Abstract
The development of small molecule agonists for class B G protein-coupled receptors (GPCRs) has been quite challenging. With proof-of-concept that exenatide, the parenterally administered peptide agonist of the glucagon-like peptide-1 (GLP1) receptor, is an effective treatment for patients with diabetes mellitus, the development of small molecule agonists could have substantial advantages. We previously reported a lead for small molecule GLP1 receptor agonist development representing the pentapeptide NRTFD. In this work, we have prepared an NRTFD derivative incorporating a photolabile benzoylphenylalanine and used it to define its site of action. This peptide probe was a full agonist with potency similar to NRTFD, which bound specifically and saturably to a single, distinct site within the GLP1 receptor. Peptide mapping using cyanogen bromide and endoproteinase Lys-C cleavage of labeled wild type and M397L mutant receptor constructs identified the site of covalent attachment of NRTFD within the third extracellular loop above the sixth transmembrane segment. This region is the same as that identified using an analogous photolabile probe based on secretin receptor sequences, and has been shown in mutagenesis studies to be important for natural agonist action of several members of this family. While these observations suggest that small molecule ligands can act at a site bordering the third extracellular loop to activate this class B GPCR, the relationship of this site to the site of action of the amino-terminal end of the natural agonist peptide is unclear.
Keywords: G protein-coupled receptor, glucagon-like peptide-1 receptor, small molecule ligand, ligand binding, photoaffinity labeling
G protein-coupled receptors (GPCRs) represent the most common target of currently approved drugs, providing access to many excitable cells throughout the body. Many of these drugs represent orally available small molecule agonists, mimicking the action of their endogenous ligands and activating intracellular signaling cascades. Of note, the vast majority of these drugs act at class A GPCRs, with the intramembranous helical bundle a common site of action. Perhaps the lessons learned by the site of action of the chromophore within rhodopsin and the site of action of biogenic amines at adrenergic receptors have informed and directed drug development at these targets.
Class B GPCRs, while sharing their heptahelical topology and their coupling to heterotrimeric G proteins with class A GPCRs, are known to be missing the signature sequences typical of class A GPCRs and are proposed to have a helical bundle conformation that is distinct from that of class A GPCRs.1 The peptide hormone-binding class B GPCRs represent potentially important targets for agonist drugs. While analogues of the agonist peptide ligands for the parathyroid hormone receptor (teriparatide) and the GLP1 receptor (exenatide) are approved for clinical use, development of small molecule agonists for receptors in this family has been more challenging.
Small molecule ligands for class B GPCRs currently in development include antagonists for CRF1,2 calcitonin gene-related peptide3 and glucagon4 receptors, and agonists for GLP15 and calcitonin6 receptors. Sites of action for most of these drugs have not yet been definitively established. The exception is the calcitonin gene-related peptide antagonists that have been shown by NMR and crystal structures to dock to the receptor ectodomain, between the receptor amino terminus and the associated RAMP protein.7,8 It has been suggested that a small molecule CRF1 receptor antagonist acts within the helical bundle of the receptor.2 While other “druggable pockets” for these receptors have also been postulated to exist,3,6 they have not yet been structurally defined. Here, we have taken advantage of a previously identified lead molecule (pentapeptide NRTFD9) for small molecule GLP1 receptor agonist development, and have utilized photoaffinity labeling to directly identify a region of the receptor spatially approximated with the docked ligand. This region is adjacent to the amino-terminal portion of the third extracellular loop above TM6 of this receptor, a region also shown by mutagenesis and photoaffinity labeling of other family members to affect natural agonist action.
