Abstract
Object
In patients with medically refractory epilepsy the accurate localization of the seizure onset zone is critical for successful surgical treatment. The object of this study was to investigate whether the degree of coupling of spontaneous brain activity as measured with functional connectivity MR imaging (fcMR imaging) can accurately identify and localize epileptic discharges.
Methods
The authors studied 6 patients who underwent fcMR imaging presurgical mapping and subsequently underwent invasive electroencephalography.
Results
Focal regions of statistically significant increases in connectivity were identified in 5 patients when compared with an ad hoc normative sample of 300 controls. The foci identified by fcMR imaging overlapped the epileptogenic areas identified by invasive encephalography in all 5 patients.
Conclusions
These results suggest that fcMR imaging may provide an effective high–spatial resolution and noninvasive method of localizing epileptic discharges in patients with refractory epilepsy.
Keywords: functional MR imaging, epilepsy, interictal discharges, functional connectivity MR imaging, localization, noninvasive localization
An important factor for success in the surgical treatment of epilepsy is the accurate localization of the seizure onset zone.25 Finding a structural lesion on MR imaging, such as mesial temporal sclerosis or a focal cortical dysplasia, improves the likelihood of controlling the illness after the surgery.12 However, in two-thirds of patients with focal epilepsy there are no identifiable brain lesions on conventional MR imaging, and it is sometimes difficult to identify the source of epileptic discharges with scalp EEG. When the location of the epileptic focus remains unclear, other diagnostic tests can be performed such as FDG-PET, ictal SPECT, and/or MEG.4,14 If uncertainty remains, iEEG with subdural grids or depth electrodes may aid in decision making.15 An accurate noninvasive and high–spatial resolution method to localize epileptic foci thus remains a major challenge.
Functional connectivity MR imaging (fcMR imaging) is based on correlating spontaneous fluctuations in blood flow across the brain, and these fluctuations are ultimately linked to neural activity.2,6,27 Previously, we and others demonstrated that fcMR imaging can be used to identify eloquent cortex (for example, motor cortex) in the presurgical evaluation of patients with brain tumors or epilepsy.20,29 In this study, we hypothesize that epileptogenic regions are characterized by altered connections to other brain regions even in the interictal state and can be localized based on functional connectivity changes in comparison with healthy controls. To test this hypothesis, we studied the feasibility of using a novel fcMR imaging approach to identify epileptic foci, and compared the results to the clinical “gold standard” for epileptic foci identification, iEEG.
Methods
Patient Population
Informed consent was obtained according to the guidelines and approval of the local institutional review board. Resting blood oxygenation level–dependent (BOLD) fMR imaging data from 6 patients with epilepsy (age range 18–24 years; 4 men, 2 women) were analyzed. The patients were undergoing routine presurgical evaluation prior to electrode implantation for localization of epileptic discharges. Three hundred healthy controls (143 men and 157 women) whose data were available in an existing fcMR imaging database21 were used as a normative comparison sample to identify aberrant connectivity patterns (age range of controls 18–33 years, mean 22.3 years). In all 6 patients undergoing presurgical evaluation, epilepsy had been diagnosed and classified based on the guidelines of the Commission on Classification and Terminology of the International League Against Epilepsy.5
MR Imaging Data Acquisition
Prior to electrode placement, we acquired MR imaging data in all patients. Scanning was performed on a 3-T TimTrio system (Siemens) using a 12-channel phased-array head coil supplied by the vendor. High-resolution 3D T1-weighted MPRAGE images were acquired for anatomical reference (TR 2530 msec, TE 3.44 msec, flip angle 7°, 1.0-mm isotropic voxels, FOV 256 × 256). Functional data were acquired using a gradient-echo echo planar pulse sequence sensitive to BOLD contrast (TR 2500 or 3000 msec, TE 30 msec, flip angle 90°, 36–43 axial slices parallel to the plane of the anterior commissure–posterior commissure, 3.0-mm isotropic voxels, 0.5-mm gap, FOV 576 × 576). The patients passively fixated on a visual cross-hair centered on a screen for each functional run, with an acquisition time ranging from 312 to 444 seconds. No additional task was performed. They were asked to remain awake and to be as still as possible. None of the patients reported a seizure within 48 hours of fMR imaging acquisition.
