | |
| Some college | 117 (38.6) | 803 (40.9) | |
| College degree | 135 (44.6) | 690 (35.2) | |
| Employed full-time for pay (missing=189) | 230 (75.4) | 1330 (67.7) | 0.01 |
| Household income (missing=252) | 0.16 | ||
| less than $25,000 | 41 (13.7) | 361 (18.9) | |
| $25,000–$49,000 | 77 (25.7) | 492 (25.8) | |
| $50,000–$74,999 | 75 (25.0) | 448 (23.5) | |
| $75,000–$99,999 | 48 (16.0) | 305 (16.0) | |
| $100,000 or more | 59 (19.7) | 300 (15.7) | |
| Married/living as married (missing=192) | 197 (65.2) | 1249 (63.6) | 0.58 |
| Smoking (missing=4) | 0.20 | ||
| Never | 181 (58.6) | 1191 (55.5) | |
| Former | 98 (31.7) | 286 (13.3) | |
| Current/recent | 30 (9.7) | 668 (31.1) | |
| Body mass index (kg/m2), range | 34.3–76.0 | 33.0–94.3 | 0.62 |
| median (Q1, Q3) | 45.4 (41.7, 51.2) | 45.9 (41.8, 51.5) | |
| Number of comorbidities (missing=1) | 0.34 | ||
| 0 | 47 (15.2) | 315 (14.7) | |
| 1 | 84 (27.1) | 547 (25.5) | |
| 2 | 92 (29.7) | 577 (26.9) | |
| 3 | 57 (18.4) | 414 (19.3) | |
| 4+ | 30 (9.7) | 294 (13.7) | |
| Pain score * (missing=195), range | 18.0–60.4 | 18.0–60.4 | 0.31 |
| median (Q1, Q3) | 39.6 (35.4, 48.5) | 39.6 (31.5, 48.5) | |
| Physical function score * (missing=194), range | 13.1–56.8 | 13.1–56.8 | 0.10 |
| median (Q1, Q3) | 37.1 (28.4, 45.8) | 39.3 (30.6, 45.8) | |
| Walking aid use (missing=219) | 37 (12.2) | 329 (17.0) | 0.04 |
| Assistance to walk 200 feet (missing=1) | <0.01 | ||
| Can walk 200 feet unassisted | 302 (97.4) | 1975 (92.0) | |
| Can walk 200 feet assisted | 8 (2.6) | 140 (6.5) | |
| Cannot walk 200 feet | 0 (0.0) | 32 (1.5) | |
| Surgical Procedure | <0.01 | ||
| Roux-en-Y Gastric Bypass | 214 (69.0) | 1524 (70.9) | |
| Roux-en-Y Gastric Bypass | 67 (21.6) | 543 (25.3) | |
| Other** | 29 (9.4) | 81 (3.8) |
Medical Outcomes Study 36-item Short-Form Health Survey score
Other procedures include Biliopancreatic Diversion with Duodenal Switch, Sleeve Gastrectomy, and Banded Gastric Bypass.
K=kilogram; m=meter;
Change in physical activity
As a group, participants were significantly more active 1 year post-operative than pre-operative (table 2). However, there was a wide variation in change in all three objectively-determined PA parameters (figure 1). For example, while the median change in steps/day was 1457 more steps/day, the change ranged from 7648 fewer steps/day to 17,205 more steps/day. Sixty percent of participants had at least one bout of high-cadence minutes lasting at least 10 minutes at post-operative vs. 39% at pre-operative (p<.0001). Still, at post-operative participants only achieved a median of 23 high-cadence minutes/week in bouts of at least 10 minutes; only 11% accumulated at least 150 high-cadence minutes/week in bouts.
Table 2.
Pre-op, post-op and pre- to post-op change in objectively measured physical activity (n=310). Median (quartiles) and ranges are presented.
| Pre-op | 1 year post-op | Pre- to post-op change* |
|
|---|---|---|---|
| Steps/day | 7563 (5570, 9575) 1552 ~ 21349 | 8788 (6655, 11149) 1502 ~ 24121 | 1457 (−276, 2822) −7648 ~ 17205 |
| Active min/day | 309 (245, 380) 74 ~ 559 | 340 (276, 413) 88 ~ 679 | 31 (−27, 81) −222 ~ 330 |
| High cadence min/week | 72 (34, 130) 0 ~ 816 | 112 (50, 185) 0 ~ 977 | 23 (−8, 72) −343 ~ 932 |
| High cadence min in bouts of at least 10 min/week | 0 (0, 26) 0 ~ 450 | 23 (0, 76) 0 ~ 680 | 1 (0, 50) −294 ~ 680 |
Calculated as post-op value minus pre-op value.
