Abstract
Patterns of atrophy in frontotemporal dementia (FTD) correlate with the clinical subtypes of behavioral variant FTD (bvFTD), semantic dementia, progressive non-fluent aphasia (PNFA) and FTD with motor neuron disease (FTD-MND). Right temporal variant FTD is associated with behavioral dyscontrol and semantic impairment, with tau abnormalities more common in right temporal bvFTD and TDP-43 accumulation in right temporal semantic dementia. However, no clinical and anatomical correlation has been described for patients with predominant right temporal atrophy and FTD-MND. Therefore, we performed a database screen for all patients diagnosed with FTD-MND at Mayo Clinic and reviewed their MRI scans to identify those with striking, dominant, right temporal lobe atrophy. For cases with volumetric MRI we performed voxel based morphometry and for those with brain tissue we performed pathological examination. Of three such patients identified, each patient had different presenting behavioral and/or aphasic characteristics. MRI, including DTI sequence in one patient, and FDG PET scan, revealed striking and dominant right temporal lobe atrophy, right corticospinal tract degeneration, and right temporal hypometabolism. Archived brain tissue was available in 2 patients; both demonstrating TDP-43 type 3 pathology (Mackenzie scheme) with predominant neuronal cytoplasmic inclusions. In one case, neurofibrillary tangles (Braak V) and neuritic plaques were also present in keeping with a diagnosis of Alzheimer's disease. There appears to be an association between FTD-MND and severe right temporal lobe atrophy. Until further characterization of such cases are determined, they may be best classified as right temporal variant FTD-MND.
Keywords: Frontotemporal dementia, Motor neuron disease, TDP-43, Voxel based morphometry (VBM), positron emission tomography (PET
(1) Introduction
Frontotemporal dementia (FTD) is clinically characterized by syndromes of progressive behavioral changes and language deficits[1, 2]. Varying degrees of atrophy can occur in the frontal and/or temporal lobes and has implications on the clinical phenotype of FTD[3]. In particular, left anterior medial and lateral temporal lobe atrophy is typically associated with semantic dementia (SMD)[4], peri-sylvian atrophy with progressive non-fluent aphasia[5], predominant frontal lobe atrophy with behavioral variant FTD (bvFTD)[6], and posterior frontal lobe atrophy with FTD with motor neuron disease (FTD-MND)[7]. In FTD, an anatomic subtype of predominant right temporal lobe atrophy[8] has been shown to be associated with both the bvFTD and the SMD syndromes[9].
Pathologically, frontotemporal lobar degeneration (FTLD) is divided into FTLD-tau, FTLD-TDP with TAR DNA-binding protein of 43 kDa deposition, and FTLD-FUS with fused in sarcoma immunoreactive inclusions[10]. One example of FTLD-TDP characterized by TDP-43 immunoreactive neuronal cytoplasmic inclusions is FTD-MND[11, 12]. These underlying pathologies can also be clinically correlated. For example, the right temporal variant of bvFTD is associated with FTLD-tau while the right temporal variant of SMD is associated with FTLD-TDP[9]. A right temporal variant of FTD-MND has not been characterized. Therefore, the aim of this study was to investigate the clinical, imaging and pathological features of FTD-MND with primarily right temporal atrophy evident on MRI, i.e. right temporal variant FTD-MND.
(1) Materials and Methods
(2) Case selection
A database search of the Mayo Clinic electronic medical record system was used to identify all patients diagnosed with both dementia and motor neuron disease between January 1, 2000 and July 31, 2010. A total of 389 cases were reviewed of which 66 had the diagnosis of FTD-MND. Patients were diagnosed with motor neuron disease based on clinical and/or electromyographic evidence of motor neuron dysfunction consistent with the El Escorial criteria[13]. Patients with solely upper motor neuron disease were included according to proposed criteria for primary lateral sclerosis (PLS)[14, 15]. Of these 66 FTD-MND patients, two reviewers (E.A.C and K.A.J) reviewed the MRI head scans to identify those in which right temporal lobe atrophy clearly exceeded that of all other brain regions. Historical data was obtained from the medical records.
This study was approved by the Mayo Clinic IRB and all patients consented to research.
