Abstract
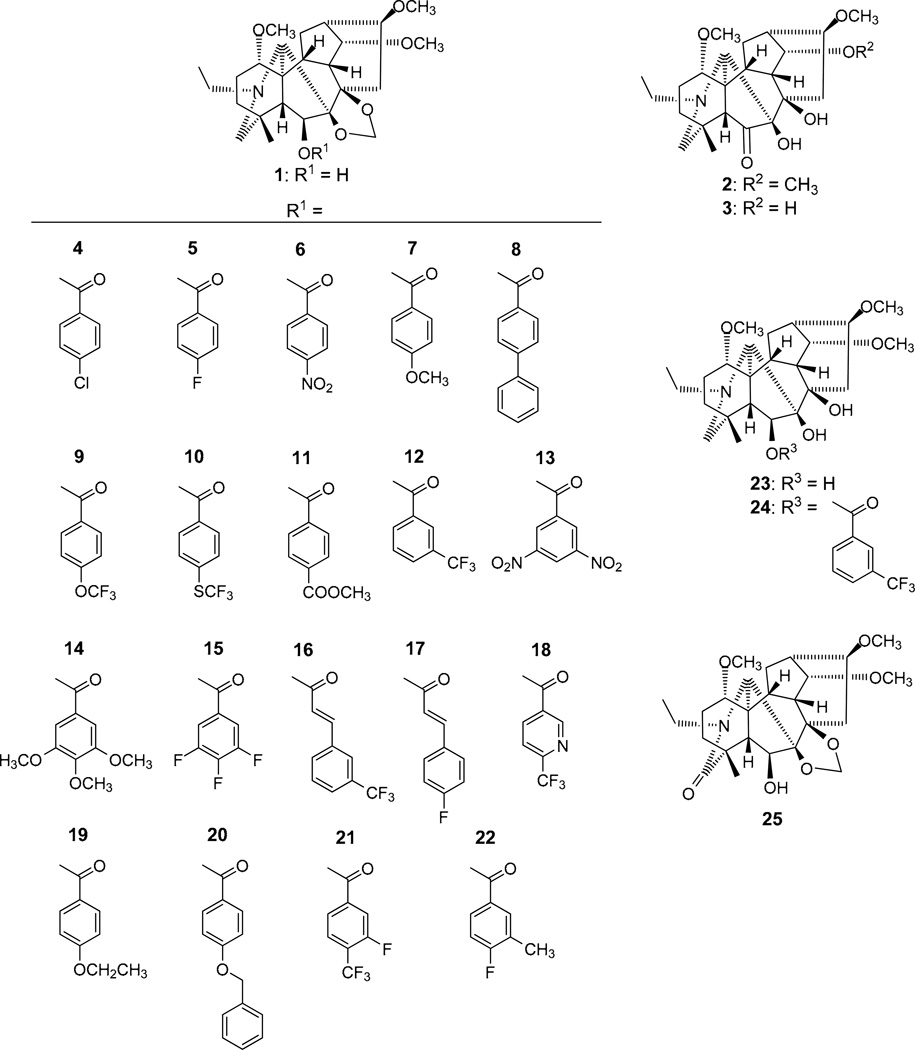
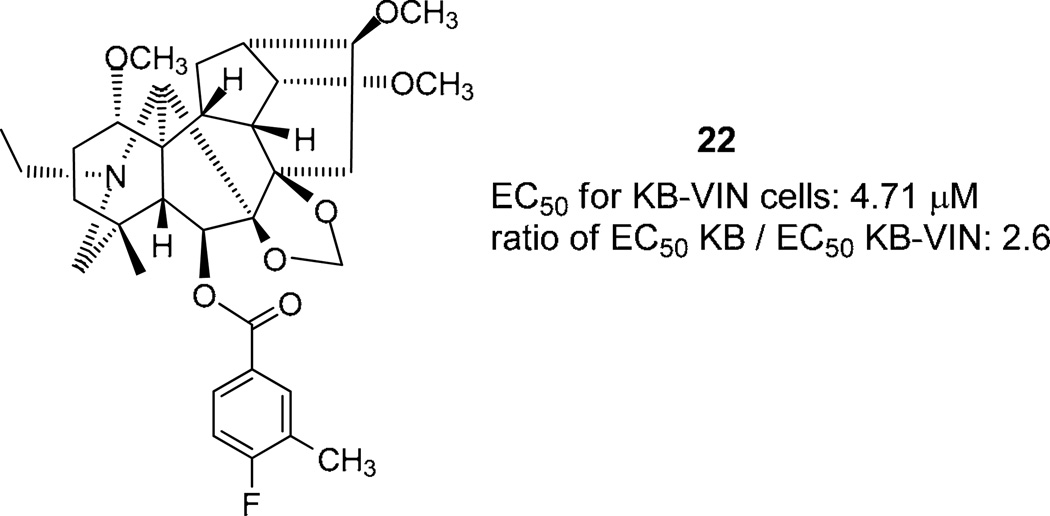
C-6 Esterifications of delpheline (1) were carried out to provide 20 new diterpenoid alkaloid derivatives (4–22, 24). Three natural alkaloids (1–3) and all synthesized compounds (4–25) were evaluated for cytotoxic activity against lung (A549), prostate (DU145), nasopharyngeal (KB), and vincristine-resistant nasopharyngeal (KB-VIN) cancer cell lines and interestingly, showed an improved drug resistance profile compared to paclitaxel. Particularly, 6-(4-fluoro-3-methylbenzoyl)delpheline (22) displayed 2.6-fold greater potency against KB-VIN cells compared with the parental non-drug resistant KB cells. 6-Acylation of 1 appears to be critical for producing cytotoxic activity in this alkaloid class and a means to provide promising new leads for further development into antitumor agents.
Keywords: Delpheline, C19-diterpenoid alkaloid, Cytotoxicity
Natural products have been the major sources of currently available anticancer drugs. According to a review of New Chemical Entities (NCE) from 1981 to 2006, approximately 73% of anticancer drugs were not purely synthetic compounds, with 47% being natural products, directly derived from natural products, or mimicking natural products in one form or another.1 Diterpenoid alkaloids have been isolated from the genera Aconitum, Consolida and Delphinium of the Ranunculaceae family, and the genus Spiraea of the Rosaceae family. Diterpenoid alkaloids are classified according to their chemical structure as C18-diterpenoid alkaloids, which have either a lappaconitine or a ranaconitine skeleton, C19-diterpenoid alkaloids, which have either an aconitine, lycoctonine, lactone-type, pyro-type, rearranged-type, or 7,17-seco-type skeleton, and