Wilcoxon matched pairs signed-rank test: p < 0.0001 for all comparisons
Figure 1.
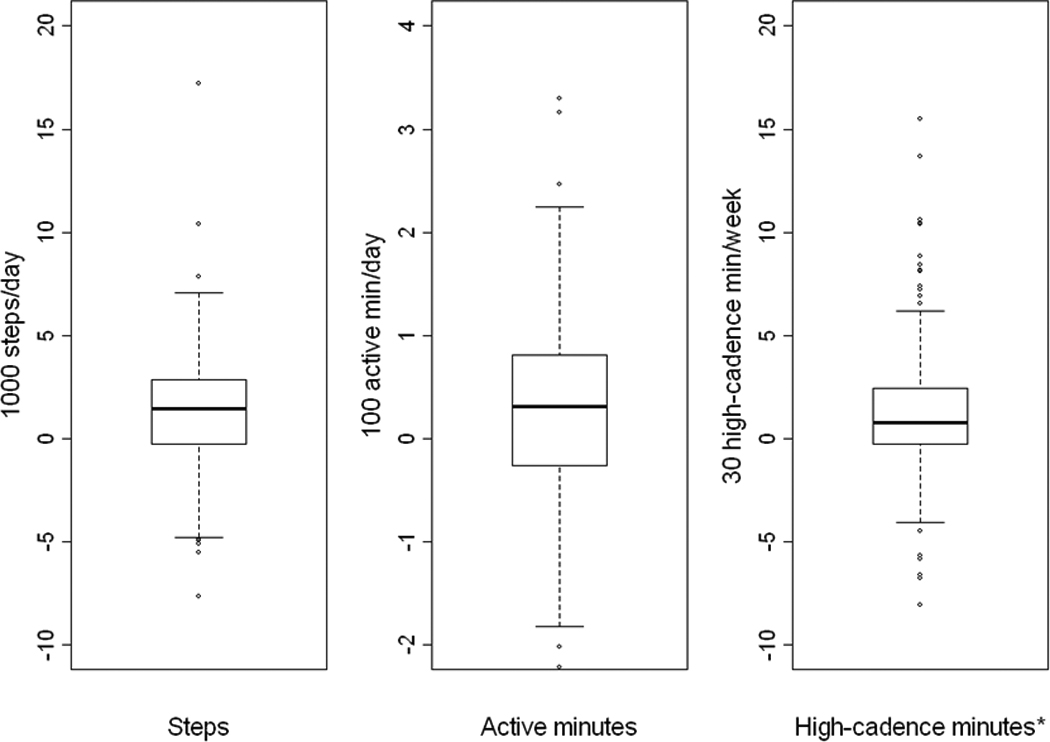
Change in objective-measured physical activity parameters from pre-op to 1 year post-op (n=310).
Footnote: *Two outliers (−343 and 932 high-cadence min/week) do not appear in the plot.
Whether “active” was defined by at least 10000 steps/day or by at least 150 high-cadence minutes/week (accumulated individually), significantly more participants changed from inactive to active than from active to inactive pre- to post-operative (p<.0001; figure 2). Similarly, significantly more participants changed from self-reporting fewer than 150 minutes/week of exercise to at least 150 minutes/week of exercise than vice versa pre- to post-operative (table 3a). More participants also reported increasing their exercise level, using home exercise equipment and participating in group exercise classes as weight loss strategies post-operative, than pre-operative, while there was no significant change in the percentage of participants who saw a personal trainer or exercise specialist, and significantly fewer reported recording their exercise daily post-operative (table 3b). Yet depending on the PA parameter, 23.6–29.0% of participants were at least 5% less active at 1 year post-operative than pre-operative. Almost half (45.5%) decreased one or more PA parameters by at least 5%.
Figure 2.