(2) Voxel-based morphometry methods
Two FTD-MND subjects had a volumetric MRI suitable for analysis (ages 59 and 64, both female); the third MRI was non-volumetric. These two subjects were matched by age and gender to a group of 10 healthy control subjects (median age = 62 (range 57–70), all female). Patterns of cerebral atrophy in the FTD-MND subjects were assessed using the automated and unbiased technique of voxel-based morphometry (VBM)[16] implemented using SPM5 (http://www.fil.ion.ucl.ac.uk/spmy). Briefly, all images were normalized to a customized template and segmented into grey matter, white matter and CSF using customized tissue probability maps and the unified segmentation routine[17] followed by the HMRF clean-up step. Grey matter images were modulated and smoothed at 8 mm full width at half maximum. A two-sampled t-test was used to assess grey matter loss in the FTD-MND subjects compared to controls. Results were assessed uncorrected for multiple comparisons at a statistical threshold of p<0.001.
(2) Pathology Methods
Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for Alzheimer's Disease[18]. After external examination of the fresh brain it was divided into right and left hemispheres. The left hemisphere was fixed in 10%–15% buffered formalin for 7–10 days and coronally sliced into 1-cm sections. Regions of interest including frontal, temporal, parietal and occipital lobes, midbrain, pons, medulla and cerebellum were sampled. Both cases had routine stains completed including Hematoxylin and Eosin, Bielschowsky and luxol fast blue. In addition, immunohistochemistry was performed with a battery of antibodies, including markers of glial pathology: glial fibrillary acid protein for astrocytes (clone GA5, 1:1000; BioGenex, San Ramon, CA) and CD68 (clone PG-M1, 1:1000; DAKO, Carpinteria, CA) for microglia. Neuronal pathology was studied with antibodies to neurofilament protein (NF-L: clone 2F11, 1:75; DAKO; NF-H: clone SMI-31, 1:2000; Sternberger Monoclonals, Lutherville, MD), ubiquitin (clone Ubi-1 [MAB1510], 1:250; Chemicon, Temecula, CA), alpha-synuclein (clone LB509, 1:200; Zymed, San Francisco, CA, or NACP98, polyclonal antibody, 1:2000; Mayo Clinic Jacksonville, FL), phospho-tau (CP13: gift from Dr. Peter Davis, Albert Einstein College of Medicine, Bronx, NY, or clone AT8, 1:1000; Innogenetics, Alpharetta, GA) and TDP-43 (1:8,000; ProteinTech Group, Chicago, IL). Pathologic diagnosis was made by one of two board certified neuropathologists (J.E.P. or D.W.D.).
TDP-43 typing was performed based on Mackenzie scheme[19] that was applied to frontotemporal neocortex and hippocampal dentate granular cells. Briefly, FTLD-TDP type 1 is based on the presence of TDP-43 immunoreactive dystrophic neurites and neuronal cytoplasmic inclusions, type 2 by a predominance of dystrophic neurites and type 3 by a predominance of neuronal cytoplasmic inclusions.
(1) Results
(2) Right temporal variant FTD-MND subjects
We identified 3 FTD-MND patients with striking and dominant right temporal lobe atrophy on MRI (Table 1). All 3 patients were female with a median age of onset of 60 (range 58–69). The average age at diagnosis was 61.5 (range 59–72) and average age at death was 63 (range 59–72). The average disease duration was 33 months (2.76 years), (range: 10–47 months). All patients had been evaluated by a behavioral neurologist with expertise in FTD (K.A.J) and all had at least 1 head MRI scan. All three patients are deceased with brain archived tissue in 2 patients.
