SUMMARY
The ability of lactoferrin to provide protection and decrease immunopathology in infectious diseases was evaluated using an aggressive aerosol model of Mycobacterium tuberculosis (MTB) infection. C57BL/6 mice were challenged with MTB strain Erdman and treated with 0.5% bovine lactoferrin added to the drinking water starting at day 0 or day 7 post infection. Mice were sacrificed at three weeks post-challenge and evaluated for organ bacterial burden, lung histopathology, and ELISpot analysis of the lung and spleen for immune cell phenotypes. Mice given tap water alone had lung log10 colony forming units (CFUs) of 7.5 ± 0.3 at week 3 post-infection. Lung CFUs were significantly decreased in mice given lactoferrin starting the day of infection (6.4 ± 0.7), as well as in mice started therapeutically on lactoferrin at day 7 after established infection (6.5 ± 0.4). Quantitative immunohistochemistry using multispectral imaging demonstrated that lung inflammation was significantly reduced in both groups of lactoferrin treated mice, with decreased foamy macrophages, increased total lymphocytes, and increased numbers of CD4+ and CD8+ cells. ELISpot analysis showed that lactoferrin treated mice had increased numbers of CD4+ IFN-γ + and IL-17 producing cells in the lung, cells that have protective functions during MTB infection. Lactoferrin alone did not alter the proliferation of MTB in either broth or macrophage culture, but enhanced IFN- γ mediated MTB killing by macrophages in a nitric oxide dependent manner. These studies indicate that lactoferrin may be a novel therapeutic for the treatment of tuberculosis, and may be useful in infectious diseases to reduced immune-mediated tissue damage.
Keywords: Lactoferrin, Tuberculosis, IL-17, Immunopathology
1. Introduction
Tuberculosis (TB) remains a significant global public health burden. There are approximately 9.27 million new cases of this disease and nearly two million deaths each year worldwide 1. Over 95% of drug-susceptible Mycobacterium tuberculosis (MTB) can be cured with a four-drug regimen of isoniazid, rifampin, pyrazinamide, and ethambutol for two months followed by four additional months of isoniazid and rifampin 2. However, the lengthy, complex drug regimen required to treat TB is difficult to administer in the resource poor countries that TB disproportionately affects. Furthermore, the efficacy of the available drug regimen is threatened by the emergence of multi-drug resistant strains 1. The last major advancement in TB therapeutics was made when rifampin was introduced in the 1960s 3. Thus, there is considerable need to develop novel agents for the treatment of TB. Enhancement of protective immune responses may represent a potential therapeutic approach.
T helper cell type I (Th1) responses are critical for host defense against MTB 4. MTB infection begins with uncontrolled growth of the TB bacilli within macrophages. An innate granuloma forms due to the accumulation of alveolar and recruited systemic macrophages. Dendritic cell presentation of TB antigens to naive CD4+ T-cells in the presence of IL-12 generates a Th1 immune response 5. Interferon-gamma (IFN-γ) production by Th1 cells activates macrophages, resulting in phagosome acidification, phagolysosome fusion, and synthesis of reactive nitrogen species that kill MTB 4. CD4+ T-cells are also essential for the generation of CD8+ cytotoxic T-cells that may play a role in control of disease progression 4. Additional immune system responses are also likely important. For example, a number of studies have highlighted the role of IL-17 in the immune response against MTB 6-8. Specifically, early enhancement of IL-17 responses by use of an IL-23 producing adenovirus during MTB infection resulted in decreased bacterial burden and reduced lung pathology 9, 10. Therefore, immunomodulatory agents useful in control of MTB infection are expected to produce a strong Th1 response, and perhaps IL-17 mediated responses.
Modulation of immune-mediated pathology while preserving essential immune responses may represent a novel therapeutic strategy for the treatment of TB. The immunocompetant host responds to MTB infection by the formation of granulomas, which may prevent dissemination of the bacilli 11. However, MTB within granulomas are protected from immune-mediated killing due to the sequestration of infected macrophages from immune effector cells 12. MTB within granulomas presumably adapt by conversion to a dormancy phenotype that includes decreased replication and changes in biochemical pathways, making the bacilli relatively resistant to the action of sterilizing antimicrobials 13, 14. Furthermore, decreasing lung immunopathology may enhance antibiotic penetration in infected tissue, thus promoting a faster response to delivered antimycobacterials 12, 15.
A number of studies suggest that lactoferrin has a number of immune modulating properties that may be favorable to the host during MTB infection 16. Lactoferrin is an iron-binding glycoprotein that is found in mucosal secretions and neutrophilic granules. It is also considered as a basic component of innate immunity, and has the potential to modulate host responses during MTB infection. Specific effects of lactoferrin include macrophage activation, enhancement of phagocytosis, and augmentation of the delayed type hypersensitivity response to a number of antigens 16. Importantly, lactoferrin enhances Th1 immune responses in a number of model systems, a response essential for host defense against MTB 16–21. Critical to the studies proposed here, lactoferrin has been shown to protect against immune-mediated tissue damage. For example, mice treated with lactoferrin had increased survival and decreased gut tissue destruction after LPS injection 22. This also holds true for damage elicited by mycobacterial antigen TDM23. Additionally, lactoferrin added to the BCG vaccine resulted in increased protection against an aerosol TB challenge, with evidence of decreased lung damage 24.
These studies explored the ability of lactoferrin to modulate lung pathology during a mouse model of MTB infection using a rapidly proliferating variant of MTB Erdman. We found that lactoferrin added to the drinking water during MTB infection decreased organ bacterial burden and lung immunopathology. Lung immune responses were explored to determine the ability of lactoferrin to promote protective Th1 and IL-17 mediated responses.
2. Materials and methods
2.1 Animals
Four week-old, female C57BL/6 mice were purchased from Jackson Laboratories. All studies were conducted under the approval from the animal ethics committee at the UTHSC, protocol AWC-08-050. Four to six mice were used per group, per time points indicated. All MTB infections occurred in biosafety level 3 facilities.