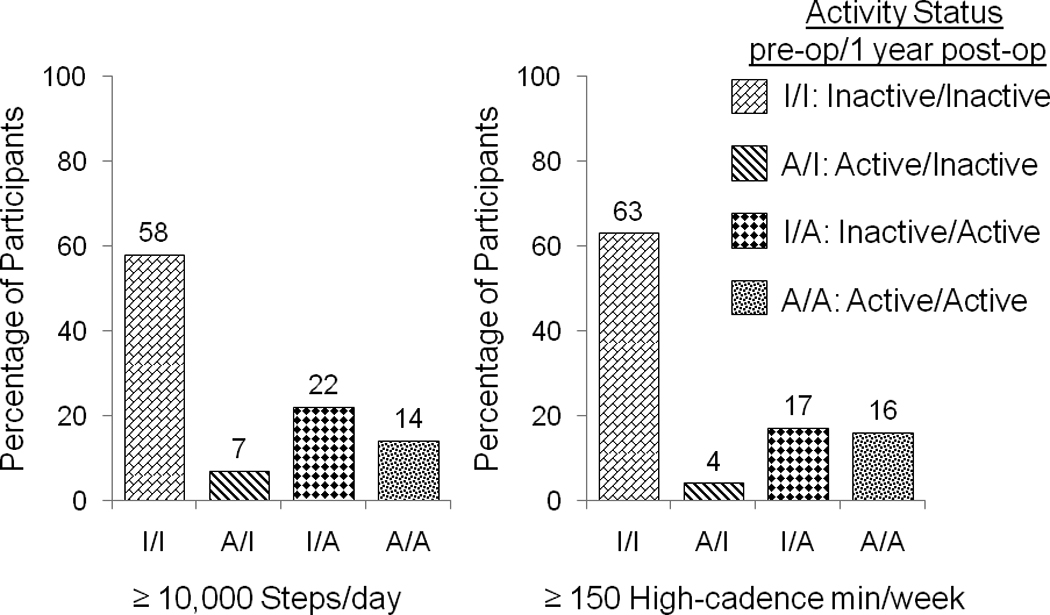
Pre- and 1 year post-operative physical activity using two criterion to define “active:” ≥ 10,000 steps/day and ≥ 150 high-cadence min/week (n=310).
Table 3.
Pre-op, post-op and pre- to post-op change in self-reported exercise
| 3a. N (%) that recorded exercise in physical activity diary (n=276)* | |||
|---|---|---|---|
| Pre-op | 1 year post-op | Pre- to post-op change: no to yes/yes to no |
|
| Some exercise | 172 (62.3%) | 196 (71.0%) | 60 (21.7%) / 36 (13.0%)‡ |
| At least 150 min/week | 82 (29.7%) | 127 (46.0%) | 68 (24.6%) / 23 (8.3%)§ |
| Walking | 127 (46.0%) | 151 (54.7%) | 60 (21.7%) / 36 (13.0%)‡ |
| Playing with kid | 21 (7.6%) | 20 (7.3%) | 11 (4.0%) / 12 (4.4%) |
| Gardening | 13 (4.7%) | 26 (9.4%) | 19 (6.9%) / 6 (2.2%)‡ |
| Weight lifting | 12 (4.4%) | 33 (12.0%) | 27 (9.8%) / 6 (2.2%)§ |
| Bicycling | 11 (4.0%) | 14 (5.1%) | 11 (4.0%) / 8 (2.9%) |
| Dancing | 9 (3.3%) | 14 (5.1%) | 11 (4.0%) / 6 (2.2%) |
| Stretching exercises | 10 (3.6%) | 20 (7.3%) | 15 (5.4%) / 5 (1.8%)† |
| Swimming | 9 (3.3%) | 6 (2.2%) | 5 (1.8%) / 8 (2.9%) |
| Bowling | 9 (3.3%) | 9 (3.3%) | 5 (1.8%) / 5 (1.8%) |
| Jogging | 3 (1.1%) | 18 (6.5%) | 18 (6.5%) / 3 (1.1%)§ |
| Aerobic dancing | 1 (0.4%) | 9 (3.3%) | 9 (3.3%) / 1 (0.4%)‡ |
| 3b. N (%) that reported using weight loss strategies involving physical activity (n=299)* | |||
|---|---|---|---|
| In the past 6 months… | Pre-op | 1 year post-op | Pre- to post-op change: no to yes/ yes to no |
| Increased your exercise level (missing=11) | 171 (59.4%) | 199 (69.1%) | 69 (24.0%) / 41 (14.2%)‡ |
| Used home exercise equipment (missing=10) | 74 (25.6%) | 103 (35.6%) | 62 (21.5%) / 33 (11.4%)‡ |
| Recorded your exercise daily (missing=7) | 55 (18.8%) | 35 (12.0%) | 23 (7.9%) / 43 (14.7%)‡ |
| Participated in group exercise classes (missing=6) | 38 (13.0%) | 61 (20.8%) | 41 (14.0%) / 18 (6.1%)‡ |
| Saw a personal trainer or exercise specialist (missing=8) | 52 (17.9%) | 48 (16.5%) | 34 (11.7%) / 38 (13.1%) |
34/310 participants did not return a physical activity diary at the pre-op and 1 year post-op assessment.