C20-diterpenoid alkaloids, which have either an arcutine, atisine, denudatine, hetidine, hetisine, kusnezoline, napelline, racemulosine, tricalysiamide, or vakognavine skeleton.2–4 The pharmacological properties of aconitine-type C19-diterpenoid alkaloids, including aconitine, hypaconitine, jesaconitine, and mesaconitine, have been studied extensively and reviewed.5,6 Two reports on the effects of C19-diterpenoid alkaloids on cancer cells have appeared in recent years. 8-O-Azeloyl-14-benzoylaconine, an aconitine-type C19-diterpenoid alkaloid, exhibited antiproliferative activity,7 and the cytotoxic effects of various C19-diterpenoid alkaloids against tumor cell lines were reported.8 Our previous study demonstrated the effects of various naturally occurring and semi-synthetic C19- and C20-diterpenoid alkaloids on growth of the A172 human malignant glioma cell line.9 Antitumor properties and radiation-sensitizing effects of various types of novel derivatives prepared from C19- and C20-diterpenoid alkaloids were also investigated.10 Two novel hetisine-type C20-diterpenoid derivatives showed significant suppressive effects against the Raji non-Hodgkin’s lymphoma cell line.11 In addition, the effects of various semi-synthetic novel hetisine-type C20-diterpenoid alkaloids on the growth of the A549 human lung cancer cell line were examined and subsequent structure-activity relationships for the anti-proliferative effects against A549 cells were considered.12 However, little information is available regarding the cytotoxic properties of lycoctonine-type C19-diterpenoid alkaloids and their chemically transformed products. Herein, we report our study results on the unique cytotoxic activities of delpheline (1), a lycoctonine-type C19-diterpenoid alkaloid, and its derivatives.