2.2. Lactoferrin and MTB
Bovine-derived lactoferrin (15 – 20% iron saturated, <0.2 endotoxin units/mg) was supplied by PharmaReview Corporation (Houston, TX). A rapidly-proliferating variant of MTB Erdman (TMC 107, American Type Cell Culture) was cultured in Middlebrook 7H9 broth with 10% supplement (5% bovine serum albumin, 2% dextrose, and 0.5% Tween 20 in distilled water) to log phase. Pelleted bacteria were resuspended in phosphate buffered saline (PBS) and diluted to 3 × 108 colony forming units (CFU) per ml using McFarland standards. Bacteria were sonicated to disperse aggregates. The bacterial CFUs were confirmed by plating serial dilutions on Middlebrook 7H11 agar plates (Remel, Lenexa, KS), which were incubated at 37 °C for 3 – 4 weeks.
2.3. Acute tuberculosis infection of mice
MTB strain Erdman was cultured to log phase as described above. C57BL/6 mice were infected using an aerosol inhalation exposure system (GLAS-COL Model #A4212 099c) to achieve an aerosol implantation of 100 CFUs. The inoculation dose was confirmed by sacrificing a subset of mice at day one post-challenge, and the lung bacterial load determined by plating lung homogenates onto 7H11 agar plates. The mice were randomized to either untreated controls or mice treated with lactoferrin at the start of infection to determine the effect of lactoferrin prior to development of histopathology, or beginning 7 days later to evaluate the impact of lactoferrin on established infection. Lactoferrin was administered in the drinking water at 5 mg/ml to give a dose of approximately 20–25 mg per mouse, assuming mice drink 4–5 ml of water per day 25, as was done in prior studies of oral lactoferrin-mediated immune modulation 26, 27. Mice were sacrificed at 1, 2, and 3 weeks post-infection. The lung, spleen, and liver were aseptically removed, placed into 5 ml of PBS, and homogenized. Serial dilutions of organ homogenates were plated onto Middlebrook 7H11 agar plates and incubated for 3 – 4 weeks at 37 °C24.
2.4 Lung histopathology analysis
Lung tissue was fixed in 10% formalin and embedded in paraffin. Five μm thick sections were stained with hematoxylin and eosin by standard methods. Acid-fast staining was performed by the Ziehl-Neelsen method. Twelve lungs from each group pooled from two different experiments at three weeks post-challenge were randomly selected for immunohistochemistry (IHC). Rat anti-mouse monoclonal antibodies (R&D Systems, Minneapolis, MN) were used to detect CD4+ and CD8+ cells, using an anti-rat horseradish peroxidase 3,3’diaminobenzidine cell and tissue staining kit according to the manufacturer’s instructions (R&D Systems).
All images of histopathology were obtained with the Nuance multispectral imaging system (CRI, Woburn, MA). This technology allows quantification of cell types in defined regions of pathology. The entire region of the immunostained lung was captured by a photographer in a blinded manner using the 10x objective, and analyzed by an independent researcher. Percentage occlusion was calculated by determining the fraction of open alveolar compartment relative to total tissue area as outlined by software analysis of captured image recognizing stained parenchyma. 10 to 12 lungs from each group were included in analysis, with one to three representative slide from each mouse utilized. Enumeration of normal lung, area percentages of macrophages and lymphocytes, and total number of CD4+ and CD8+ cells was done using the tissue and cell segmenting abilities of Inform software (CRI) as detailed by the manufacturer’s instructions.
2.5. Lung cytokine expression
Total lung RNA was isolated by homogenizing lung tissue in 1 ml of RNA-Bee (Tel-Test, Friendswood, TX), followed by addition of 0.1 ml of chloroform (Sigma)28–30. The samples were mixed, incubated on ice for 15 min, and centrifuged for 15 min at 13,000 rpm. The supernatants were removed and placed into an equal volume of isopropanol for overnight precipitation. The samples were centrifuged at 13,000 rpm for 15 min. The resulting RNA pellets were washed with 75% ethanol and resuspended in water with 1 mM EDTA. cDNA was generated and quantified by the Taqman assay as previously described 31. Data are expressed as fold change expression relative to naive controls after normalization to β-actin 32.
2.6. Preparation of lung digests and ELISpot analysis
Lung tissue from infected mice was removed, minced, and incubated at 37°C with 30 μg/ml DNAse (Roche Diagnostics, Mannheim, Germany) and 1 mg/ml type I collagenase (Worthington Biochemical Corporation, Lakewood, NJ) for 60 min on a rotating shaker. The lung digests were passed through a 40 μm filter (Fisher) followed by centrifugation at 1500 rpm. Red blood cells were lysed with ACK lysing buffer (Lonza, Walkersville, MD). The cells were washed 2 times with PBS and counted. One hundred μl of lung digests or splenocytes (2 × 105 cells) (see below) were examined for IL-17 producing cells (eBioscience, San Diego, CA) and CD4+ IFN-γ cells (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
2.7. Recall response to MTB antigens
Recall responses by splenocytes to heat-killed MTB was performed to evaluate lactoferrin modulation of the systemic immune response to MTB. Spleens were harvested from infected mice at the times points designated above. The tissues were minced using a glass homogenizer. Red blood cells were lysed with ACK lysing buffer (Lonza), followed by washing twice with PBS. Cells were resuspended in Dulbecco’s modified eagle’s medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% FBS, 0.01% HEPES (Sigma), and 0.01% L-arginine (Sigma), and plated into 24-well tissue culture plates at a concentration of 2 × 106 cells/ml. Heat-killed MTB was added at a MOI of 10:1, generated by autoclaving MTB suspended in 1xPBS at 121°C for 10 mins. Subsets of cells were stimulated with 2 μg/ml conA or 10 ng/ml LPS as controls. Supernatants were collected at 72 hours post-stimulation, filtered with a 2 μm filter, and analyzed by ELISA for IL-17, IFN-γ, IL-10, IL-12p40, TNF-α, IL-6, and TGF-β (R&D Systems).