The pentapeptide NRTFD was synthesized as previously reported.9 A novel NRTFD derivative, Bpa-Y-NRTFD (Bpa-NRTFD), incorporating a p-benzoyl-L-phenylalanine (Bpa) for crosslinking and a tyrosine for radioiodination, was synthesized using manual solid-phase techniques and purified by reversed-phase HPLC.10 Expected molecular masses were verified by mass spectrometry. This peptide and GLP1 (used as radioligand for binding assay) were radioiodinated oxidatively to yield specific radioactivities of 2000 Ci/mmol.10
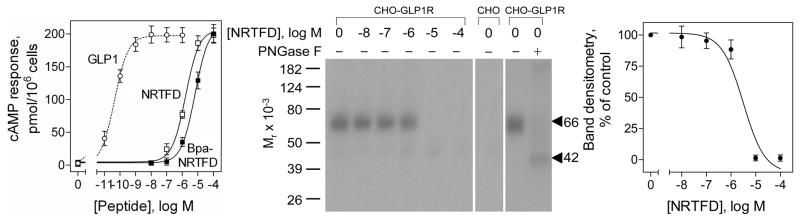
The biological activity of Bpa-NRTFD was determined by examining its ability to stimulate concentration-dependent cAMP responses in human GLP1 receptor expressing (CHO-GLP1R) cells9 using a cAMP assay previously published.11 Figure 1 shows that Bpa-NRTFD was a fully efficacious agonist, stimulating intracellular cAMP responses in GLP1 receptor-bearing CHO-GLP1R cells in a concentration-dependent manner, with both potency and efficacy similar to that of NRTFD, although much less potent than GLP1 (EC50 values: GLP1, 44 ± 9 pM; NRTFD, 1.6 ± 0.5 μM; Bpa-NRTFD, 6.4 ± 1.5 μM). Like NRTFD,9 Bpa-NRTFD did not competitively inhibit GLP1 radioligand binding to its receptor when used in concentrations as high as 100 μM. The affinity of Bpa-NRTFD for the GLP1 receptor was determined based on the ability of NRTFD to competitively inhibit photoaffinity labeling of the receptor with the radioiodinated NRTFD analogue described below.
Figure 1.
Characterization of the Bpa-NRTFD probe. Left shows curves of intracellular cAMP responses to increasing concentrations of GLP1, NRTFD and Bpa-NRTFD in CHO-GLP1R cells. Data points represent absolute values expressed as means ± S.E.M. of at least three independent experiments performed in duplicate. Middle shows typical autoradiographs of gels used to separate the products of affinity labeling of CHO-GLP1R cell membranes by Bpa-NRTFD in the absence and presence of increasing concentrations of unlabeled NRTFD, the negative control of attempting to label non-receptor-bearing CHO cell membranes, and the identification of the product of deglycosylation of the labeled receptor band. Right shows densitometric analysis of the peptide competition for receptor labeling that was performed using NIH ImageJ software in three similar experiments.
Plasma membranes were prepared from CHO cells expressing wild type and mutant receptors using discontinuous sucrose gradient centrifugation.11 Photoaffinity labeling of these membranes were performed following the procedures previously reported.11 Figure 1 also shows that Bpa-NRTFD covalently labeled the receptor specifically and saturably, with labeling inhibited by NRTFD in a concentration-dependent manner (IC50 value, 3.1 ± 0.8 μM). The labeled receptor migrated at Mr = 66,000 and shifted to Mr = 42,000 after deglycosylation with PNGase F, similar to the GLP1 receptor labeled by natural ligand probes.11 No bands were detected in affinity-labeled non-receptor-bearing CHO cell membranes (Fig. 1).
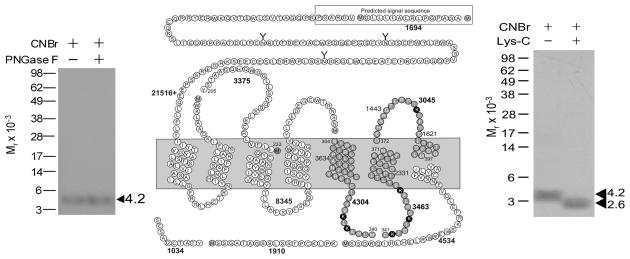
Peptide mapping with cyanogen bromide (CNBr) and endoproteinase Lys-C (Lys-C) and N-glycosidase F (PNGase F) were performed following the procedures previously reported.11 The apparent molecular weights of the radioactive bands on the gels were determined by interpolation on a plot of the mobility of the appropriate ProSieve protein markers (Cambrex, Rockland, ME) versus the log values of their apparent masses. Figure 2 shows that CNBr cleavage of the labeled GLP1 receptor yielded a band migrating at approximate Mr = 4,200 that did not further shift after deglycosylation. Given the molecular mass of the radioiodinated probe (1,172 Da) and non-glycosylated nature of the labeled fragment, four fragments could be candidates (calculated masses of fragments of 3045, 3375, 3463, and 4304, none of which include sites of glycosylation, and all of which are exposed to the extracellular milieu). These were the receptor region Tyr205-Met233 including the first extracellular loop (ECL1), Asn304-Met340 including the fifth transmembrane domain (TM5), Cys341-Met371 including TM6, and Asp372-Met397 including ECL3. Figure 2 also shows that further Lys-C cleavage of the Mr = 4,200 CNBr fragment of the labeled GLP1 receptor yielded a band migrating at Mr = 2,600. These excluded the Tyr205-Met233 fragment including ECL1 as the candidate because it did not contain a lysine residue.