McNemar’s test for agreement:
p < 0.05;
p < 0.01;
p < 0.001
12/310 participants did not complete any of these questions at the pre-op and 1 year post-op assessment.
McNemar’s test for agreement:
p < 0.01
Pre-operative predictors
Many pre-operative factors were related to 1 year PA in unadjusted analyses (data not shown). However, in multivariable analyses once any of the pre-operative PA parameters was entered, most other factors dropped out. In addition to pre-operative PA, which was significantly positively associated with 1 year post-operative PA (table 4), less pain at pre-operative was significantly associated with more steps and high-cadence minutes post-operative. Not having asthma and increasing exercise as a weight loss strategy pre-operative were also significantly associated with more high-cadence minutes post-operative. Adjusting for sex, age, BMI and surgical procedure, the only factor independently associated with achieving at least 10,000 steps/day at 1 year post-operative was pre-operative steps (OR=1.5, 95% CI=(1.3–1.7), per 1000 steps/day). Similarly, the only factor independently associated with achieving at least 150 high-cadence minutes/week at 1 year post-operative was pre-operative high-cadence minutes (OR=1.6, 95% CI=(1.4–1.9), per 30 high-cadence minutes/week). Finally, only pre-operative BMI was significantly independently associated with decreasing steps/day by at least 5% at 1 year post-operative, when adjusting for sex, age, and surgical procedure (OR = 0.62, 95% CI=(0.40–0.96) per 10 kg/m2).
Table 4.
Pre-op predictors of 1 year post-op physical activity.
| Steps/day n=304† |
Active minutes/day n=310† |
High Cadence minutes/week n=294† |
||||
|---|---|---|---|---|---|---|
| Estimates | p-value‡ | Estimates | p-value‡ | Estimates | p-value‡ | |
| Sex (ref. male) | −291.5 | 0.37 | −15.0 | 0.15 | 13. 2 | 0.23 |
| Age, years | 0.6 | 0.96 | −0.5 | 0.26 | 0.5 | 0.39 |
| Body mass index, kg/m2 | −3.0 | 0.90 | −0.6 | 0.41 | −0.1 | 0.95 |
| Surgical procedure (ref. RYGB) | ||||||
| LAGB | −764.9 | 0.13 | −20.1 | 0.18 | 4.0 | 0.78 |
| Other procedures§ | −162.5 | 0.76 | 8.4 | 0.64 | 11.1 | 0.50 |
| Pre-op PA (same measure as outcome) | 0.7 | <0.0001 | 0.6 | <0.0001 | 0.7 | <0.0001 |
| Bodily pain (SF-36 score per 10 points) | 394.2 | 0.03 | 19.1 | 0.01 | ||
| Asthma (ref. no asthma) | −25.2 | 0.03 | ||||
| Increased exercise in past 6 months (ref. didn’t increase exercise) | 25.6 | 0.03 | ||||
Sample included in each analysis is slightly less than the sample of 310 due to missing covariates.
Generalized Estimating Equations.
Other procedures include Biliopancreatic Diversion with Duodenal Switch, Sleeve Gastrectomy, and Banded Gastric Bypass.
Ref.=reference; kg=kilograms; m=meters; RYGB=Roux-en-Y gastric bypass; LAGB=Laparoscopic adjustable gastric band.