2.8. Proliferation of MTB in broth and macrophage culture in the presence of lactoferrin
MTB were grown in 7H9 broth alone, with 100 μg/ml lactoferrin, or with 1 mg/ml of lactoferrin. Bacteria were sonicated every 4 hours, the OD600 obtained, and serial dilutions plated on 7H11 agar plates. Plates were incubated at 37 °C for 3 – 4 weeks.
For proliferation studies in macrophages, the J774A.1 (ATCC TIB-67) cell line was cultured in antibiotic-free DMEM with 2% FBS in 24-well tissue culture plates at a concentration of 5 × 105 cells/ml. MTB Erdman were added at a MOI of 1:1. Subsets of cells were given combinations of 10 ng/ml recombinant IFN-γ (Cell Sciences, Canton, MA), 100 μg/ml lactoferrin, and 1 mM of the NO synthetase inhibitor N-mono-methyl-arginine (Sigma). The cells were lysed every 48 hours with 0.05% SDS, neutralized with 15% bovine serum albumin (Sigma), and the lysates plated onto 7H11 agar plates. The cell supernatants were also removed, filtered with a 0.2 μm filter and examined for IL-6, TNF-α, IL-12p40, and TGF-β production by ELISA (R&D Systems). Production of NO was assessed by the Griess reaction as previously described 33. Briefly, 50 μl of the culture supernatants were mixed with 50 μl of Griess reagent (1% sulfanilamide and 0.1% naphthyl-ethylenediamine dihydrochloride in 1 N HCL). The optical density at 550 nm was determined. NaNO2 was used to produce a standard curve.
2.9. Statistics
The data are shown as the mean ± SD, with the exception of standard error analysis for Figure 1 growth curves from tissue. Two-way ANOVA was used to determine the differences between groups by use of GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). A p-value of less than 0.05 was defined as statistically significant.
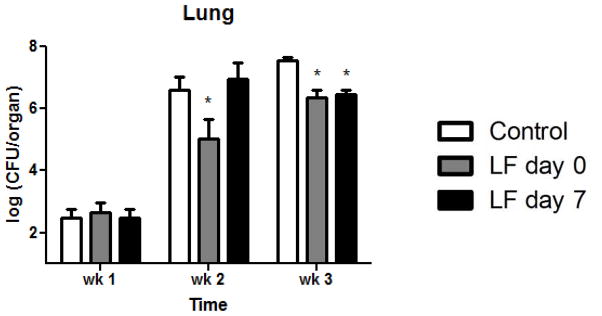
Figure 1. Decreased lung bacterial burden in lactoferrin treated mice.
Mice treated with lactoferrin starting at day 0 had significantly reduced bacterial burden in the lung at weeks 2 and 3 post-challenge compared to mice given tap water. Mice started on lactoferrin at day 7 had significantly decreased lung CFUs at week 3. Data are presented as the mean and SEM, n = 6 mice per group, per time point. *p < 0.05 with comparisons to control mice.
3. Results
3.1. Oral Lactoferrin treatment decreases bacterial burden in a mouse model of MTB infection
Bacterial CFUs in the lung are shown in Figure 1. Specifically, control mice administered tap water had lung log10 CFUs of 2.5 ± 0.7 at week 1, 6.6 ± 0.9 at week 2, and 7.5 ± 0.3 at week 3. In comparison, the mice treated with lactoferrin at the start of infection had significantly fewer lung CFUs at week 2 (5.0 ± 1.4) and week 3 (6.4 ± 0.7). Mice treated with lactoferrin beginning at day 7 after established infection at day 7 had significantly reduced lung CFUs at week 3 (6.5 ± 0.4). Bacterial growth in the spleen was not observed until week 2 and was not significantly different between the two groups (data not shown). Mice treated with lactoferrin at day 0 had significantly reduced liver CFUs at weeks 1 and 3 compared to control mice while mice given lactoferrin at day 7 had significantly decreased liver bacterial loads only at week 3 (not shown).
3.2. Lactoferrin reduces MTB induced lung immunopathology
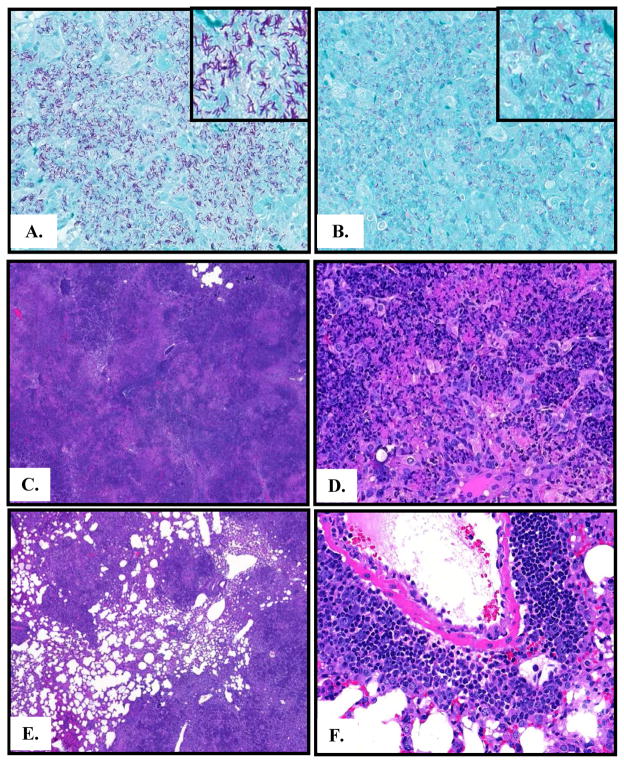
Lung tissue was performed by the Zhiel-Neelsen method (Figure 2A&B) and by hematoxylin and eosin staining (Figure 2C – F). Control mice administered tap water had numerous acid-fast bacilli in the lung at three weeks post-challenge. However, mice treated with lactoferrin had markedly fewer organisms visible on acid-fast staining in regions with comparable histopathology. Control mice given tap water have severe inflammation with nearly complete lung occlusion and large areas of pulmonary edema (Table 1 and Figure 2C). High power images demonstrate a predominance of macrophages, cellular debris, and tissue beginning to undergo necrosis (Figure 2D). Few areas of lymphocytes were observed, with relatively few CD4+ and CD8+ cells visualized with IHC (Table 1).