Table 1: Clinical characteristics of 3 patients with right temporal variant FTD-MND
| Case | Gender | Age at onset | Age at diagnosis | Age at death | Diseas e Duration (yrs) | Family History | Behavioral symptoms | Cognitive and other symptoms | Motor symptoms |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | 72 | 72 | 3.41 | Father with parkinsonian disorder and dementia | Personality change with loss of empathy and insight; apathy, abnormal eating behaviors | Mild word finding difficulty, decreased speech output, logopenia | Mixed UMN and LMN |
| 2 | F | 58 | 59 | 59 | 0.89 | Autosomal dominant MND | Ritualistic behaviors, became fearful | Anomia progressing to mutism | Mixed UMN and LMN |
| 3 | F | 62 | 64 | 66 | 3.98 | No | Indecisive | Loss of semantic knowledge, topographagnosi a prosopagnosia | Asymmetric UMN |
Abbreviations: F, Female; MND, Motor neuron disease; UMN, upper motor neuron; LMN, lower motor neuron
(2) Brief clinical histories
Case 1 was a right-handed woman who presented at age 72 after a 3 year history of confusion, behavioral changes and speech difficulty. Her daughter reported personality changes; specifically she had become apathetic and lacked empathy. She began to crave sweets with an increased appetite and developed abnormal eating habits such as stuffing more food into her mouth that she could chew, resulting in choking. Dysphagia was her initial motor symptom and she then began falling with weakness in all extremities. Speech and language examination revealed a mixed spastic/flaccid dysarthria without aphasia. She scored 31/38 on the Short Test of Mental Status (STMS)[20], 43/72 on the frontal behavioral inventory[21] and 9/10 on a test of facial recognition (nl ≥ 9). There was no focal weakness. Fleeting fasciculations were observed in the gastrocnemius muscles only. She had evidence of spasticity in all four limbs and a Babinski sign was elicited. EMG revealed a diffuse neurogenic process affecting motor neurons or their axons. An MRI head scan revealed predominant right anterior medial temporal lobe atrophy (Figure 1). Her diagnosis was FTD-MND. She died a few months after evaluation with approximately 3 ½ years illness duration. A brain only autopsy was performed.
Figure 1.
Axial MRI fluid attenuation inversion recovery (FLAIR) sequence demonstrating significantly greater right than left anterior medial temporal lobe atrophy and increased signal change (Cases 1–3).
Case 2 was 58 when she began having difficulty recalling names. Her father had ALS at age 57 with paternal uncle and aunt diagnosed with ALS in their 60–70's. She had progressively less spontaneous speech and at the time of presentation she was anarthric. Her husband described early behavioral changes; she had become fearful of being alone and developed ritualistic behavior such as watching the same motion picture every night. Six months after the onset of her cognitive symptoms she began dragging her left leg. Her motor symptoms rapidly progressed over the next few months until she was unable to ambulate without assistance and had significant dysphagia. Cognitive examination was limited due to anarthria. Fasciculations were observed over pectoralis and deltoid muscles only. She had evidence of spasticity in all four limbs as well as a Babinski sign. There was electrophysiological evidence for neurogenic changes affecting motor neurons. MRI revealed prominent right anterior temporal lobe atrophy (Figure 1). Her diagnosis was FTD-MND. She died within a month of evaluation with illness duration of less than 1 year. A brain only autopsy was performed.
Case 3 was a 64 year-old left handed woman with a past medical history of manic-depressive episodes for over 20 years. She presented after a 2 year history of falls with associated left arm and leg weakness. Cognitive and behavioral changes developed one year after motor symptoms. She became indecisive and had trouble with directions. She had difficulty recognizing faces of individuals she knew. On bedside cognitive examination she received a score of 31/38 on the STMS[20]. She had prominent prosopagnosia and loss of semantic knowledge. Motor examination revealed left upper and lower limb, upper motor neuron type weakness. She had asymmetric left greater than right spasticity and a Babinski sign on the right. No fasciculations or muscle atrophy was observed anywhere. Serial MRIs showed progressive right temporal atrophy (Figure 1) with diffusion tensor imaging showing decreased right corticospinal tract fiber track density compared to the left (Figure 2). F-18 fluorodeoxyglucose PET scan showed right temporal and motor cortex hypometabolism (Figure 3). Her diagnosis was FTD-MND (FTD-PLS variant)[22] although a diagnosis of SMD-MND could not be excluded. She died 2 years after evaluation with approximate 4-year illness duration. An autopsy was refused by the family.
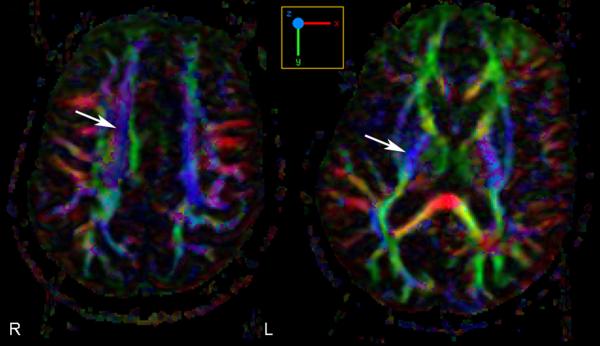
Figure 2.