Factors associated with change in physical activity
Factors independently associated with change in PA as a continuous variable are shown in table 5. After controlling for sex, age, BMI, and surgical procedure, pre-operative PA was significantly inversely associated with change in the corresponding PA parameter. In addition, recording exercise within the past 6 months at post-operative was significantly positively associated with change in steps and change in active minutes; fewer role limitations due to emotional problems (SF-36 score) at post-operative was significantly positively associated with change in active minutes; and having a LAGB vs. RYGB, greater percentage weight loss from pre- to post-operative, and not smoking at post-operative were significantly positively associated with change in high-cadence minutes.
Table 5.
Factors associated with change* in physical activity from pre-op to 1 year post-op.
| Steps/day n=292† |
Active minutes/day n=297† |
High Cadence minutes/week n=300† |
||||
|---|---|---|---|---|---|---|
| Pre-op factors | Estimates | p-value‡ | Estimates | p-value‡ | Estimates | p-value‡ |
| Sex (ref. male) | −391.8 | 0.23 | −15.6 | 0.13 | 4.9 | 0.64 |
| Age, years | −11.1 | 0.41 | −0.6 | 0.15 | −0.1 | 0.78 |
| Body mass index, kg/m2 | −15.1 | 0.53 | −0.7 | 0.28 | −0.6 | 0.44 |
| Surgical procedure (ref. RYGB) | ||||||
| LAGB | −497.1 | 0.30 | −17.3 | 0.17 | 37.9 | 0.02 |
| Other procedures§ | 24.0 | 0.97 | 21.5 | 0.35 | 10.6 | 0.50 |
| Pre-op PA (same measure as outcome) | −0.3 | <0.0001 | −0.4 | <0.0001 | −0.3 | <0.0001 |
| Change factors | ||||||
| Percentage weight loss (per 10%) | 21.0 | 0.01 | ||||
| 1 yr Post-op factors | ||||||
| Recorded exercise daily in past 6 months (ref. didn’t record exercise) | 743.5 | 0.047 | 29.4 | 0.01 | ||
| Role limit emotional (SF-36 score per 10 points) | 11.5 | 0.01 | ||||
| Smoking status (ref. non-smoker) | −45.4 | 0.003 | ||||
Calculated as post-op value minus pre-op value; positive change indicates an increase in physical activity.
Sample included in each analysis is slightly less than the sample of 310 due to missing covariates.
Generalized Estimating Equations.
Other procedures include Biliopancreatic Diversion with Duodenal Switch, Sleeve Gastrectomy, and Banded Gastric Bypass.
Ref.=reference; kg=kilograms; m=meters; RYGB=Roux-en-Y gastric bypass; LAGB=Laparoscopic adjustable gastric band.
DISCUSSION
This is the first study to examine pre- to post-operative changes in objectively-measured PA in a large cohort (n=310) of adults undergoing bariatric surgery. In addition to examining daily steps, which can be accumulated at low, moderate or vigorous intensity PA, this study examined high-cadence minutes, a proxy measure for ambulatory MVPA, and active minutes, the inverse of sedentary time. This allowed us to better understand how different aspects of PA change pre- to post-operative. Activity diary data was also presented to provide insight into participants’ exercise habits.
Change in physical activity
As a group, participants were more active 1 year post- than pre-operative. While the degree of change was less than what would be expected based on previous studies of self-reported PA,(1) it was consistent with changes in time spent exercising as recorded in participants’ PA diaries. For example, using cut points of at least 10000 steps/day or at least 150 high-cadence minutes/week to define active, approximately 20% of participants were active pre- and 35% were active post-operative. Similarly, the percentage of participants reporting at least 150 minutes of exercise/week increased from 30% to 46% pre- to post-operative.
When PA was dichotomized using steps or high-cadence minutes, only 4–7% of participants were identified as active pre- but inactive post-operative. This is consistent with the study by Bond and colleagues (2009), described earlier(16). However, in the current study, when change in objectively-measured PA was evaluated on a continuous scale, roughly a fourth of participants decreased their PA by at least 5%, indicating a sizeable portion of patients are actually less active 1 year after having surgery. There was a similar pattern of change across all three PA parameters suggesting similar degrees of change in MVPA and time up and about performing activities of daily living.
1 year post-operative physical activity
At both pre- and post-operative more participants were categorized as active when self-reported exercise, rather than objectively determined PA, was utilized. For example 46% of participants reported exercising at least 150 minutes per week, while only 33% accumulated 150 high-cadence minutes/week and only 11% accumulated 150 high-cadence minutes/week in bouts of at least 10 minutes. Given that activity monitors record steps from all ambulatory activities, not just exercise, the direction of the discrepancy might be surprising. However, there are several possible explanations: 1) participants over-reported the duration of exercise, 2) participants reported exercise performed at low-intensity, 3) participants achieved MVPA at a cadence below the threshold of 80 steps/minute or by doing non-ambulatory PA.