MR Imaging Processing: Functional Connectivity Analysis
Functional data were preprocessed according to the steps described previously.27 To measure the degree of local and remote functional connectivity of each voxel, a voxel-wise whole-brain correlation analysis was performed. 3,26 Specifically, the BOLD low-frequency signal time course of each voxel from the subject’s brain was correlated to every other voxel time course, at the local and remote level. Then Pearson correlation coefficients were averaged and assigned to the given voxel. We chose a neighborhood strategy to define local and remote limits. This computationally efficient approach, which is a derived measure of betweenness centrality in graph theory, has demonstrated robust estimates of connectivity profiles that characterize normal cortical regions.26 For local connectivity, all voxels in the 3-mm–radius sphere surrounding the seed voxel were included. For remote connectivity, all the voxels outside a 25-mm–radius sphere were included. This gap between local and remote distances excluded possible overlap between the indexes. The degree of coupling was calculated by summing the number of voxels above a correlation threshold of 0.25 both locally and remotely. To determine statistically significant differences between the patients and controls, we calculated the Z value at each voxel in the individual patients using our ad hoc normative control sample of subjects. The Z score of each voxel in each patient’s map is given by Zi = (Xi − X̄/σ, where X̄ is the mean coupling and σ is the standard deviation of the degree of our normative sample at that particular target voxel. Therefore, we produced local and remote connectivity Z score maps for each patient that indexed aberrant connectivity. This analysis was done without knowledge of the results from the iEEG.
Magnetoencephalography
As part of the routine evaluation of surgical epilepsy patients, whole-head MEG was obtained using a Elekta-Neuromag device (see Knake et al.13 for acquisition details). Equivalent current dipole (ECD) source analysis was performed on the interictal discharges using a single-layer boundary element model. The ECDs were fused to the MPRAGE anatomical volume from each patient’s MR imaging. Epileptic discharges were localized by experienced magnetoencephalographers, and the lobar localizations of interictal discharges were determined.
Intracranial EEG Monitoring
To localize the seizure event foci, intracranial electrodes (2 mm diameter, 1 cm separation, Ad-Tech) were implanted through a small craniotomy. The number of electrodes per patient ranged from 48 to 126. The MPRAGE anatomical volume was coregistered with a postimplantation CT image using an automatic rigid-body registration algorithm. In some patients, the registration required manual adjustment due to mild brain shifts resulting from the craniotomy, such as subdural and epidural fluid accumulations. As part of the routine clinical evaluation, patients stayed in the hospital 6–9 days, and physiological data were continuously monitored during this period. Recordings around the seizure events were analyzed by an epileptologist who identified the electrodes corresponding to the seizure onset and frequent interictal spikes (electrodes identified in Fig. 1). All the iEEG interpretation was done without knowledge of the fcMR imaging results.
Fig. 1.