Diffusion tensor imaging with fiber tracking demonstrates asymmetric decreased fiber track density within the right corticospinal tract compared to the left corticospinal tract. Arrows point to the corona radiata on the left and posterior limb of the internal capsule on the right (Case 3)
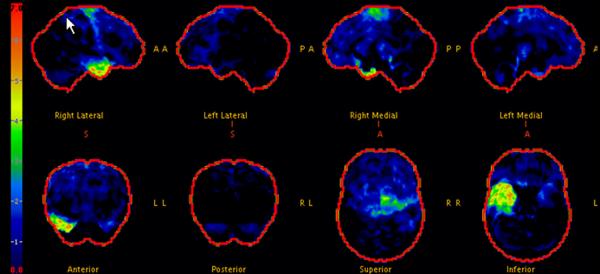
Figure 3.
F-18 fluorodeoxyglucose PET scan shows asymmetric FDG hypometabolism in the right temporal lobe and right motor cortex. There is otherwise normal cortical, basal ganglia and cerebellar metabolism (Case 3).
(2) Voxel based morphometry results
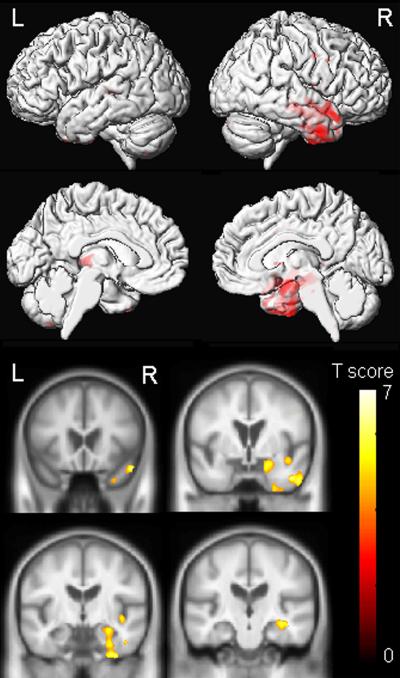
The FTD-MND patients (Cases 2 & 3) showed grey matter loss relatively restricted to the right temporal lobe, involving the temporal pole, amygdala, hippocampus, fusiform gyrus and inferior temporal gyrus (Figure 4). Some loss was also observed in the right insula and posterior frontal lobe.
Figure 4.
Voxel-based morphometry demonstrates significant right greater than left anterior temporal lobe atrophy (Cases 2 and 3; p=0.001 uncorrected).
(2) Pathology Results
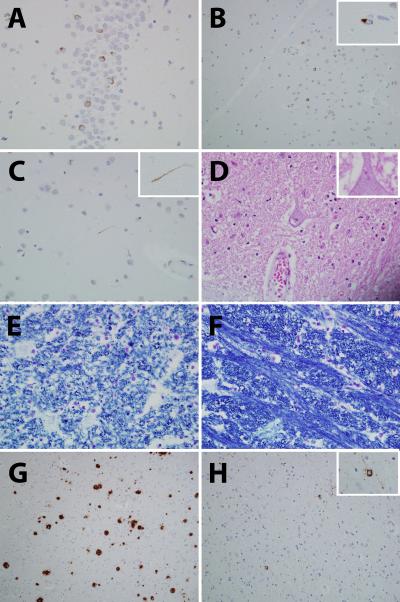
The left fixed hemibrain weights were 580g (Case 1) and 560g (Case 2). In both cases there were features consistent with FTLD-MND (Figure 5). Specifically, there was loss of Betz cells in motor cortex and degeneration of the descending corticospinal tract with pallor and discoloration of the medial peduncle and the medullary pyramids. There were TDP-43 immunoreactive neuronal cytoplasmic inclusions in the dentate gyrus of the hippocampus and frontotemporal neocortex. Rare to sparse dystrophic neuritis were observed consistent with FTLD-TDP type 3 pathology[19]. The hypoglossal nucleus showed mild to moderate neuronal loss with Bunina bodies identified in the remaining neurons. In addition, there were features of Alzheimer's disease in Case 2, with relative hippocampal sparing. There were moderate to frequent diffuse plaques, sparse to moderate neuritic plaques and sparse neurofibrillary tangles. The burden and distribution of these lesions met criteria for Braak and Braak stage V[23], CERAD probable[18], and NIA-Reagan high likelihood Alzheimer's disease (AD)[24].
