Abstract
There is currently an unmet need for the development of small-molecule therapeutics for norovirus infection. The piperazine scaffold, a privileged structure embodied in many pharmacological agents, was used to synthesize an array of structurally-diverse derivatives which were screened for anti-norovius activity in a cell-based replicon system. The studies described herein demonstrate for the first time that functionalized piperazine derivatives possess anti-norovirus activity. Furthermore, these studies have led to the identification of two promising compounds (6a, 9l) that can be used as a launching pad for the optimization of potency, cytotoxicity, and drug-like characteristics.
Keywords: norovirus, inhibition, piperazine derivatives
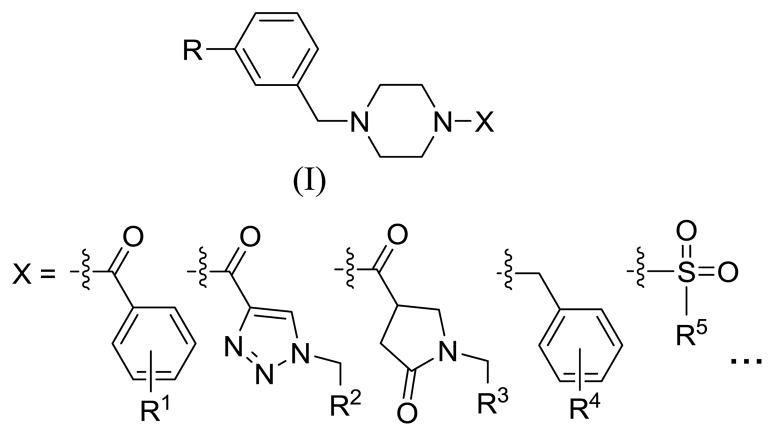
Noroviruses belong to the Norovirus genus of the Caliciviridae family and are the most common cause of acute viral gastroenteritis in the U.S. and worldwide.1–3 Noroviruses are highly contagious and outbreaks in cruise ships, schools, hospitals and nursing homes, are associated with significant morbidity and mortality. Although infection by noroviruses is generally self-limiting, the disease constitutes an important health problem and a potential bioterrorism threat because of its highly contagious nature and morbidity. The problem is further compounded by a dearth of small-molecule therapeutics or vaccines. Indeed, only a limited number of studies aimed at the discovery of therapeutics for norovirus infection have been reported in the literature.4–6 We have recently described the inhibition of noroviruses by cyclosulfamide derivatives and have used a scaffold hopping strategy to identify additional series of compounds with anti-norovirus activity.7–9 During the course of those studies, a cyclosulfamide-based piperazine hit was identified that exhibited noteworthy anti-norovirus activity. The piperazine scaffold is a privileged structure10–12 capable of binding to multiple receptors with high affinity. It is a recurring structural motif in a large number of biologically active molecules.13 Based on the forgoing, we hypothesized that functionalized piperazine derivatives may exhibit anti-norovirus activity. To explore this hypothesis, small, focused libraries of piperazine derivatives were synthesized and screened for anti-norovirus activity using a replicon-based system. We describe herein the results of synthetic and biochemical studies related to the discovery of piperazine derivatives (structure (I), Figure 1) as anti-norovirus agents.
Figure 1.
General structure of piperazine derivatives.
A series of structurally-diverse piperazine derivatives was synthesized in order to develop preliminary structure-activity relationship studies and to identify a hit suitable for use in a hit-to-lead optimization campaign.14–15 The anti-norovirus effects of the synthesized compounds16 were examined in NV replicon-harboring cells (HG23 cells)17–20 and the results are summarized in Table 1.
Table 1.
| Compound | ED50 (μM)a | TD50(μM) |
|---|---|---|
| 1a | 8.2 | 18.5 |
| 1b | 2.8 | 11.4 |
| 1c | > 20 | N/Db |
| 1d | > 20 | N/D |
| 1e | > 20 | N/D |
| 1f | 7.8 | 95.3 |
| 1g | > 20 | N/D |
| 1h | > 20 | N/D |
| 1i | > 20 | N/D |
| 3a | 8.5 | 62.5 |
| 3b | >20 | N/D |
| 3c | >20 | N/D |
| 3d | >20 | N/D |
| 3e | >20 | N/D |
| 6a | 7.2 | 155.4 |
| 6b | > 20 | N/D |
| 6c | > 20 | N/D |
| 6d | > 20 | N/D |
| 9a | 3.8 | 23.5 |
| 9b | 5.5 | 15.6 |
| 9c | > 20 | N/D |
| 9d | > 20 | N/D |
| 9f | > 20 | N/D |
| 9g | 4.5 | 25.4 |
| 9h | > 20 | N/D |
| 9i | 5.6 | 75.4 |
| 9j | > 20 | N/D |
| 9k | 8.5 | 31.5 |
| 9l | 6.7 | 121.2 |
| 9m | 12.5 | 118.7 |
average of two independent experiments;
N/D: not done due to high ED50 values.