Although there was also substantial variation in 1 year PA level, regardless of PA measure, the majority of participants were insufficiently active. While the amount of PA required to maintain weight loss is variable and may largely depend on dietary intake(26), studies of non-surgical weight loss have shown that a substantial increase in PA level is required to maintain weight loss in previously obese adults(27;28). Such studies have led the International Association for the Study of Obesity to recommend 60- to 90-minutes of moderate intensity PA (or lesser amounts of vigorous intensity PA) a day to prevent weight regain(5).
Pre-operative predictors
Many pre-operative factors were related to 1 year PA in unadjusted analyses. However, many were also related to pre-operative PA so they were not independently associated with 1 year PA once pre-operative PA was considered. In addition to pre-operative PA, less pain, not having asthma, and having increased PA as a weight loss strategy prior to surgery were also independently associated with higher PA at 1 year post-operative. Thus, increasing PA level before surgery and addressing bodily pain and asthmatic symptoms before and in the early post-operative period may positively influence post-operative PA. However, an observational study with self-reported PA in 42 LAGB patients found that pre-operative PA was not significantly associated with 2 year post-operative PA, while perceived benefits of exercise and confidence to exercise were (r=.36 and r=.35, respectively; both p<.05)(29). Thus, more work is needed to confirm these relationships.
While we tested for differences in sociodemographics, health behaviors, physical health and mental health indicators between those who did and did not have a decrease in PA, lower BMI was the only factor independently related to a decrease in steps of at least 5%. This finding suggests patients who are less severely obese at time of surgery are not exempt from decreasing their PA following surgery. In addition, this study, along with the study by Bond and colleagues, which found no significant differences in pre-operative demographics, BMI, PA level or health related quality of life between those who increased their PA (inactive to active; n=68) versus those who remained inactive (n=39)(16), suggests it may be very difficult to identify patients in the pre-operative period who are likely to decrease their PA or fail to increase their PA.
Factors associated with change in physical activity
To gain better insight into what might be affecting change in PA from pre- to post-operative we examined whether change in other factors, as well as factors measured at the 1 year assessment were associated with change in PA. Adjusting for pre-operative sex, age, BMI, surgical procedure and PA, fewer role limitations due to emotional problems, greater weight loss post-operative, and not smoking and recording exercise daily post-operative were positively associated with change in at least one PA parameter. While it is possible that increases in PA led to fewer role limitations due to emotional problems and greater weight loss post-operatively, the inverse could also be true. On the other hand, not smoking and recording exercise daily post-operative likely had a positive influence on change in PA level. Thus, in addition to encouraging patients to be more physically active, medical professions should consider encouraging smoking cessation and PA self-monitoring behaviors, such as keeping an exercise diary, especially given that this weight loss strategy was actually less common post- than pre-operative.
Strengths and limitations
A major strength of this study is the sample size (n=310). While participants were only a subsample of the LABS-2 cohort, for the most part they were representative, reflecting the fact that over 80% of exclusions were due to factors unrelated to participant characteristics (e.g., lack of activity monitors), whereas the higher prevalence of walking limitations among those excluded from the current analysis reflects the physical activity assessment exclusion criteria (i.e. wheel chair use, walking limitation not directly related to obesity such as sprained ankle or multiple sclerosis). Another strength of this study is that PA was measured with a high-quality activity monitor that is reliable and valid in obese populations. In addition, both the monitor wear protocol and data processing protocol followed best practices(30), and analysis utilized three PA parameters that measured different aspects of ambulatory PA. While the accuracy of the PA monitor to measure non-ambulatory activities (e.g., swimming, water jogging, weight lifting, bicycling) is unknown, the PA diary data indicated these activities were uncommon and were done with similar frequencies pre- and post-operatively.
