Abstract
INTRODUCTION
Adult survivors of childhood lower-extremity sarcoma are largely physically inactive, a behavior which potentially compounds their health burden. Altering this behavior requires understanding those factors that contribute to their physical inactivity. Therefore, this investigation sought to identify factors associated with inactivity in this subpopulation of cancer survivors.
METHODS
Demographic, personal, treatment and physical activity information from adult survivors of childhood lower-extremity sarcomas was obtained from the Childhood Cancer Survivor Study (CCSS) cohort. Generalized linear models were used to identify variables that best identified those individuals who were physically inactive.
RESULTS
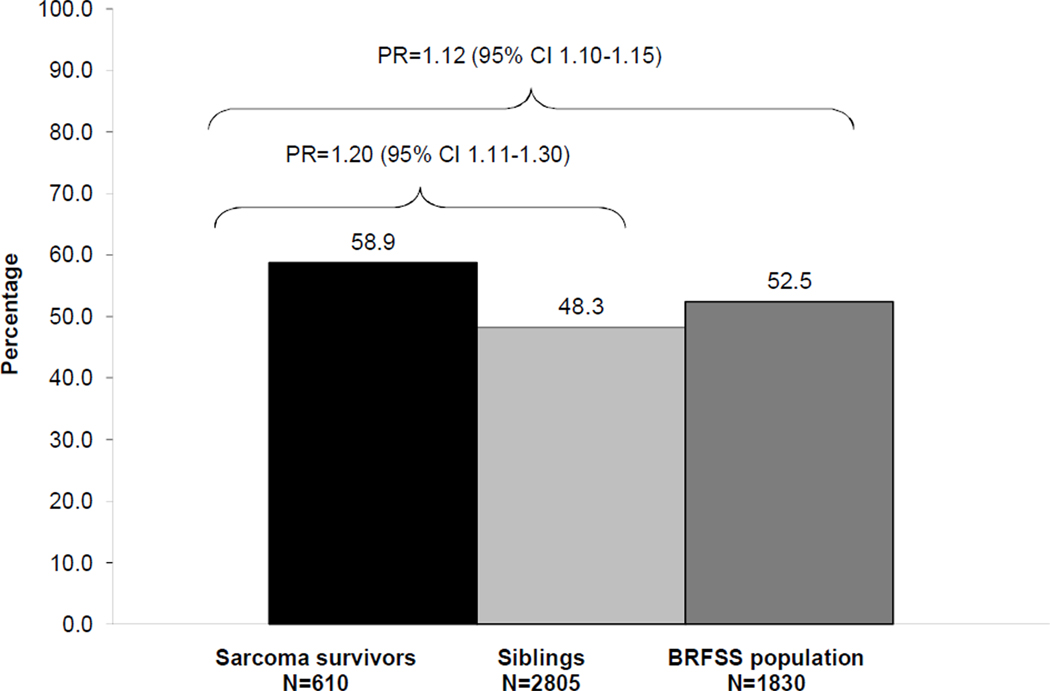
Only 41% of survivors met Center for Disease Control (CDC) activity guidelines. Survivors were 1.20 (95% CI 1.11–1.30) more likely compared to CCSS sibling cohort and 1.12 (95% CI 1.10–1.15) times more likely than the general population to fail to meet CDC guidelines. Significant predictors of physical inactivity included female sex, hemipelvectomy surgery, and platinum and vinca alkaloid chemotherapy.
CONCLUSIONS
The primary findings of this study are that survivors of childhood onset lower-extremity sarcoma are 1) highly likely to be physically inactive and 2) less likely than their siblings or the general population to regularly exercise. This study has identified treatment related risk factors associated with inactivity that will help health and wellness practitioners develop successful exercise interventions to help these survivors achieve recommended levels of physical activity for health.
IMPLICATIONS FOR CANCER SURVIVORS
These results suggest that physical activity interventions for adult survivors of childhood lower-extremity sarcomas should be sex specific and responsive to unique physical late effects experienced by these survivors.
Keywords: Childhood cancer, physical activity, exercise, late-effects, sedentary
INTRODUCTION
Significant improvements in treatment interventions and medical care have increased 5 year survival rates among children with lower extremity sarcoma to over 65 percent [1–4]. Unfortunately, even after successful treatment, survivors face an increased risk for cardiovascular, orthopedic, neurologic, and functional abnormalities, many of which are the result of either the cancer or its treatment [3, 5–11]. Emerging evidence now suggests that substantial numbers of these survivors are also physically inactive. We recently reported that over 50% of adult survivors of childhood cancers did not meet the minimum physical activity guidelines established by the Centers for Disease Control (CDC) (30 minutes of moderate intensity physical activity on five or more days of the week or 20 minutes of vigorous intensity physical activity on three or more days of the week) [12].