Figure 2. Reduced lung histopathology in lactoferrin treated mice.
A. Acid-fast staining in control mice three weeks post-infection demonstrates numerous acid-fast bacilli. B. A similar region of histopathology in mice given lactoferrin at day 0 shows markedly fewer bacteria. C. Control mice demonstrate nearly complete lung occlusion, 40X. D. High power image from control mice shows large numbers of macrophages, edema, and cellular debris, 400X. E. Mice treated with lactoferrin have significantly less areas of inflammation and occlusion, 40X. F. Mice treated with lactoferrin have clusters of lymphocytes, fewer areas of foamy macrophages, and less edema, 400X; representative histopathology shown. n = 6 mice per group.
Table 1.
Lactoferrin decreases lung immunopathology during MTB infection
| Control | LF at day 0 | LF at day 7 | |
|---|---|---|---|
| % lung occlusion | 89.4 ± 4.8 | *71.6 ± 6.0 | *79.0 ± 1.8 |
| % macrophages | 79.5 ± 11.5 | *33.0 ± 16.8 | *52.3 ± 8.3 |
| % lymphocytes | 4.1 ± 5.6 | *22.6 ± 7.4 | *25.9 ± 8.1 |
| CD4+ lymphocytes | 175.3 ± 292.6 | *1425.0 ± 589.0 | *1843 ± 511.1 |
| CD8+ lymphocytes | 30.0 ± 27.3 | *379.2 ± 237.3 | *598.8 ± 488.3 |
p< 0.05 compared to control mice
In contrast, mice treated with lactoferrin had significantly reduced lung occlusion compared to control mice (Figure 4E and Table 1). There was significantly decreased overall lung inflammation, less pulmonary edema and occluded vasculature, and reduced areas of macrophages in mice started on lactoferrin at day 0 and day 7 post-infection. Clusters of lymphocytes were frequently observed (Figure 2E). The majority of the lymphocytes were CD4+ cells; abundant CD8+ cells were observed as well (Table 1).
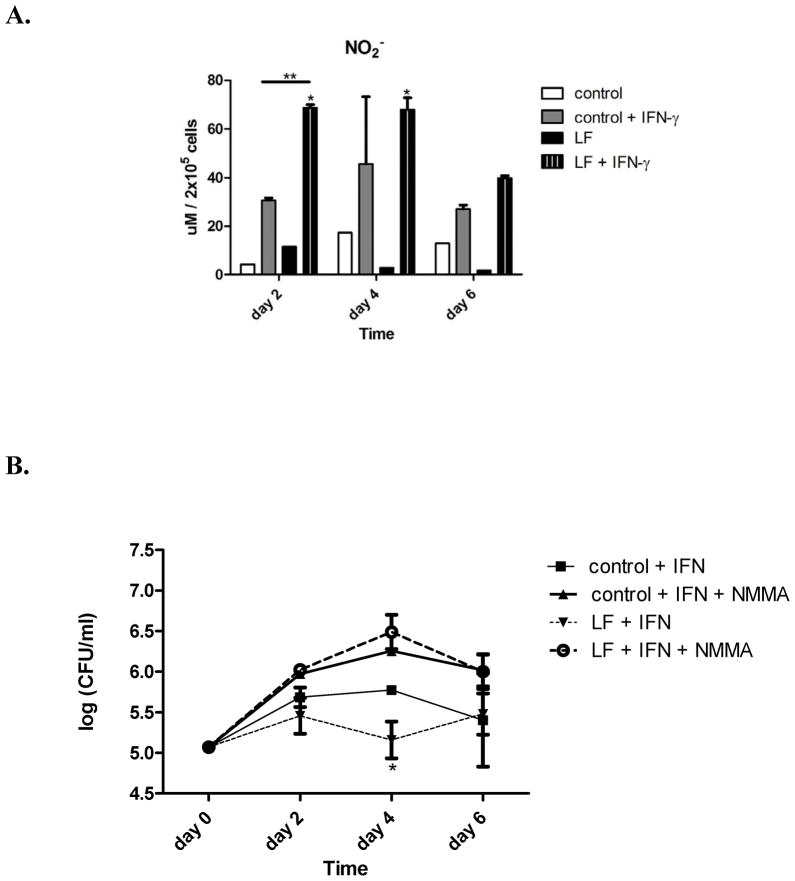
Figure 4. Lactoferrin enhances IFN- γ mediated NO production.
A. NO production was measured in the supernatants from infected J774 cells using the Griess reaction. Macrophages treated with the combination of lactoferrin and IFN-γ had markedly enhanced NO production. * p < 0.05, with comparison to control macrophages. ** p < 0.05 with comparison to control macrophages activated with IFN-γ . B Macrophages. were treated with 1mM of the nitric oxide synthase inhibitor n-monomethyl-l-arginine (NMMA) with various combinations of 100 μg/ml lactoferrin (LF) and 10 ng/ml IFN-γ .