Conclusion
This study adds to the literature by highlighting the tremendous variability in change in PA level from pre- to 1 year post-operative, which should raise awareness that many adults who undergo bariatric surgery do not increase their PA level and in fact some decrease their PA level. In addition, this study reveals that the majority of adults who undergo bariatric surgery are insufficiently active 1 year post-operative to reap the many benefits of regular PA, and in particular to help prevent weight regain. Although gains in PA may be smaller among patients with higher pre-operative PA, pre-operative PA had the strongest positive association with post-operative PA. In addition, reporting an increase in PA in the 6 months prior to surgery had an independent positive association with post-operative PA. Thus, future work should examine whether increasing pre-operative PA among insufficiently active bariatric surgery candidates increases their post-operative PA level.
Acknowledgements
LABS personnel contributing to the study include:
Columbia University Medical Center, New York, NY: Paul D. Berk, MD, Marc Bessler, MD, Amna Daud, Harrison Lobdell IV, Jemela Mwelu, Beth Schrope, MD, PhD, Akuezunkpa Ude, MD Cornell University Medical Center, New York, NY: Michelle Capasso, BA, Ricardo Costa, BS, Greg Dakin, MD, Faith Ebel RD, MPH, Michel Gagner, MD, Jane Hsieh BS, Alfons Pomp, MD, Gladys Strain, PhD Mt. Sinai Medical Center, New York, NY: W. Barry Inabnet, MD East Carolina Medical Center, Greenville, NC: Rita Bowden, RN, William Chapman, MD, FACS, Lynis Dohm, PhD, John Pender MD, Walter Pories, MD, FACS Neuropsychiatric Research Institute, Fargo, ND: Jennifer Barker, MBA, Michael Howell, MD, Luis Garcia, MD, FACS, MBA, Kathy Lancaster, BA, Erika Lovaas, BS, James E. Mitchell, MD, Tim Monson, MD, Oregon Health & Science University: Chelsea Cassady, BS, Clifford Deveney, MD, Katherine Elder, PhD, Andrew Fredette, BA, Stefanie Greene, Jonathan Purnell, MD, Robert O’Rourke, Lynette Rogers, MD, Chad Sorenson, Bruce M. Wolfe, MD, Legacy Good Samaritan Hospital, Portland, OR: Emma Patterson, MD, Mark Smith, MD, William Raum, MD, Lisa VanDerWerff, PAC, Jason Kwiatkowski, PAC, Jamie Laut Med Sacramento Bariatric Medical Associates, Sacramento, CA: Iselin Austrheim-Smith, CCRP, Laura Machado, MD University of Pittsburgh Medical Center, Pittsburgh, PA: Chris Costa, BA Anita P. Courcoulas, MD, MPH, FACS, Jessie Eagleton, BS, George Eid, MD, William Gourash, MSN, CRNP, Lewis H. Kuller, MD, DrPH, Carol A. McCloskey, MD, Ramesh Ramanathan, MD, Rebecca Search, MPH, Eleanor Shirley, MA University of Washington, Seattle, WA: David E. Cummings, MD, E. Patchen Dellinger, MD, Hallie Ericson, BA, David R. Flum, MD, MPH, Katrina Golub, MPH, CCRC, Brant Oelschlager, MD, Skye Steptoe, MS, CCRC, Tomio Tran, Andrew Wright, MD Virginia Mason Medical Center, Seattle, WA: Lily Chang, MD, Stephen Geary, RN, Jeffrey Hunter, MD, Anne MacDougall, BA Ravi Moonka, MD, Olivia A. Seibenick, CCRC, Richard Thirlby, MD Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Abi Adenijii, MS, Steven H. Belle, PhD, MScHyg, Lily (Jia-Yuh) Chen, MS, Michelle Fouse, BS, Jesse Hsu, MS, Wendy C. King, PhD, Kevin Kip, PhD, Kira Leishear, MS, Laurie Iacono, MFA, Debbie Martin, BA, Rocco Mercurio, MBA, Faith Selzer, PhD, Abdus Wahed, PhD National Institute of Diabetes and Digestive and Kidney Diseases: Mary Evans, Ph.D, Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD National Cancer Institute: David E. Kleiner, MD, PhD
Source of support:
This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wendy C King, University of Pittsburgh, Graduate School of Public Health, Pittsburgh.
Jesse Y Hsu, University of Pittsburgh, Graduate School of Public Health, Pittsburgh.
Steven H Belle, University of Pittsburgh, Graduate School of Public Health, Pittsburgh.
Anita P Courcoulas, University of Pittsburgh Medical Center, Pittsburgh.