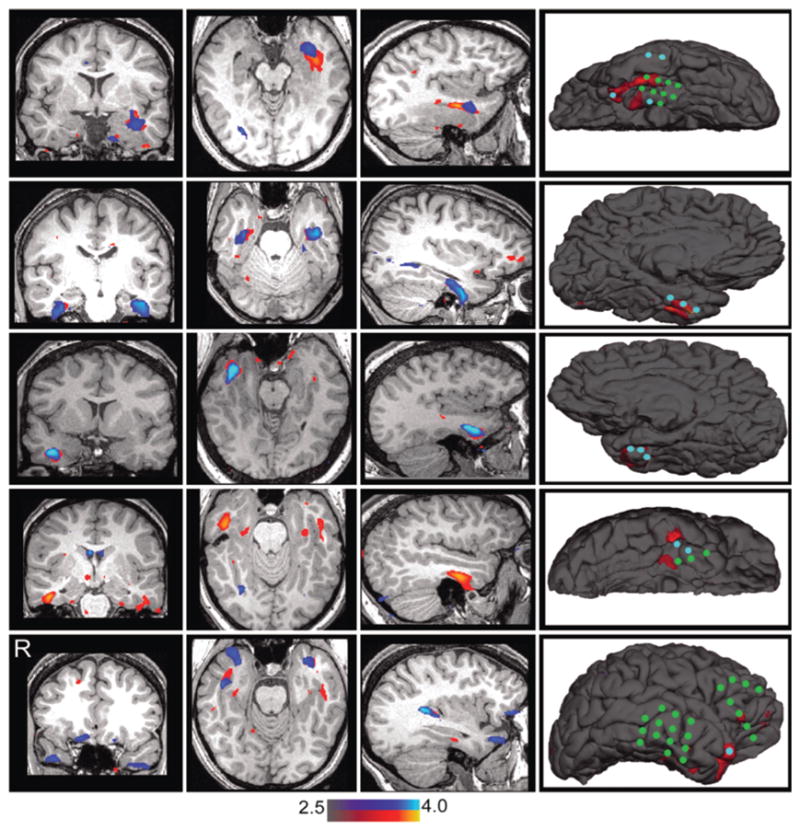
Epileptic foci localized based on degree of functional connectivity. Each row shows the functional abnormality scores of 1 patient (Cases 1, 3, 4, 5, and 6 in Table 1). Remote (blue) and local (red) functional connectivity differences revealing abnormal cortex are displayed, both representing Z-scores compared with a normative sample of 300 healthy individuals. The first 3 columns illustrate the functional abnormality in 3 orthogonal views. In the fourth column, the functional abnormality scores (remote and local combined) were rendered on the surface and compared with iEEG findings. The blue circles in the map indicate the iEEG electrodes corresponding to seizure onset. The green circles indicate the electrodes corresponding to frequent interictal discharges.
Results
Statistically significant differences were found in the functional connectivity maps in 5 of 6 patients (Fig. 1). In these 5 individuals the detected connectivity abnormalities fell within a 5-mm radius of the iEEG electrodes corresponding to the seizure onset zone. In 2 patients (Cases 3 and 6 in Table 1, corresponding to the second and fifth rows in Fig. 1), we found functional connectivity abnormalities in both hemispheres, although stronger in one of them. Since the iEEG grids only covered one hemisphere, we could not determine if there were any discharges contralateral to the iEEG. However, the stronger functional connectivity abnormality corresponded to the hemisphere where the iEEG electrodes were placed in both patients. No significant functional abnormality was found in one patient despite identifying ictal activity on the iEEG in the frontal lobe. Finally, it is important to note that, although the local and remote connectivity increases spatially matched one another in several patients (results shown in rows 1–3 in Fig. 1), local degree estimates appear more accurate for foci detection, especially when compared with the iEEG overlap. Data on surgical outcomes were not available in these cases.
TABLE 1.
Diagnosis, structural MR imaging, MEG localization, invasive recordings, and fcMR imaging results
| Case No. | MRI Clinical Report | MEG | iEEG | fcMRI* |
|---|---|---|---|---|
| 1 | normal | lt temporal | lt temporal | lt temporal |
| 2 | normal | rt frontal; rt parietal | rt frontal | undetermined |
| 3 | possible lt hippocampal-sclerosis | lt temporal | lt temporal | bilateral temporal |
| 4 | rt mesial temporal sclerosis | rt temporal | rt temporal | rt temporal |
| 5 | normal | rt temporal; rt frontal | rt temporal | rt temporal |
| 6 | normal | rt temporal | rt temporal | bilateral temporal |
Lobar location using criteria of p > 0.01 (uncorrected) and cluster size > 5 contiguous voxels.
Discussion
We demonstrate the feasibility of using fcMR imaging to identify epileptic foci during interictal periods in patients with medically refractory epilepsy. The fcMR imaging technique is based on calculating the intrinsic correlations of spontaneous BOLD signal across the brain to identify regions with an abnormal degree of functional connectivity. We found distinct differences in connectivity patterns in many of the patients and also found that this abnormal connectivity overlapped the epileptogenic areas identified by iEEG. This proof-of-concept suggests that fcMR imaging may be useful for identifying epileptic foci with the advantage of being noninvasive and having a high spatial resolution.