3.3. Lactoferrin enhances IFN-γ mediated killing of MTB and modulates cytokine production in macrophage culture
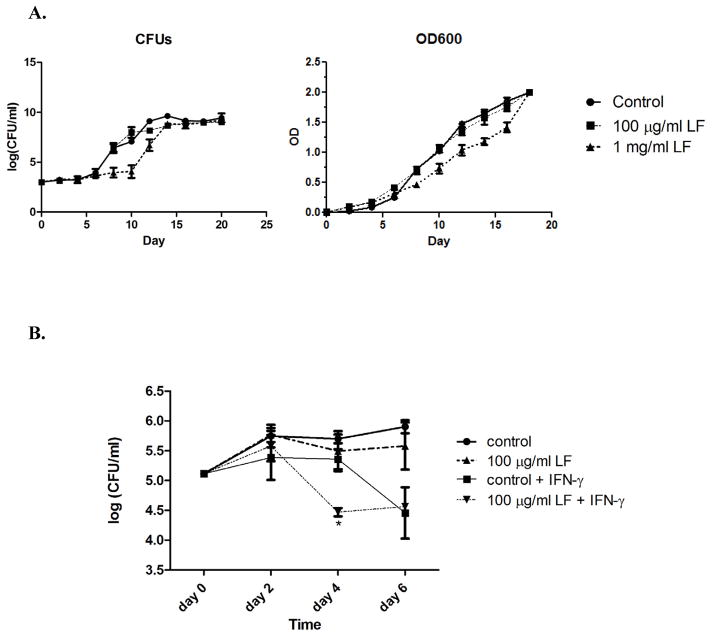
Studies were undertaken to determine if lactoferrin has a direct effect on MTB proliferation. The results of lactoferrin’s effect on MTB growth in broth culture are shown in Figure 3A. A physiologic concentration of lactoferrin (100 μg/ml) did not alter the growth of MTB in 7H9 broth using either the OD600 or CFUs. Only a very high, non-physiologic concentration (1mg/ml) had a slight inhibitory effect on MTB growth, possibly due to iron sequestration.
Figure 3. MTB proliferation in broth and macrophage culture in the presence of lactoferrin.
A. MTB were grown in 7H9 broth alone, with 100 μg/ml lactoferrin, or 1 mg/ml lactoferrin. The OD600 values and bacterial CFUs both show that physiologic concentrations of lactoferrin (100 μg/ml) do not alter MTB growth. Only very high, non-physiologic concentrations of lactoferrin have a slight impact of MTB growth. B. J774 macrophages were cultured in DMEM, infected with MTB at a MOI of 1:1, and treated with various combinations of lactoferrin and IFN- γ. Lactoferrin alone did not impact MTB proliferation in macrophages. However, there was enhanced early killing by the combination of lactoferrin and IFN-γ .
MTB proliferation with or without lactoferrin in macrophage culture was also assessed (Figure 3B). Lactoferrin alone did not affect MTB growth in naive J774 cells. However, there was enhanced early killing when lactoferrin was used in combination with IFN-γ. This effect is possibly mediated by NO, as macrophages given the combination of lactoferrin and IFN-γ produced significantly more NO compared to control and activated macrophages (Figure 4A). The addition of the NO synthetase inhibitor N-mono-methyl-arginine abolished the enhanced MTB killing effect of lactoferrin and IFN-γ (Figure 4B).
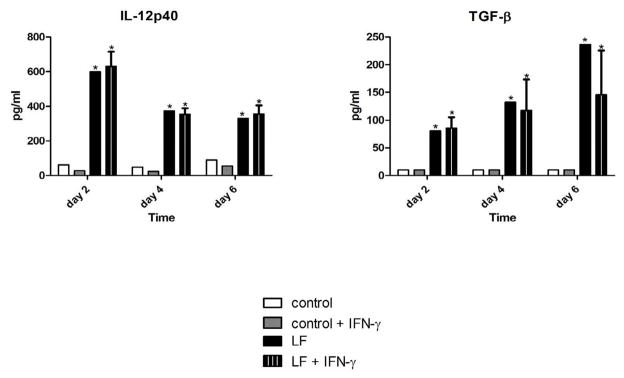
Lactoferrin modulated cytokine production from infected macrophages (Figure 5). There was significantly increased TNF-α production when lactoferrin was added to macrophages activated with IFN-γ (data not shown). Of potential importance, lactoferrin significantly increased IL-12p40 production, a cytokine of critical importance for the induction of Th1 responses 34, 35. TGF-β synthesis was also significantly increased, a cytokine that has a number of regulatory functions 36.
Figure 5. Lactoferrin modulates cytokine production by infected macrophages.
J774 cells were cultured in DMEM, infected with MTB at a MOI of 1:1, and treated with various combinations of 100 μg/ml lactoferrin and 10 ng/ml IFN-γ . Supernatants were collected at the indicated time points, filtered with a 0.2 μm filter, and analyzed for IL-12p40 and TGF-β by ELISA. * p < 0.05 with comparisons to control macrophages.
3.4. Lactoferrin modulates lung cytokine expression in MTB infected mice
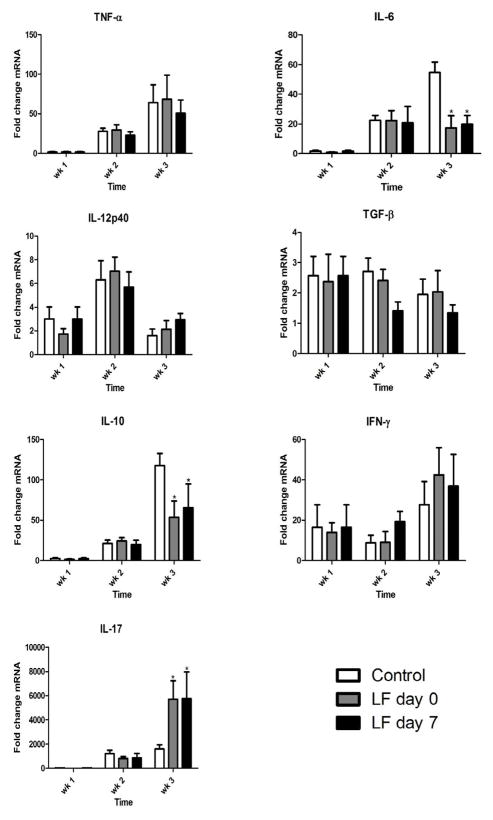
Lung cytokine expression of TNF-α, IL-6, IL-12p40, TGF-β, IL-10, IFN-γ, and IL-17 was evaluated by Taqman qPCR (Figures 6). Control mice increased expression of all cytokines over the three-week time course, with the exception of IL-12p40 expression that peaked at week 2. Lactoferrin administered both at day 0 and day 7 post-challenge significantly decreased expression of the proinflammatory cytokine IL-6. IL-10, a cytokine with inhibitory actions on cell-mediated immunity, was also decreased by lactoferrin treatment. There was a non-significant trend towards increased IFN-γ expression in the lactoferrin treated mice. IL-17 expression was significantly increased by lactoferrin treatment. There were no significant differences in lung expression of TNF-α, IL-12p40, or TGF-β between control and lactoferrin treated mice.