The proportions of sarcoma survivors in this cohort who did not meet minimum activity guidelines were some of the highest from the Childhood Cancer Survivorship Study (CCSS); for example 56.8% of women with soft tissue sarcoma, 57.4% of men with osteosarcoma and 69.0% of women with Ewing sarcoma histories reported not meeting minimum activity guidelines. [12]. Although we identified amputation and anthracycline therapy as important treatment related risk factors for inactivity, the high correlation between cancer type and treatment did not allow us to specifically characterize a phenotype of the sarcoma survivor at greatest risk for a poor activity outcome in this cohort [12]. The ability to characterize the risk profile for inactivity among lower extremity sarcoma survivors by type of amputation, and or by chemotherapeutic agent will provide clinicians and researchers with important information for both early rehabilitation referral and targeted intervention study design.
A growing body of literature demonstrates that participation in exercise programs improves the physiological and psychological status of cancer survivors across the entire spectrum of cancer care including long-term survivorship [13]. Successful programs are often dependent on an understanding of barriers faced by survivors of cancer [14–16]. The constellation of potential barriers in adult survivors of childhood sarcomas has not been reported, despite the fact that these survivors have some of the highest rates of inactivity found in cancer survivors [12]. This information is necessary for the development of programs that can successfully alter the activity patterns of these survivors. Therefore, the purpose of this study was to identify specific treatment, demographic, and personal factors that are associated with suboptimal physical activity behaviors in adult survivors of childhood lower-extremity sarcoma.
METHODS
Study population
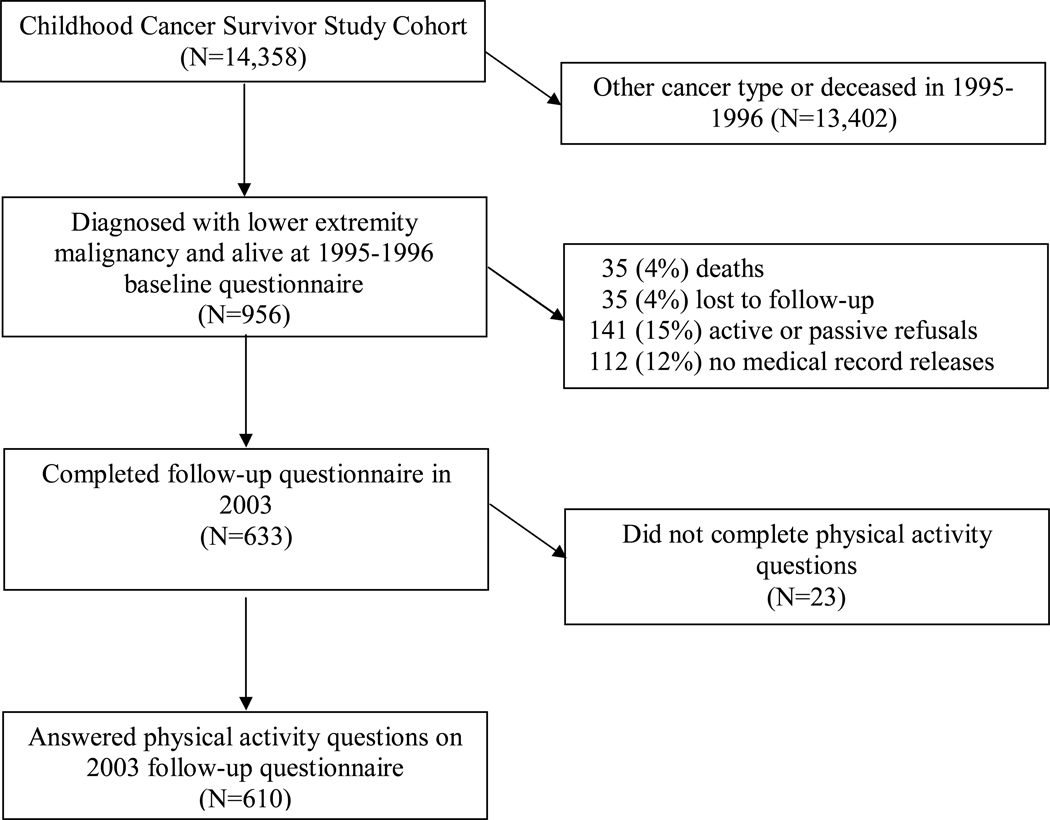
The current analysis was completed using data from survivors of lower-extremity sarcoma who were members of the Childhood Cancer Survivor Study (CCSS) cohort. The CCSS was designed to gain new knowledge about the long-tem effects of childhood cancer and treatment. It is hoped that new knowledge will provide information to help design treatment protocols and intervention strategies to increase survival and minimize harmful health effects. The study design has been described previously [17]. Briefly, this cohort includes over 14,000 childhood cancer survivors who lived for five or more years after diagnosis, were diagnosed with one of eight different malignancies between 1970 and 1986 when younger than 21 years of age, and were treated at one of 25 participating institutions in the United States and one institution in Canada. This cohort has been followed since 1994 and has been contacted approximately every two years and asked questions about their health and well-being. For the current analyses, we only included individuals who had been diagnosed with a pelvic or lower extremity (International Classification of Disease – Oncology (ICD-O) site codes C40.2, C40.3, C41.4, C41.8, C49.2, C49.5) bone or soft tissue sarcoma who were alive and completed the 2003 physical activity follow-up questionnaire, and who consented to medical record abstraction (Figure 1) [18]. A random sample of siblings of all study participants was also contacted for enrollment into the CCSS sibling cohort. Siblings completed questionnaires similar to those completed by survivors at each time point. Approval for study documents and questionnaires was obtained from each participating institution’s Institutional Review Board prior to contacting potentially eligible participants. Consent was obtained from study participants or their guardians at study entry and again at age of majority for participants who were younger than age 18 years at study enrollment.