If we use the lobar localization of ictal discharges on iEEG as a reference, we can estimate the preliminary diagnostic accuracy of this method. The overall approximate sensitivity is 0.83, the specificity is 0.91, the false positive rate is 0.10, and the false negative rate is 0.15. These numbers are biased in that all of the patients tested had epileptic discharges, so a larger number of patients that do not have discharges will be required to obtain true diagnostic accuracy.
Abnormal functional connectivity has been proposed to be involved in the pathogenesis of epilepsy for decades.23 Functional connectivity as measured by fcMR imaging has been shown to be abnormal in other studies. 1,9,18,19,22,28 For example, Bettus et al.1 found a decrease in functional connectivity in affected hippocampal structures and an increase in functional connectivity between hippocampal structures in the healthy hemisphere. The methods used were different from ours in that statistical differences in the correlation coefficients for targeted regions were explored, rather than measures of local and remote coupling. Another study demonstrated disrupted functional connectivity in the language cortex in patients with temporal lobe epilepsy as compared with healthy controls.22 This change in functional connectivity might relate to plasticity of the language system due to damaged cortex, rather than a direct effect of the epileptic discharges. Moreover, Laufs et al.18,19 noted that the default network was disrupted at the group level in patients with left temporal lobe epilepsy using connectivity measures and EEG-fMR imaging. Wagner et al.28 found decreased connectivity in the hippocampus of epilepsy patients, which correlated with decreased memory performance.
Abnormal resting connectivity has also been shown in other brain disorders distinct from epilepsy, in particular Alzheimer disease, where coupling between the hippocampal formation and cortical regions is disrupted.8,10 Further exploration will be required to determine whether it is possible to separate fcMR imaging differences linked to the seizure focus from broader network alterations that may emerge from long-standing epilepsy.
Relevant to this possibility, there were bilateral increases in both local and remote coupling in 2 of our patients. This observation might indicate that the fcMR imaging is detecting abnormal functional connectivity in the entire epileptic network, as has been reported in previous fMR imaging studies (see Gotman7 for a review). When bilateral increases in connectivity are found on fcMR imaging, especially in the temporal lobe, it might be useful to compare the results with those of other neuroimaging methods such as interictal PET or ictal SPECT.
The fcMR imaging technique differs methodologically from fMR imaging with simultaneous scalp EEG, which has had a major impact recently in the neuroimaging of epilepsy.17 Functional MR imaging with simultaneous EEG inside an MR imaging scanner makes it possible to correlate the EEG with BOLD-fMR imaging.11,17 In patients with epilepsy, EEG is typically performed continuously during fMR imaging acquisition, and the images are selectively averaged based on the timing of the epileptic discharges.24 The results are sometimes ambiguous since a similar-appearing discharge may lead to an “activation” pattern (positive BOLD response) or a “deactivation” (negative BOLD response) relative to the ongoing background activity16 Abnormalities in functional connectivity patterns as measured here offer a complementary approach for identifying epileptic cortex. In this study, our hypothesis of altered BOLD connectivity reflecting epileptic discharges is supported by the spatial overlap of the fcMR imaging and iEEG, particularly for the local coupling. However, it is also interesting that remote connectivity often matches local connectivity, although not always, perhaps reflecting other network properties distant from the epileptic onset foci.
We cannot rule out the possibility that some of the connectivity abnormalities relate to structural changes due to the effects of long-standing epilepsy. Two of the patients had confirmed MR imaging–diagnosed mesial temporal sclerosis (see Table 1). It is not clear how this might affect our results. Moreover, it is widely reported that long-standing epilepsy may result in hippocampal volume loss and cortical thinning. We have no evidence that these potential confounds exist in our results. Another limitation of the current fcMR imaging analysis is that the underlying neural activity is unknown. Simultaneous EEG would be a useful measure to determine the underlying state of the patient’s epileptic discharges at the time of the scan and would provide a more direct comparison of the two approaches in analyzing fMR imaging.