Figure 6. Lung expression of proinflammatory mediators in MTB-infected mice treated with lactoferrin.
Expression of TNF-α, IL-6, IL-12p40, TGF-β, IL-10, IFN-γ , and IL-17 mRNA was quantified in the lung in mice challenged with MTB and treated with or without lactoferrin. Data are expressed as fold change relative to naive mice after normalization to β-actin. Data are presented as the mean with SD, n = 6 mice per group, per time point. * p < 0.05, comparisons are made to control mice.
3.5. Increased Th1 and IL-17 producing cells in the lung and spleens of lactoferrin treated mice
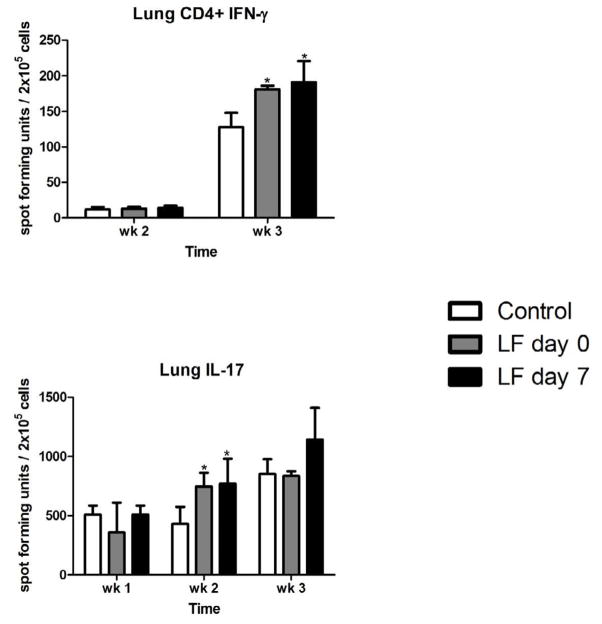
Lung and spleen homogenates were examined for CD4+ IFN-γ + and IL-17 producing cells by ELISpot analysis (Figure 7). The numbers of CD4+ IFN-γ+ cells in the lungs were increased at week 3 in all groups examined, but significantly greater in the lactoferrin treatment groups. CD4+ IFN-γ+ cells in the spleen were not significantly different between the groups (data not shown). IL-17 producing cells were also evaluated (Figure 7). The total number of IL-17+ cells increased in the lungs over the three-week observation period. There were significantly increased levels of IL-17 producing cells in the lung at week 2 in the lactoferrin treated mice. The spleen had relatively few IL-17 producing cells, with no significant differences between the groups (data not shown).
Figure 7. CD4+ IFN-γ + and IL-17 producing cells in the lung of control and lactoferrin treated mice.
Lung digests were incubated with heat-killed MTB for 48 hours. CD4+ IFN-γ + and IL-17 producing cells were enumerated by ELISpot analysis. Comparisons made to control mice, n = 4 mice per group per time point. * p < 0.05.
3.6. MTB-specific responses by splenocytes are altered by lactoferrin treatment
Splenocytes from control and lactoferrin treated mice were isolated and stimulated with heat-killed MTB for 72 hours. Supernatant TNF-α, IL-6, IL-12p40, TGF-β, IL-10, IFN-γ, and IL-17 levels were measured. Splenocytes from both lactoferrin treatment groups had significantly elevated IL-12p40 at week 2; levels were 434 pg/ml for lactoferrin given at day 0 and 462 pg/ml when given at day 7, compared to 236 pg/ml for control infected mice. There was also significant IFN-γ production at week 3; 2289 pg/ml for lactoferrin given at day 0 and 2408 pg/ml when given at day 7, compared to 1677 pg/ml for control infection splenic recall response. The mice given lactoferrin at day 7 after infection also had increased TNF-α and IL-10 at week 3 and no differences in splenocyte synthesis of IL-6, TGF-β, or IL-17 in response to heat-killed MTB (not shown).
4. Discussion
TB continues to be a major cause of morbidity and mortality due to infectious diseases. Treatment of this disease requires 6 – 9 months of anti-TB chemotherapy that is difficult to administer in the regions of the world that have the highest burden of TB cases. Thus, there is a considerable need to develop novel agents for the treatment of TB both to shorten the treatment time and combat the emergence of drug resistant organisms. Increasing evidence suggests that immune modulating therapies that target granulomas and enhance protective immune responses may be useful as adjunct therapeutics for the treatment of TB 12.
Early innate events are critical towards development of the protective niche required for the organism’s survival within the host. MTB has several molecules with innate immunostimulatory activity, making use of ligands for the toll-like receptors, C-type lectins, and NOD-like receptors (intracellular pattern recognition receptors) to trigger host events37, 38. Cytokines, such as TNF-α and induced chemokines are critical for development of the granulomatous response39, 40. Lactoferrin, a mediator of local and systemic responses during infectious assault16, 22, 41, was hypothesized to play a role in modulation of innate host responses during MTB infection. Indeed, it has shown to be effective to alter macrophage responses to both MTB and to BCG17, 20, and augment the development of MTB-specific delayed type hypersensitivity21, 42.