Delpheline (1), pacinine (2) and yunnadelphinine (3) were purified from the seeds of Delphinium elatum cv. Pacific Giant by a previously described procedure.13,14 Delpheline (1) was reacted with various acyl chlorides at 80°C in pyridine to give C-6 substituted benzoyl and cinnamoyl derivatives (4–22). Delpheline (1) and 6-(3-trifluoromethylbenzoyl)delpheline (12) were hydrolyzed under acidic conditions to provide the 7,8-demethylene derivatives, 7,8-demethylenedelpheline (23)15 and 7,8-demethylene-6-(3-trifluoromethylbenzoyl)delpheline (24), respectively. Oxidation of delpheline (1) with KMnO4–acetone gave 19-oxodelpheline (25).
The three natural alkaloids (1–3) and all synthesized compounds (4–25) were evaluated for cytotoxic activity against four human tumor cell lines [lung carcinoma (A549), prostate carcinoma (DU145), nasopharyngeal (KB) and multi-drug resistant variant expressing P-glycoprotein (KB-VIN)]. Paclitaxel was used as a positive control (data shown in Table I).
Table 1.
| A549 | DU145 | KB | KB-VIN | |
|---|---|---|---|---|
| 1 | >20 | >20 | >20 | >20 |
| 2 | >20 | >20 | >20 | >20 |
| 3 | >20 | >20 | >20 | >20 |
| 4 | 14.8 ± 3.76 | 7.40 ± 1.16 | 8.85 ± 2.02 | 8.27 ± 1.62 |
| 5 | >20 | 15.6 ± 5.39 | >20 | 15.0 ± 6.51 |
| 6 | >20 | 17.2 ± 3.26 | >20 | 17.7 ± 3.51 |
| 7 | >20 | >20 | >20 | >20 |
| 8 | >20 | 17.1 ± 11.4 | >20 | 17.4 ± 7.42 |
| 9 | 18.7 ± 6.57 | 20.3 ± 7.06 | 20.1 ± 7.63 | 18.9 ± 4.95 |
| 10 | >20 | 16.6 ± 12.7 | >20 | 17.9 ± 4.16 |
| 11 | >20 | >20 | >20 | >20 |
| 12 | >20 | 12.6 ± 2.96 | 14.9 ± 4.93 | 11.9 ± 3.26 |
| 13 | >20 | >20 | 19.1 ± 4.78 | 20.3 ± 2.66 |
| 14 | >20 | >20 | >20 | >20 |
| 15 | 19.9 ± 10.1 | 16.9 ± 6.74 | 14.6 ± 7.05 | 6.80 ± 4.99 |
| 16 | 10.2 ± 2.62 | 15.1 ± 5.97 | >20 | 9.10 ± 1.51 |
| 17 | >20 | >20 | >20 | >20 |
| 18 | >20 | >20 | >20 | 18.7 ± 5.17 |
| 19 | 20.0 ± 0.89 | 15.6 ± 2.56 | 14.8 ± 3.27 | 6.50 ± 2.16 |
| 20 | 14.1 ± 2.93 | 13.2 ± 5.68 | 6.76 ± 1.74 | 4.22 ± 1.09 |
| 21 | 16.5 ± 2.24 | 11.3 ± 7.9 | 5.44 ± 1.75 | 4.40 ± 0.83 |
| 22 | >20 | 19.8 ± 4.58 | 12.1 ± 7.83 | 4.71 ± 1.44 |
| 23 | >20 | >20 | >20 | >20 |
| 24 | >20 | >20 | >20 | >20 |
| 25 | >20 | >20 | >20 | >20 |
| Paclitaxel | 0.0064 ± 0.0013 | 0.0059 ± 0.0019 | 0.0060 ± 0.0008 | 0.76 ± 0.22 |
Cytotoxicity as EC50 values for each cell line, the concentration of compound that caused 50% reducation in absorbance at 515 nm relative to untreated cells using sulforhodamine B assay.