Conclusions
In summary, we demonstrate the feasibility of a simple approach to identify and localize epileptic cortex using fcMR imaging. Although promising, this is a small study without postsurgical outcomes. Larger studies with seizure frequency after surgery will be required to validate the method. The method presented here may benefit the overall health care of patients suffering from epilepsy since it is noninvasive and does not require an additional imaging modality. Different MR imaging modalities are already part of the standard evaluation for patients with medically refractory epilepsy, and implementing this method requires adding a single 5- to 10-minute series to existing MR imaging epilepsy protocols.
Acknowledgments
This work was supported by National Institute on Aging Grant AG021910, National Center for Research Resources Grant P41RR14074, National Institute of Mental Health Grant K08MH067966, a Harvard Catalyst Pilot Grant, and the Howard Hughes Medical Institute.
Abbreviations used in this paper
- BOLD
blood oxygenation level–dependent
- EEG
electroencephalography
- fcMR imaging
functional connectivity MR imaging
- fMR imaging
functional MR imaging
- iEEG
invasive EEG
- MEG
magnetoencephalography
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Stufflebeam, Sepulcre, Buckner. Acquisition of data: Stufflebeam, Tanaka. Analysis and interpretation of data: Stufflebeam, Liu, Sepulcre, Buckner, Madsen. Drafting the article: Stufflebeam, Sepulcre, Buckner. Critically revising the article: Stufflebeam, Liu, Sepulcre, Buckner. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Stufflebeam, Liu.
References
- 1.Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 3.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascino GD. Surgical treatment for extratemporal epilepsy. Curr Treat Options Neurol. 2004;6:257–262. doi: 10.1007/s11940-004-0017-4. [DOI] [PubMed] [Google Scholar]
- 5.Commission on Classification and Terminology of the International League Against Epilepsy: Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 6.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 7.Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49 (Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA. 2010;23:409– 421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- 10.Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill RA, Chiappa KH, Huang-Hellinger F, Jenkins BG. EEG during MR imaging: differentiation of movement artifact from paroxysmal cortical activity. Neurology. 1995;45:1942–1943. doi: 10.1212/wnl.45.10.1942-a. [DOI] [PubMed] [Google Scholar]
- 12.Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49:758–764. doi: 10.1111/j.1528-1167.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 13.Knake S, Halgren E, Shiraishi H, Hara K, Hamer HM, Grant PE, et al. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res. 2006;69:80–86. doi: 10.1016/j.eplepsyres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Knowlton RC. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behav. 2006;8:91–101. doi: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008;64:25– 34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27:488–497. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakow K, Woermann FG, Symms MR, Allen PJ, Lemieux L, Barker GJ, et al. EEG-triggered functional MRI of interictal epileptiform activity in patients with partial seizures. Brain. 1999;122:1679–1688. doi: 10.1093/brain/122.9.1679. [DOI] [PubMed] [Google Scholar]
- 18.Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2007;28:1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K. Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia. 2006;47:444–448. doi: 10.1111/j.1528-1167.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. Laboratory investigation. J Neurosurg. 2009;111:746–754. doi: 10.3171/2008.10.JNS08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci USA. 2008;105:6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan RM, Parry AM, Arida RM, Matthews PM, Davies B, Castell LM. Effects of elevated plasma tryptophan on brain activation associated with the Stroop task. Psychopharmacology (Berl) 2007;190:383–389. doi: 10.1007/s00213-006-0609-7. [DOI] [PubMed] [Google Scholar]
- 24.Schomer DL, Bonmassar G, Lazeyras F, Seeck M, Blum A, Anami K, et al. EEG-Linked functional magnetic resonance imaging in epilepsy and cognitive neurophysiology. J Clin Neurophysiol. 2000;17:43–58. doi: 10.1097/00004691-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 26.Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol. 2010;6:e1000808. doi: 10.1371/journal.pcbi.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner K, Frings L, Halsband U, Everts R, Buller A, Spreer J, et al. Hippocampal functional connectivity reflects verbal episodic memory network integrity. Neuroreport. 2007;18:1719–1723. doi: 10.1097/WNR.0b013e3282f0d3c5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Johnston JM, Fox MD, Leuthardt EC, Grubb RL, Chicoine MR, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery. 2009;65 (6 Suppl):226–236. doi: 10.1227/01.NEU.0000350868.95634.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]