Figure 2.
CNBr and Lys-C cleavage of the labeled GLP1 receptor. Left shows a representative autoradiograph of a NuPAGE gel used to separate the products of CNBr cleavage with and without PNGase F treatment. Middle shows a diagram of the predicted sites of CNBr and sequential Lyc-C cleavage of the GLP1 receptor along with the masses. Right shows a representative autoradiograph of a NuPAGE gel used to separate the products of sequential Lys-C cleavage. These data suggest the three lysine-containing CNBr fragments are candidates for the domain of labeling by Bpa-NRTFD (grey cycles).
To determine which of the three remaining CNBr fragments (Asn304-Met340, Cys341-Met371, and Asp372-Met397) included the site of covalent attachment, a GLP1 receptor construct was prepared in which residue Met397 was changed to a leucine (M397L) to eliminate a site for CNBr cleavage using QuikChange Site-directed Mutagenesis (Stratagene, LaJolla, CA) with wild type receptor as template. A CHO-K1 cell line stably expressing this construct was established using previously published procedures.9 This M397L GLP1 receptor construct had similar affinity to bind GLP1 as wild type receptor (Ki values in nM: WT, 4.4 ± 1.0; M397L, 6.9 ± 0.9), as determined in a radioligand competition-binding assay previously described.11 This construct also responded to GLP1 to elicit intracellular cAMP responses similar to wild type receptor, exhibiting similar levels of background and maximal activity, as well as potency (EC50 values in pM: WT, 19 ± 1; M397L, 27 ± 4). Further, this construct signaled in response to NRTFD stimulation similarly to wild type receptor (EC50 values in μM: WT, 1.7 ± 0.6; M397L, 2.2 ± 0.8), and it was specifically and saturably labeled by Bpa-NRTFD (data not shown).
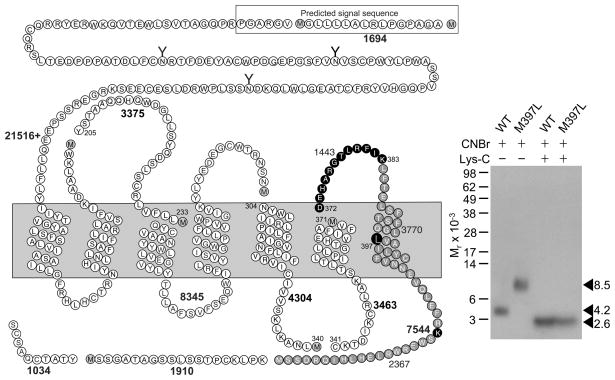
Figure 3 shows that CNBr cleavage of the labeled M397L mutant receptor yielded a much bigger fragment (Mr = 8,500) than that resulting from cleavage of labeled wild type receptor (Mr = 4,200). This definitively identified that the receptor Asp372-Met397 fragment including ECL3 contained the site of labeling for Bpa-NRTFD. Further Lys-C cleavage of the Mr = 8,500 CNBr fragment from the labeled M397L mutant receptor yielded a band migrating at Mr = 2,600, similarly to the band from Lys-C cleavage of the Mr = 4,200 CNBr fragment from the labeled wild type GLP1 receptor (Fig. 3), indicating the amino-terminal Asp372-Lys383 segment of ECL3 contained the site of labeling of Bpa-NRTFD.
Figure 3.
CNBr and Lys-C cleavage of the mutant M397L GLP1 receptor labeled with Bpa-NRTFD. Left shows a diagram of the predicted sites of sequential CNBr and Lyc-C cleavage of the M397L mutant receptor along with the masses. Right shows a typical autoradiograph of a gel to separate the products of sequential CNBr and Lys-C cleavage of labeled wild type and M397L GLP1 receptor. These data identified the amino-terminal Asp372-Lys383 segment of ECL3 containing the site of labeling.
Understanding the molecular basis of ligand binding and activation of class B GPCRs should facilitate rational design of drugs that can act at this family of receptors. In the absence of three-dimensional structures of intact receptors, we previously used photoaffinity labeling to understand how natural GLP1 interacts with its receptor.11 In this work, we have again utilized this technique to explore how another ligand, representing the pentapeptide agonist, NRTFD,9 interacts with this receptor.