Figure 5.
TDP-43 immunoreactive neuronal cytoplasmic inclusions were observed in the dentate nucleus of the hippocampus (A) and frontal neocortex (B); rare dystrophic neurites were identified (C). Bunina bodies were identified in surviving neurons of the hypoglossal nucleus (D). There was evidence of degeneration of the descending corticospinal tract which was easily appreciated when comparing fiber density of the pyramids of the medulla (E) to fiber density of the medial lemniscus (F). Panels A–F were taken from Case 2, although similar features were observed in Case 1. In Case 2 there were also neuritic plaques (CERAD Probable) (G) and neurofibrillary tangles (Braak stage V) (H) in keeping with an NIA-Regan high likelihood of Alzheimer's disease. TDP-43 (A–C), Hematoxylin & Eosin (D), Luxol fast blue (E–F), beta-amyloid (G) and phosphorylated tau (H).
(1) Discussion
In this study we describe the existence of an anatomic variant of FTD-MND that we refer to as right temporal variant FTD-MND. All three patients had clinical, electrophysiological and/or neuropathological evidence of degeneration of motor neurons and/or descending corticospinal tract while imaging studies reveal striking and dominant right anterior medial temporal lobe atrophy.
Interesting, all patients were women, presenting in the sixth and seventh decade which is typical of the FTD syndromes[25]. The disease courses were rapid with a median duration of less than three years, consistent with previous reports of FTLD-MND disease duration[26, 27]. Case 3, had the longest duration of almost 4 years which is more typical of the FTD-PLS variant of FTD-MND[22].
All three patients in this study had features consistent with FTD including behavioral and personality change, and aphasia. Typical features included loss of empathy, apathy, indecisiveness, ritualism, and abnormal eating behaviors. Symptoms of language impairment included difficulty naming in two cases with evidence of semantic impairment in case 3. Case 2 was anarthric and therefore difficult to assess for aphasia. In retrospect, assessment of writing would have been helpful. In case 1 the Frontal Behavioral Inventory score was in keeping with a diagnosis of FTD based on the recommended cutoff score for a diagnosis of FTD[21]. All three patients also had clinical features consistent with MND. Cases 1 and 2 had clinical features of upper and lower motor neuron disease while case 3 had features solely of upper motor neuron disease. Evidence of lower motor neuron disease was also demonstrated with EMG in cases 1 and 2. Two of the three patients had testing of facial recognition. Case 1 did well on testing while case 3 did poorly. Since prosopagnosia has been linked to the fusiform gyrus in SMD[28], one can speculate that the fusiform gyrus was relatively spared in case 1.
The imaging characteristic of all three cases was in keeping with our designation of right temporal variant FTD-MND as expected based on inclusion criteria. We were also able to demonstrate dominant right temporal lobe hypometabolism on FDG-PET scan, which also revealed evidence of motor cortex hypometabolism. Motor cortex hypometabolism has been demonstrated in cases of PLS where it is referred to as the stripe of PLS[29]. In addition to motor cortex dysfunction, we were also able to demonstrate corticospinal tract abnormalities with diffusion tensor imaging showing reduced fiber density on the right compared to the left, in the descending corticospinal tract. Interestingly, this patient was left-handed although her prominent symptoms were prosopagnosia and topographagnosia which is more common in patients with predominant right temporal lobe atrophy[30].
We also performed VBM in two of the three patients that had completed a volumetric head MRI scan. And although we had limited power given the number of patients, we were still able to demonstrate striking right temporal lobe volume loss in the two patients compared to controls. We also observed subtle volume loss of the posterior frontal lobe atrophy, a region that we have previously identified as being atrophic in a larger cohort of FTLD-MND subjects[7].
Pathological studies in patients with right temporal lobe FTD, whether right temporal bvFTD or right temporal SMD are lacking. However, there is some evidence to suggest that right temporal bvFTD is associated with FTLD-tau while right temporal SMD is associated with FTLD-TDP[9]. At the same time, there is excellent evidence that FTD-MND is associated with FTLD-TDP pathology[12, 31]. Not surprisingly therefore was the demonstration of FTLD-TDP pathology in both of our cases that went to autopsy. Furthermore, the histological characteristic of our cases was that of FTLD-TDP type 3 pathology (Mackenzie scheme)[19] which is characteristic of most cases of FTLD-MND[11, 12, 19]. Unfortunately, we do know whether the FTLD or AD pathology was the main cause of the right temporal lobe loss in case 2 although one could speculate that the AD pathology had less of an impact on the right temporal volume loss, since the AD pathology, specifically neurofibrillary tangle burden in the hippocampus was absent to minimal.