Lung carcinoma (A549), prostate carcinoma (DU145), nasopharyngeal (KB) and multi-drug resistant variant expressing P-glycoprotein (KB-VIN).
Interestingly, the natural alkaloids (1–3) and synthetic analogs that did not contain a C-6 ester group (23, 25) were inactive (EC50 > 20 µM). Thus, acylation of the C-6 hydroxy group of 1 was necessary for cytotoxic activity. The methylenedioxy group of a lycoctonine-type C19-diterpenoid alkaloid might also be necessary for an inhibitory effect against A549, DU145, KB and KB-VIN cell lines, as compound 24 lost activity against all cell lines compared with the related compound 12.
Among the C-6 esterified compounds, 4 [6-(4-chlorobenzoyl)delpheline], 20 [6-(4-benzyloxybenzoyl)delpheline], and 21 [6-(3-fluoro-4-trifluoromethylbenzoyl)delpheline] exhibited the highest average potency over all cell lines (average EC50 9.83, 9.57, and 9.41 µM, respectively). However, compound 16 [6-(3-trifluoromethylcinnamoyl)delpheline] showed significantly increased cytotoxic activity (EC50 10.2 µM) against A549 cells compared with 4, 20, and 21, but was generally less potent against DU145, KB, and KB-VIN cells. Compounds 7, 11, 14, and 17 were inactive against all four cancer cell lines, while 5, 6, 8–10, 13, and 18 showed very limited potency.
The most striking observations from the data in Table 1 were the degree and relative ratio of KB/KB-VIN potency. Among the four cancer cell lines tested, the highest potency was found against the KB-VIN cell line by compounds 20–22 (EC50 4.22, 4.40, and 4.71 µM, respectively), followed by compounds 19, 15, 4, 16, and 12 (EC50 6.50, 6.80, 8.27, 9.10, and 11.9 µM, respectively). Generally, all of the active compounds showed the highest potency against the KB-VIN cell line compared with the other three cancer cell lines tested. Moreover, compounds 4, 12, 20, and 21 displayed similar potency against the KB and KB-VIN cell lines (ratio of EC50 KB / EC50 KB-VIN: 1.07, 1.25, 1.60, and 1.24, respectively). Even more notably, compounds 15, 19, 16, and 22 showed over twofold selectivity between the two cell lines, with highest cytotoxic activity against the KB-VIN cell line (ratio of EC50 KB / EC50 KB-VIN: 2.15, 2.28, 2.31, and 2.57, respectively).
The identity of the substituent on the C-6 acyl group affected the cytotoxic potency. For instance, the compounds with the highest potency against the KB-VIN cell line contained chloro (4), fluoro (15, 21, 22), trifluoromethyl (12, 16, 21), ethoxy (19), or benzyloxy (20) substituents on the acyl group. Against the KB-VIN cell line, compounds 21 and 22 with both fluoro and trifluoromethyl/methyl groups were more potent than 12 with only a single trifluoromethyl group and even more potent than 5 with a single fluoro group. Similarly, compound 16 [6-(3-trifluoromethylcinnamoyl)delpheline] showed increased cytotoxic activity against most cell lines compared with the related fluorinated compounds 17 [6-(4-fluorocinnamoyl)delpheline] and 18 [6-(6-trifluoromethylnicotinoyl)delpheline]. Moreover, compounds with nitro, methoxy, phenyl, trifluoromethoxy, trifluoromethythio, and methyl carboxylate groups on a C-6 benzoate ester were generally less potent.