The molecular basis of ligand binding and activation of class B GPCRs is still not well understood due to the lack of a three-dimensional structure of intact receptors. Recent NMR and crystal structures of amino-terminal domains of several receptors in this family, including the GLP1 receptor, have suggested a consistent structural theme for docking of the carboxyl-terminal region of natural peptide ligands, although there are inconsistencies in the absolute poses of the ligands relative to their receptor amino-terminal domains.12 However, how the flexible amino-terminal regions of the natural ligands interact with their receptors and the orientations of the amino-terminal domains of these ligands with the core domains of their receptors are not clear.
To date, the best model for docking a natural ligand to an intact class B GPCR is that of secretin occupation of its receptor that is based on extensive direct spatial approximation constraints coming from photoaffinity labeling.13 In this model, multiple spatial approximations have been established between residues scattered throughout the pharmacophore of secretin in positions 1, 2, 5, 6, 12, 13, 15, 16, 18, 20, 21, 22, 23, 24, 25 and 26. Of these, the amino-terminal positions 1, 2 and 5 were demonstrated to interact with the body of the secretin receptor13. Similar observations based on photoaffinity labeling data have been made for calcitonin,14 parathyroid hormone15 and GLP111 occupation of their respective receptors. Based on these studies, a two-domain mechanism has been proposed for activation of class B GPCRs by their natural ligands.
An interesting set of agonist ligands is based on structures intrinsic to a portion of the receptor amino-terminal domains.16 The first such sequence to be reported was between the second and third conserved cysteine residues in the amino terminus of the secretin receptor.16 Extensive analysis was performed to determine the shortest sequence to possess full agonist activity represented WDN, with this activity enhanced by cyclization and by derivatization with lipids.16 That peptide was also extended with a photolabile benzoylphenylalanine and used in photoaffinity labeling studies, where it was found to label a region of the secretin receptor above TM6 in ECL3.16 Although the original hypothesis proposed was that this portion of the secretin receptor might act as a natural endogenous agonist ligand, binding to and activating the receptor core,16 that hypothesis was recently disproven when it was established that the two domains could not establish the necessary spatial approximation with each other.17 Nevertheless, that peptide continues to represent a lead for small molecule agonist development.
The potential importance of such a lead has been further advanced by observations of similar full agonist activity for peptide fragments from analogous regions of the VPAC1, calcitonin, and GLP1 receptors.9,16 This suggests that there may be a consistent structural theme for these agonists and for their mode of action.
The GLP1 receptor is an important drug target with proven clinical significance for diabetes mellitus. GLP1 has multiple antidiabetic actions and the ability to lower body weight.18 GLP1 is, however, rapidly degraded by dipeptidyl peptidase 4 (DPP4)18 and more stable GLP1 analogues such as exenatide and liraglutide are currently being used as drugs. DPP4 inhibitors to prevent GLP1 degradation is also used, but the efficacy of this approach may be limited by lack of the specificity. Small molecule agonists for the GLP1 receptor have been recently reported,5 but their potential as new drugs has yet to be determined. Recently, a smaller peptide consisting of 11 amino acids has been shown to have similar potency to full-length GLP1, and can potentially be delivered via inhalation.19 The current project is focused on another small molecule lead compound, NRTFD, representing the fragment from the GLP1 receptor that was shown to possess full agonist activity.9
NRTFD is fully active as a linear peptide, unlike the cyclic WDN peptide derived from the secretin receptor.9 Of further interest, NRTFD acts as a full agonist not only at the GLP1 receptor, but also the secretin receptor.9 Molecular modeling predicts a salt bridge between the arginine and aspartic acid within NRTFD resulting in a three-dimensional structure that was shown to be similar to cyclic WDN.9
Here, we have localized the site of action of NRTFD using direct photoaffinity labeling. It is notable that the site labeled represents ECL3 of the GLP1 receptor, the region that is analogous to that labeled by cyclic WDN in the secretin receptor.16 This is also the region labeled by amino-terminal probes of several class B natural ligands, including secretin,20 calcitonin,14 and parathyroid hormone,15 suggesting that this region likely contributes to an important drug binding pocket or pockets.
The site of action of NRTFD has been postulated to be distinct from the site of action of the natural agonist, GLP1.9 This was based on the observations that a high affinity peptide antagonist competitively blocked the agonist action of GLP1, but not that of NRTFD.9 However, this antagonist represents the carboxyl-terminal region of the peptide and may only compete for the binding to the receptor amino terminus, and not compete with the biologically active amino-terminal end of GLP1. The other evidence for distinct site of action was the additivity of agonist action of GLP1 and NRTFD up to the maximum level stimulated by each alone.9 Here, too, the evidence for true allosteric action must be considered to be soft. Unfortunately, the low binding affinity of NRTFD precludes the performance of the classical assay evaluating the influence of infinite dilution with orthosteric ligand on the kinetics of dissociation of bound ligand to establish its allosteric mode of action.