Lactoferrin treated mice demonstrated a decrease in lung bacterial CFUs and a reduction in bacterial dissemination to the liver. These favorable effects were evident even when the mice were treated one week post-infection, indicating that lactoferrin has the potential as a novel agent for the treatment of TB. This decrease in bacterial burden is not likely due to a direct effect of lactoferrin on MTB because physiologic concentrations of lactoferrin did not alter MTB proliferation in either broth or macrophage culture. Thus, we hypothesize that the mechanism of bacterial reduction is due to immune modulation. Indeed, mice treated with oral lactoferrin had higher numbers of Th1 cells in the lung at three weeks after infection. Lactoferrin has been shown to increase cell-mediated immune responses in a number of infectious disease models 16, 43. Enhancement of antigen presenting cell activity is one potential mechanism by which lactoferrin may promote Th1 responses. Lactoferrin increased expression of MHC II and the CD86:CD80 ratio in BCG-infected macrophages and dendritic cells, and resulted in increased production of IFN-γ from overlaid CD3+ and CD4+ cells compared to cells cultured without lactoferrin 17, 20, 44. Lactoferrin may also enhance Th1 responses by modulation of antigen presenting cell cytokine production 45. Lactoferrin was demonstrated to increase production of IL-12 in a number of studies 17, 20, 42, 44, 46, including the data presented here. IL-12 is an essential cytokine for induction of IFN-γ from naive T-cells and enhancing production from mature Th1 cells 34, 35. Furthermore, lactoferrin appeared to enhance IFN-γ mediated bacterial killing in macrophage culture; this effect is possibly due to enhancement of NO production. Other studies have reported increased NO production by lactoferrin, both in macrophage culture and in vivo 47, 48. IFN-γ induced NO synthesis by macrophages is considered a crucial antimycobacterial activity 49. Thus, we hypothesize that a major mechanism of lactoferrin’s favorable effect during MTB infection is the generation of IFN-γ producing cells; IFN-γ in turn acts synergistically with lactoferrin to enhance macrophage killing of MTB, possibly through NO production.
Lactoferrin increased IL-17 producing cells in the lung in addition to enhancement of IFN-γ mediated responses. To our knowledge, this is the first report of lactoferrin modulation of IL-17 responses. Several studies suggest that IL-17 synthesizing cells may play an important role in MTB host defense 6–8. Early enhancement of IL-17 responses by use of an IL-23 producing adenovirus during MTB infection resulted in decreased bacterial burden and reduced lung pathology 9. Thus, an early increase in IL-17 responses in the lactoferrin treated mice may have contributed to the reduction in bacterial CFUs and lung histopathology. The mechanisms by which IL-17 may be protective during MTB challenge include augmentation of bacterial killing by enhancement of IFN-γ responses that activate macrophages, direct stimulation of phagocytic cells, recruitment of neutrophils, and increasing expression of antimicrobial peptides that have activity against MTB 9, 50.
Mice treated with lactoferrin had a marked reduction in lung immunopathology in addition to a decrease in bacterial CFUs. Modulation of MTB-induced inflammatory pathology has been proposed as a mechanism to decrease the treatment time for TB 12, an approach successfully used in animal studies and human clinical trials. For example, the TNF-α reducer thalidomide used in combination with anti-TB antibiotics reduced mortality, brain pathology, and leukocytosis in a rabbit model of TB meningitis compared to anti-TB chemotherapy alone 51. A clinical trial exploring the combination of etanercept, a soluble TNF-α receptor, with TB antibiotics in patients co-infected with HIV and TB reported increased bacterial clearance and improved chest-rays with the combination therapy 52. A second clinical trial in individuals with HIV and TB demonstrated a higher rate of sputum culture conversion when high dose prednisolone was used as the immunomodulator in conjunction with TB antimicrobial chemotherapy 53. In addition to an overall reduction in lung pathology, lactoferrin-treated mice had a decreased percentage of macrophages, an increased percentage of lymphocytes, and increased numbers of CD4+ and CD8+ cells, suggesting an increase in immune cells with protective effects during MTB infection.
In light of the increased emergence of drug resistant organisms and the increasing incidence of TB, it is essential to develop new agents for the treatment of TB. It is noteworthy that lactoferrin reduced bacterial burden, accompanied by an increase in certain proinflammatory responses while decreasing overall lung immunopathology. Lactoferrin has a number of advantages over the current immunomodulators in use because it does not suppress the immune system and has a proven safety record in a number of animal models and human clinical trials 54–57. These investigations indicate that lactoferrin has potential as a novel therapeutic for the treatment of TB.
Acknowledgments
This work was supported in part by NIH grants 1R41GM079810-03A and R42-AI051050-05. This work was presented in part at the Texas Tuberculosis Research Symposium (TTRS) 2011, Galveston, TX, sponsored by the University of Texas Medical Branch (UTMB Health), Galveston, TX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. WHO report 2009. Global tuberculosis control. Epidemiology, strategy, financing. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.CDC. Treatment of tuberculosis. MMWR. 2003;52:1–77. [PubMed] [Google Scholar]
- 3.Cynamon MH. Chemotherapeutic agents for mycobacterial infections. In: Friedman LN, editor. Tuberculosis: current concepts and treatment. New York: CRC Press; 2001. pp. 301–30. [Google Scholar]
- 4.Co DO, Hogan LH, Kim SI, Sandor M. Mycobacterial granulomas: keys to a long-lasting host-pathogen relationship. Clin Immunol. 2004;113:130–6. doi: 10.1016/j.clim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Rao A, Avni O. Molecular aspects of T-cell differentiation. Br Med Bull. 2000;56:969–84. doi: 10.1258/0007142001903634. [DOI] [PubMed] [Google Scholar]
- 6.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–22. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 8.Wozniak TM, Ryan AA, Britton WJ. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006;177:8684–92. doi: 10.4049/jimmunol.177.12.8684. [DOI] [PubMed] [Google Scholar]
- 9.Happel KI, Lockhart EA, Mason CM, Porretta E, Keoshkerian E, Odden AR, Nelson S, Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–8. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–60. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell Microbiol. 2010;12:301–9. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 13.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82. ix. doi: 10.1016/j.ccm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–63. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Roy E, Lowrie DB, Jolles SR. Current strategies in TB immunotherapy. Curr Mol Med. 2007;7:373–86. doi: 10.2174/156652407780831557. [DOI] [PubMed] [Google Scholar]