George M Eid, University of Pittsburgh Medical Center, Pittsburgh.
David R Flum, University of Washington, Seattle.
James E Mitchell, Neuropsychiatric Institute, Fargo.
John R Pender, East Carolina Medical Center, Greenville.
Mark D Smith, Legacy Good Samaritan Hospital, Portland.
Kristine J Steffen, Neuropsychiatric Institute, Fargo.
Bruce M Wolfe, Oregon Health and Science University, Portland.
References
- 1.Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev. 2010;12:366–377. doi: 10.1111/j.1467-789X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 2.Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obes Surg. 2010;20:657–665. doi: 10.1007/s11695-010-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Heart Lung and Blood Institute TG. Bethesda, MD: National Heart Lung and Blood Institute; 1998. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: The Evidence Report; pp. 56–94. [Google Scholar]
- 4.Rippe JM, Hess S. The role of physical activity in the prevention and management of obesity. J Am Diet Assoc. 1998;98(10 Suppl 2):S31–S38. doi: 10.1016/s0002-8223(98)00708-1. [DOI] [PubMed] [Google Scholar]
- 5.Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–114. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.U.S.Department of Health and Human Services. Washington, DC: U.S. Department of Health and Human Services; 2008. Physical Activity Has Many Health Benefits. 2008 Physical Activity Guidelines for Americans; pp. 7–14. [Google Scholar]
- 7.King WC, Belle SH, Eid GM, et al. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2008;4:721–728. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond DS, Jakicic JM, Vithiananthan S, et al. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surg Obes Relat Dis. 2010;6:72–78. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond DS, Jakicic JM, Unick JL, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity (Silver Spring) 2010;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colles SL, Dixon JB, O'Brien PE. Hunger control and regular physical activity facilitate weight loss after laparoscopic adjustable gastric banding. Obes Surg. 2008;18:833–840. doi: 10.1007/s11695-007-9409-3. [DOI] [PubMed] [Google Scholar]
- 11.Josbeno DA, Jakicic JM, Hergenroeder A, Eid GM. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surg Obes Relat Dis. 2008;6:361–366. doi: 10.1016/j.soard.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Melanson EL, Knoll JR, Bell ML, et al. Commercially available pedometers: considerations for accurate step counting. Prev Med. 2004;39:361–368. doi: 10.1016/j.ypmed.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Schneider PL, Crouter SE, Lukajic O, Bassett DR., Jr Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc. 2003;35:1779–1784. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 14.Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994;26:1446–1452. [PubMed] [Google Scholar]
- 15.Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord. 1997;21:380–386. doi: 10.1038/sj.ijo.0800417. [DOI] [PubMed] [Google Scholar]
- 16.Bond DS, Phelan S, Wolfe LG, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity (Silver Spring) 2009;17:78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- 17.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boone DA, Coleman KL. Use of a step activity monitor in determining outcomes. J Prosthet Orthot. 2006;18(1S):86–92. [Google Scholar]
- 19.King WC, Leishear K, Mitchell JE, Belle SH. Determining activity monitor wear time: an influential decision rule. J Phys Act Health. 2011;8:566–580. doi: 10.1123/jpah.8.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 21.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Raynor HA, Jeffery RW, Ruggiero AM, Clark JM, Delahanty LM. Weight loss strategies associated with BMI in overweight adults with type 2 diabetes at entry into the Look AHEAD (Action for Health in Diabetes) trial. Diabetes Care. 2008;31:1299–1304. doi: 10.2337/dc07-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 24.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 25.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–171. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 26.Cox TL, Malpede CZ, Desmond RA, et al. Physical activity patterns during weight maintenance following a low-energy density dietary intervention. Obesity (Silver Spring) 2007;15:1226–1232. doi: 10.1038/oby.2007.144. [DOI] [PubMed] [Google Scholar]
- 27.Ewbank PP, Darga LL, Lucas CP. Physical activity as a predictor of weight maintenance in previously obese subjects. Obes Res. 1995;3:257–263. doi: 10.1002/j.1550-8528.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 28.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wouters EJ, Larsen JK, Zijlstra H, van Ramshorst B, Geenen R. Physical activity after surgery for severe obesity: the role of exercise cognitions. Obes Surg. 2010 doi: 10.1007/s11695-010-0276-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37(11 Suppl):S582–S588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]