In conclusion, NRTFD and cyclic WDN may represent lead compounds for the development of small molecule agonists acting at analogous sites within class B GPCRs. The data from the current study provide insights into the binding pocket for docking these molecules. It will be important to modify these ligand molecules in attempt to improve their affinities and potencies, and to examine their specificity for binding to members of this receptor family. Once that is accomplished, stability, absorption and bioavailability studies should determine whether these might be therapeutically useful or whether they can help to guide a small molecule agonist development program.
Acknowledgments
This work was supported by grants from American Diabetes Association (1-08-RA-42) and the National Institutes of Health (DK46577). The authors thank M.L. Augustine and A.M. Ball for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. Mol Pharmacol. 2003;63:1256. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 2.Hoare SR, Brown BT, Santos MA, Malany S, Betz SF, Grigoriadis DE. Biochem Pharmacol. 2006;72:244. doi: 10.1016/j.bcp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Mallee JJ, Salvatore CA, LeBourdelles B, Oliver KR, Longmore J, Koblan KS, Kane SA. J Biol Chem. 2002;277:14294. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 4.Petersen KF, Sullivan JT. Diabetologia. 2001;44:2018. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 5.Murphy KG, Bloom SR. Proc Natl Acad Sci USA. 2007;104:689. doi: 10.1073/pnas.0610679104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong M, Cox RF, Miller LJ. J Biol Chem. 2009;284:21839. doi: 10.1074/jbc.M109.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koth CM, Abdul-Manan N, Lepre CA, Connolly PJ, Yoo S, Mohanty AK, Lippke JA, Zwahlen J, Coll JT, Doran JD, Garcia-Guzman M, Moore JM. Biochemistry. 2010;49:1862. doi: 10.1021/bi901848m. [DOI] [PubMed] [Google Scholar]
- 8.ter Haar E, Koth CM, Abdul-Manan N, Swenson L, Coll JT, Lippke JA, Lepre CA, Garcia-Guzman M, Moore JM. Structure. 2010;18:1083. doi: 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Dong M, Gao F, Pinon DI, Miller LJ. Mol Endocrinol. 2008;22:1489. doi: 10.1210/me.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers SP, Pinon DI, Miller LJ. Int J Pept Protein Res. 1988;31:429. doi: 10.1111/j.1399-3011.1988.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Pinon DI, Miller LJ, Dong M. J Biol Chem. 2010;285:24508. doi: 10.1074/jbc.M110.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Trends Biochem Sci. 2009;34:303. doi: 10.1016/j.tibs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Dong M, Lam PC, Pinon DI, Hosohata K, Orry A, Sexton PM, Abagyan R, Miller LJ. J Biol Chem. 2011;286:23888. doi: 10.1074/jbc.M111.245969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong M, Pinon DI, Cox RF, Miller LJ. J Biol Chem. 2004;279:31177. doi: 10.1074/jbc.M404113200. [DOI] [PubMed] [Google Scholar]
- 15.Bisello A, Adams AE, Mierke DF, Pellegrini M, Rosenblatt M, Suva LJ, Chorev M. J Biol Chem. 1998;273:22498. doi: 10.1074/jbc.273.35.22498. [DOI] [PubMed] [Google Scholar]
- 16.Dong M, Pinon DI, Asmann YW, Miller LJ. Mol Pharmacol. 2006;70:206. doi: 10.1124/mol.105.021840. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Lam PC, Pinon DI, Orry A, Sexton PM, Abagyan R, Miller LJ. J Biol Chem. 2010;285:9919. doi: 10.1074/jbc.M109.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker DJ. Nat Clin Pract Endocrinol Metab. 2005;1:22. doi: 10.1038/ncpendmet0017. [DOI] [PubMed] [Google Scholar]
- 19.Qian F, Mathias N, Moench P, Chi C, Desikan S, Hussain M, Smith RL. Int J Pharm. 2009;366:218. doi: 10.1016/j.ijpharm.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Dong M, Li Z, Pinon DI, Lybrand TP, Miller LJ. J Biol Chem. 2004;279:2894. doi: 10.1074/jbc.M310407200. [DOI] [PubMed] [Google Scholar]