- 16.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–73. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol. 2009;21:1185–97. doi: 10.1093/intimm/dxp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SA, Arora R, Kruzel ML, Actor JK. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c) Tuberculosis (Edinb) 2009;89 (Suppl 1):S49–54. doi: 10.1016/S1472-9792(09)70012-5. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5:591–9. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009;91:76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25:6730–43. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24:33–44. doi: 10.1023/a:1006935908960. [DOI] [PubMed] [Google Scholar]
- 23.Welsh KJ, Hwang SA, Hunter RL, Kruzel ML, Actor JK. Lactoferrin modulation of mycobacterial cord factor trehalose 6-6'-dimycolate induced granulomatous response. Transl Res. 2010;156:207–15. doi: 10.1016/j.trsl.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–34. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Healey MC. The immunosuppressive effects of dexamethazone adminstered in drinking water to C57BL/6 mice infected with Cryptosporidium parvum. J Parasitol. 1993;79:626–30. [PubMed] [Google Scholar]
- 26.Artym J, Zimecki M, Kruzel ML. Reconstitution of the cellular immune response by lactoferrin in cyclophosphamide-treated mice is correlated with renewal of T cell compartment. Immunobiology. 2003;207:197–205. doi: 10.1078/0171-2985-00233. [DOI] [PubMed] [Google Scholar]
- 27.Artym J, Zimecki M, Kruzel ML. Effect of lactoferrin on the methotrexate-induced suppression of the cellular and humoral immune response in mice. Anticancer Res. 2004;24:3831–6. [PubMed] [Google Scholar]
- 28.Abbott AN, Guidry TV, Welsh KJ, Thomas AM, Kling MA, Hunter RL, Actor JK. 11beta-hydroxysteroid dehydrogenases are regulated during the pulmonary granulomatous response to the mycobacterial glycolipid trehalose-6,6'-dimycolate. Neuroimmunomodulation. 2009;16:147–54. doi: 10.1159/000204227. [DOI] [PubMed] [Google Scholar]
- 29.Guidry TV, Hunter RL, Jr, Actor JK. Mycobacterial glycolipid trehalose 6,6'-dimycolate-induced hypersensitive granulomas: contribution of CD4+ lymphocytes. Microbiology. 2007;153:3360–9. doi: 10.1099/mic.0.2007/010850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–40. [PubMed] [Google Scholar]
- 31.Welsh KJ, Abbott AN, Hwang SA, Indrigo J, Armitige LY, Blackburn MR, Hunter RL, Jr, Actor JK. A role for tumour necrosis factor-alpha, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6'-dimycolate induced granulomatous response. Microbiology. 2008;154:1813–24. doi: 10.1099/mic.0.2008/016923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Carranza C, Juarez E, Torres M, Ellner JJ, Sada E, Schwander SK. Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contacts. Am J Respir Crit Care Med. 2006;173:238–45. doi: 10.1164/rccm.200503-411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–31. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–88. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. 2009;206:2865–8. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–11. [PubMed] [Google Scholar]
- 40.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–7. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 41.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 2007;61:261–7. [PubMed] [Google Scholar]
- 42.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–86. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi K, Wakabayashi H, Shin K, Takase M. Bovine lactoferrin: benefits and mechanism of action against infections. Biochem Cell Biol. 2006;84:291–6. doi: 10.1139/o06-054. [DOI] [PubMed] [Google Scholar]
- 44.Wilk KM, Hwang SA, Actor JK. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postepy Hig Med Dosw (Online) 2007;61:277–82. [PMC free article] [PubMed] [Google Scholar]
- 45.Takakura N, Wakabayashi H, Yamauchi K, Takase M. Influences of orally administered lactoferrin on IFN-gamma and IL-10 production by intestinal intraepithelial lymphocytes and mesenteric lymph-node cells. Biochem Cell Biol. 2006;84:363–8. doi: 10.1139/o06-056. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi H, Takakura N, Yamauchi K, Tamura Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin Vaccine Immunol. 2006;13:239–45. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med. 2002;196:1507–13. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem Mol Biol Int. 1997;43:79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 49.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsenova L, Sokol K, Freedman VH, Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis. 1998;177:1563–72. doi: 10.1086/515327. [DOI] [PubMed] [Google Scholar]
- 52.Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, Ducar C, Millard M, Mayanja-Kizza H, Whalen C, Okwera A. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1–associated tuberculosis. AIDS. 2004;18:257–64. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 53.Mayanja-Kizza H, Jones-Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, Whalen CC. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis. 2005;191:856–65. doi: 10.1086/427995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzoni P, Decembrino L, Stolfi I, Pugni L, Rinaldi M, Cattani S, Romeo MG, Messner H, Laforgia N, Vagnarelli F, Memo L, Bordignon L, Saia OS, Maule M, Gallo E, Mostert M, Magnani C, Quercia M, Bollani L, Pedicino R, Renzullo L, Betta P, Ferrari F, Magaldi R, Mosca F, Stronati M, Farina D. Lactoferrin and prevention of late-onset sepsis in the pre-term neonates. Early Hum Dev. 2010;86 (Suppl 1):59–61. doi: 10.1016/j.earlhumdev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, Stolfi I, Decembrino L, Laforgia N, Vagnarelli F, Memo L, Bordignon L, Saia OS, Maule M, Gallo E, Mostert M, Magnani C, Quercia M, Bollani L, Pedicino R, Renzullo L, Betta P, Mosca F, Ferrari F, Magaldi R, Stronati M, Farina D. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 56.Tamano S, Sekine K, Takase M, Yamauchi K, Iigo M, Tsuda H. Lack of chronic oral toxicity of chemopreventive bovine lactoferrin in F344/DuCrj rats. Asian Pac J Cancer Prev. 2008;9:313–6. [PubMed] [Google Scholar]
- 57.Ueno H, Sato T, Yamamoto S, Tanaka K, Ohkawa S, Takagi H, Yokosuka O, Furuse J, Saito H, Sawaki A, Kasugai H, Osaki Y, Fujiyama S, Sato K, Wakabayashi K, Okusaka T. Randomized, double-blind, placebo-controlled trial of bovine lactoferrin in patients with chronic hepatitis C. Cancer Sci. 2006;97:1105–10. doi: 10.1111/j.1349-7006.2006.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]