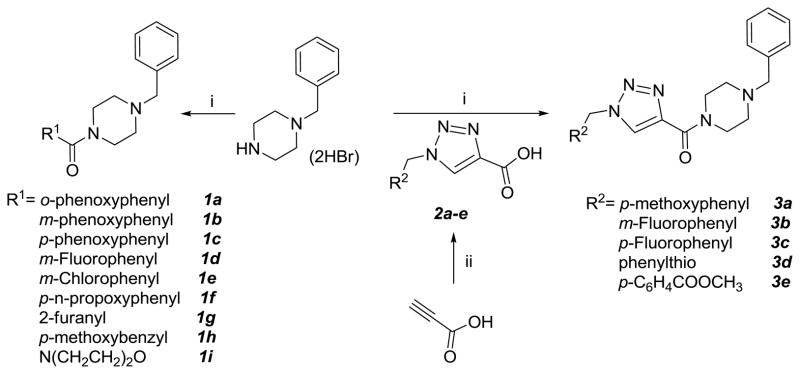
Benzyl piperazine was initially coupled to a series of carboxylic acids to generate compounds 1a-i (Scheme 1) which were subsequently screened in a cell-based replicon system. A few of the compounds had low μM anti-norovirus activity (compounds 1a-1b and 1f, Table 1), with compound 1f having a better therapeutic index than the other two compounds. Furthermore, anti-norovirus activity was found to be very sensitive to the nature of the ring substituent. These observations provided preliminary support of the hypothesis that suitably-functionalized piperazine derivatives possess anti-norovirus activity.
Scheme 1.
Reagents and reaction condictions: i) R1COOH or 2/EDCI/DMF, then 1-benzyl piperazine dihydrobromide/TEA; ii)R2CH2N3/sodium ascorbate/CuSO4/t-BuOH/H2O.
A series of substituted 1H-1,2,3-triazole-4-carboxylic acids 2a-e were then prepared using click chemistry methodology21–23 from propargylic acid and the corresponding azides. Subsequent coupling to 1-benzyl piperazine dihydrobromide gave compounds 3a-e (Scheme 1) which were found to be inactive.
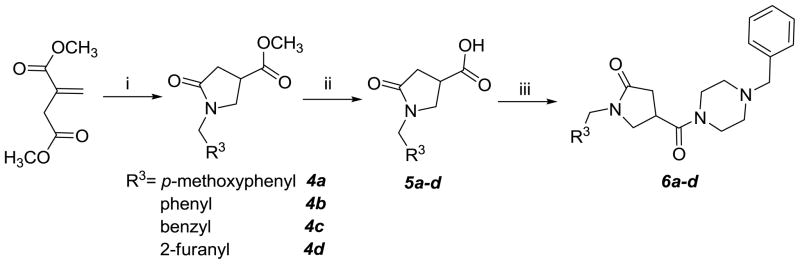
The triazole ring was then replaced by γ-lactam ring. Thus, compounds 4a-d were constructed using dimethyl itaconate and the corresponding primary amines24. Subsequent hydrolysis of 4a-d with 10% potassium hydroxide gave compounds 5a-d which were coupled to 1-benzyl piperazine to give compounds 6a-d (Scheme 2) of which the (p-methoxyphenyl) substituted derivative 6a had a therapeutic index of ~22. Thus, the replacement of the triazole ring by a γ-lactam ring was highly beneficial.
Scheme 2.
Reagents and reaction condictions: i) R3CH2NH2/CH3OH/r.t. to reflux; ii) 10% KOH/THF; iii) EDCI/DMF/1-benzyl-piperazine dihydrobromide/TEA.