In a recent report a patient with MRI features of right greater than left temporal lobe atrophy presenting with aphasia, prosopagnosia, and reduced semantic fluency, the authors made a diagnosis of SMD-MND, instead of FTD-MND[32]. Unfortunately, pathology was not performed. However, there is a very strong association between FTLD-MND and FTLD-TDP type 3 pathology (Mackenzie scheme)[11, 12, 19], and between SMD and FTLD-TDP type 2 pathology[11, 12, 19]. Therefore, although right temporal lobe atrophy can be a feature of SMD[30], the diagnosis of FTD-MND was appropriate in our cases 1 and 2 and is consistent with the pathological findings of FTLD-TDP type 3 pathology in both cases. We cannot exclude the possibility that case 3 would have shown FTLD-TDP type 2 pathology. In fact, SMD-MND was considered a possible diagnosis as this patient had prosopagnosia and loss of semantic knowledge without evidence of lower motor neuron disease. However, we echo the sentiments that SMD is a very distinct clinical syndrome and that caution should be made in making a diagnosis of SMD when atypical features are present[33].
Our study is limited by the small number of patients, the lack of pathologic diagnosis in one patient and that according to established protocol only one hemisphere was pathologically analyzed. However, in this rare disorder, clinical, anatomic and pathologic correlation can add to our understanding of the spectrum of FTLD proteinopathies.
Acknowledgements
This study was supported by NIH grant R01 AG037491. The authors would like to acknowledge Dr. Clifford R. Jack, Jr. for the use of his laboratory to perform the VBM analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
(1) References
- [1].Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- [2].Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- [3].Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- [4].Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49:433–442. [PubMed] [Google Scholar]
- [5].Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology. 1997;39:556–559. doi: 10.1007/s002340050466. [DOI] [PubMed] [Google Scholar]
- [6].Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whitwell JL, Jack CR, Jr., Senjem ML, Josephs KA. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. neurology. 2006;66:102–104. doi: 10.1212/01.wnl.0000191395.69438.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–213. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- [9].Josephs KA, Whitwell JL, Knopman DS, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. neurology. 2009;73:1443–1450. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- [13].Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- [14].Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992;115(Pt 2):495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- [15].Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. neurology. 2006;66:647–653. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- [16].Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [17].Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- [18].Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- [19].Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62:281–288. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- [21].Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- [22].Josephs KA, Dickson DW. Frontotemporal lobar degeneration with upper motor neuron disease/ primary lateral sclerosis. neurology. 2007;69:1800–1801. doi: 10.1212/01.wnl.0000277270.99272.7e. [DOI] [PubMed] [Google Scholar]
- [23].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [24].Working Group Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- [25].Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- [26].Josephs KA, Knopman DS, Whitwell JL, et al. Survival in two variants of taunegative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- [27].Hu WT, Seelaar H, Josephs KA, et al. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol. 2009;66:1359–1364. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Josephs KA, Whitwell JL, Vemuri P, et al. The anatomic correlate of prosopagnosia in semantic dementia. neurology. 2008;71:1628–1633. doi: 10.1212/01.wnl.0000334756.18558.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Claassen DO, Josephs KA, Peller PJ. The stripe of primary lateral sclerosis: focal primary motor cortex hypometabolism seen on fluorodeoxyglucose F18 positron emission tomography. Arch Neurol. 2010;67:122–125. doi: 10.1001/archneurol.2009.298. [DOI] [PubMed] [Google Scholar]
- [30].Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- [31].Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- [32].Kim SH, Seo SW, Go SM, et al. Semantic dementia combined with motor neuron disease. J Clin Neurosci. 2009;16:1683–1685. doi: 10.1016/j.jocn.2009.05.005. [DOI] [PubMed] [Google Scholar]
- [33].Warren JD. Comment on “Semantic dementia combined with motor neuron disease”. J Clin Neurosci. 2010;17:1223. doi: 10.1016/j.jocn.2009.12.025. [DOI] [PubMed] [Google Scholar]