In conclusion, we have synthesized various C-6 acylated derivatives of 1, a lycoctonine-type C19-diterpenoid alkaloid. All derivatives were screened against four tumor cell lines, and the C-6 acylated was found to be critical for cytotoxic activity. Compounds 4, 12, 20, and 21 displayed the greatest potency over all four tested cell lines. While these four compounds showed comparable potency against both KB and KB-VIN cancer cell lines, some compounds showed tumor-selective activity. Compounds 15, 16, 19, and 22 exhibited greater inhibitory activity against drug-resistant KB-VIN cells (2.15–2.57 fold) than the parental KB cells. Continued studies are merited to further develop these promising new leads as antitumor agents, particularly with enhanced tumor selectivity.
Figure 1.
Figure 2.
Acknowledgments
This study was supported by grant CA17625-32 from the National Cancer Institute awarded to K. H. Lee. This study was also supported in part by the Department of Health Cancer Research Center of Excellence (DOH-100-TD-C-111-05).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Newman DJ, Cragg GM. J. Nat. Prod. 2007;70:461. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 2.Wang FP, Chen QH, Liu XY. Nat. Prod. Rep. 2010;27:570. doi: 10.1039/b916679c. [DOI] [PubMed] [Google Scholar]
- 3.Wang FP, Chen QH. In: The Alkaloids. Cordell GA, editor. Vol. 69. New York: Elsevier Science; 2010. pp. 1–623. [Google Scholar]
- 4.Wang FP, Chen QH, Liang XT. In: The Alkaloids. Cordell GA, editor. Vol. 67. New York: Elsevier Science; 2007. pp. 1–78. [Google Scholar]
- 5.Amiya T, Bando H. In: The Alkaloids. Brossi A, editor. Vol 34. San Diego: Academic Press; 1988. pp. 95–179. [Google Scholar]
- 6.Joshi BS, Pelletier SW. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol 13. Amesterdam: Pergamon; 1999. pp. 289–370. [Google Scholar]
- 7.Chdoeva A, Bosc J-J, Guillon J, Decendit A, Petraud M, Absalon C, Vitry C, Jarry C, Robert J. Bioorg. Med. Chem. 2005;13:6493. doi: 10.1016/j.bmc.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 8.de Ines C, Reina M, Gavin JA, Gonzalez-Coloma A. Z. Naturforsch. C. 2006;61:11. doi: 10.1515/znc-2006-1-203. [DOI] [PubMed] [Google Scholar]
- 9.Wada K, Hazawa M, Takahashi K, Mori T, Kawahara N, Kashiwakura I. J. Nat. Prod. 2007;70:1854. doi: 10.1021/np070270w. [DOI] [PubMed] [Google Scholar]
- 10.Hazawa M, Wada K, Takahashi K, Mori T, Kawahara N, Kashiwakura I. Invest. New Drugs. 2009;27:111. doi: 10.1007/s10637-008-9141-4. [DOI] [PubMed] [Google Scholar]
- 11.Hazawa M, Wada K, Takahashi K, Mori T, Kawahara N, Kashiwakura I. Invest. New Drugs. 2011;29:1. doi: 10.1007/s10637-009-9327-4. [DOI] [PubMed] [Google Scholar]
- 12.Wada K, Hazawa M, Takahashi K, Mori T, Kawahara N, Kashiwakura I. J. Nat. Med. 2011;65:43. doi: 10.1007/s11418-010-0452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bando H, Wada K, Tanaka J, Kimura S, Hasegawa E, Amiya T. Heterocycles. 1989;29:1293. [Google Scholar]
- 14.Wada K, Yamamoto T, Bando H, Kawahara N. Phytochemistry. 1992;31:2135. [Google Scholar]
- 15.Narzullaev AS, Yunusov MS, Sabirov SS. Chemistry of Natural Compounds. 1989;25:43. [Google Scholar]