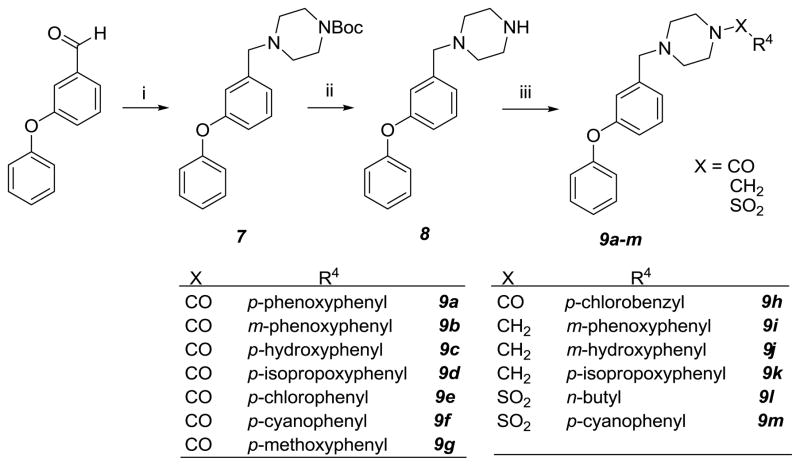
To further probe how the nature of the substituents attached to the piperazine scaffold affects anti-norovirus activity, a series of (m-phenoxy)phenyl substituted piperazine derivatives was synthesized (compounds 9a-m, Scheme 3). Reductive amination of m-phenoxybenzaldehyde and 1-Boc-piperazine, followed by removal of the Boc with trifluoroacetic acid, gave compound 8. Compound 8 was either acylated with EDCI activated carboxylic acid, or alkylated using reductive amination with substituted benzaldehyde and sodium triacetoxyborohydride, or sulfonylated with sulfonyl chloride in the presence of triethylamine to give compounds 9a-m (Scheme 3). Several derivatives were found to possess anti-norovirus activity, however, potency and toxicity were highly sensitive to structural variations. The best compound in this group, tertiary sulfonamide 9l, had a therapeutic index of 18.
Scheme 3.
Reagents and reaction conditions: i) 1-Boc-piperazine/NaHB(OAc)3/CH2Cl2; ii) TFA/CH2Cl2; iii) EDCl/R4COOH/DMF or R4CHO/NaHB(OAc)3/CH2Cl2 or R4SO2Cl/TEA/CH2Cl2.
In summary, this preliminary report demonstrates for the first time that functionalized piperazine compounds exhibit noteworthy anti-norovirus activity in a cell-based system. Two first-generation piperazine derivatives (compounds 6a and 9l) have been identified that could potentially serve as a starting point for further optimization studies in conjunction with mechanism of action studies aimed at identifying the molecular target(s) with which these compounds interact. Taken together, these results hold significant promise for the development of inhibitors directed against norovirus infection.
Supplementary Material
Acknowledgments
The generous financial support of this work by the National Institutes of Health (AI081891) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergmann RJ, Gebhardt J, Gould E, Tucker P, Unge T, Hilgenfeld R, Neyts J. Antiviral Research. 2010;87:162. doi: 10.1016/j.antiviral.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo HL, Ajami N, Atmar RL, DuPont HL. Discov Med. 2010;10:61. [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL. Food Environ Virol. 2011;2:117. doi: 10.1007/s12560-010-9038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guiard J, Fiege B, Kitov PI, Peters T, Bundle DR. Chemistry. 2011;17:7438. doi: 10.1002/chem.201003414. [DOI] [PubMed] [Google Scholar]
- 5.Rademacher C, Guiard J, Kitov PI, Fiege B, Dalton KP, Parra F, Bubdle DR, Peters T. Chemistry. 2011;17:7442. doi: 10.1002/chem.201003432. [DOI] [PubMed] [Google Scholar]
- 6.Rohayem J, Bergmann M, Gebhardt J, Gould E, Tucker P, Mattevi A, Unge T, Higenfeld R, Neyts J. Antiviral Res. 2010;87:162. doi: 10.1016/j.antiviral.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou D, Tiew KC, He G, Mandadapu SR, Aravapalli S, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2011;19:5975. doi: 10.1016/j.bmc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2011;19:5749. doi: 10.1016/j.bmc.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Eur J Med Chem. 2011 doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Comb Chem High Throughput Screen. 2004;7:473. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- 11.Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barelier S, Krimm I. Curr Opin Chem Biol. 2011;15:469. doi: 10.1016/j.cbpa.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Berkheij M, van der Sluis L, Sewing C, den Boer DJ, Terpstra JW, Hiemstra H, Iwema Bakker W, Ivan den Hoogenband A, van Maarseveen JH. Tetrahedron Lett. 2005;46:2369. [Google Scholar]
- 14.Gillespie P, Goodnow RA. Ann Rep Med Chem. 2004;39:293. [Google Scholar]
- 15.Wunberg T, Hendrix M, Hillisch A, Lobell M, Meier H, Schmeck C, Wild H, Hinzen B. Drug Discov Today. 2006;11:175. doi: 10.1016/S1359-6446(05)03700-1. [DOI] [PubMed] [Google Scholar]
- 16.All compounds were characterized by 1H NMR and HRMS and had a >95% purity.
- 17.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Virology. 2006;2:463. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Chang KO, George DW. J Virol. 2007;22:12111. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang KO. J Virol. 2009;83:8587. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y, Thapa M, Hua DH, Chang KO. Antiviral Res. 2011;89:165. doi: 10.1016/j.antiviral.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Woodward R, Kedenburg JP, Liu X, Chen M, Fang L, Sun D, Wang PG. J Med Chem. 2008;51:7417. doi: 10.1021/jm8005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour TS, Jin H. Bioorg Med Chem Lett. 1991;1:757. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.