Figure 1.
A description of how CCSS cohort participants were chosen for this retrospective analysis.
Outcomes
The primary outcome of interest for the current analysis was whether or not participants met the Centers for Disease Control and Prevention (CDC) Physical Activity Guidelines [19]. Participants were asked six questions from the Behavioral Risk Factor Surveillance System (BRFSS) Survey about the frequency and intensity of physical activity (Figure 2). For analysis, we calculated the average number of minutes per day and number of days per week of both moderate and vigorous intensity physical activity and constructed a binary variable to indicate whether or not individuals met CDC Physical Activity Guidelines. Those who did not report at least 30 minutes of moderate intensity physical activity on five or more days of the week, or at least 20 minutes of vigorous intensity physical activity on three or more days per week, were classified as not meeting the CDC Guidelines for Physical Activity (physical inactivity). As in our previous analysis [12], a three-to-one population-based sample was selected for comparison, frequency matched on age and gender, from individuals who answered the same six questions on the 2003 BRFSS survey [20].
Figure 2.
Behavioral Risk Factor Surveillance Survey questions answered by study participants in the Childhood Cancer Survivor Study 2003 Questionnaire.
Diagnosis and treatment
Diagnosis and treatment information was obtained from the medical records at each of the treating institutions. For these analyses, the following variables were considered for possible association with the outcome: 1) age at diagnosis; 2) type of surgery used to the lower extremity sarcoma, classified as hemipelvectomy, above the knee amputation, below the knee amputation, limb salvage, or no bone surgery; 3) lung surgery; 4) chest or lung radiation; 5) local radiation; and 6) chemotherapy types (vinca-alkaloids, anthracyclines, bleomycin, platinum). Vinca alkaloid exposure was treated as a dichotomous variable. Anthracyclines, bleomycin and platinum were evaluated as both dose tertiles and as dichotomies.
Demographic and personal variables
Demographic and personal variables were selected based on previous literature that documented physical activity correlates in both healthy populations [21.22], and in persons with mobility limitations [23], and were classified according to participants’ answers to questions on both the initial baseline questionnaire and the 2003 follow-up questionnaire. Sex, race/ethnicity (white, black, Hispanic, other), obesity status in 2003 (body mass index (BMI) ≥ 30 kilograms per meter squared after accounting for the loss of all or a portion of a limb) [24], smoking status in 2003 (yes or no), and current age were evaluated along with treatment variables as predictors of physical inactivity. A history of depressive symptoms [25], measured at baseline (1995–1996), current pain and or anxiety as a result of the cancer, and annual household income were also considered as potential predictors inactivity. Participants with T-scores of 63 or higher on the Brief Symptom Inventory–18 (BSI-18) were classified as depressed [26]. Cancer pain and cancer anxiety were dichotomized as none/mild or moderate/more than moderate. Annual household income was dichotomized as less than $40,000 per year or $40,000 or more per year.
Statistical methods
Descriptive statistics were calculated for the diagnosis, treatment, demographic, and personal factors and are reported as medians and ranges and frequencies and percents. The distribution of each of these variables was normal; however medians and ranges were reported to give the reader a sense of the middle and extremes of these variables. The percentages of sarcoma survivors, siblings and population based comparison group members who were physically inactive were calculated and compared using generalized linear models with a binomial distribution and a log link to obtain age adjusted prevalence ratios (PR) and 95% confidence intervals (95% CI). Generalized estimating equations with robust variance estimates were used to account for intra-family correlation between siblings [27]. The percentage of sarcoma survivors who were physically inactive was compared by treatment, demographic and personal factors variables. Generalized linear models were used to evaluate predictors of physical inactivity. These models, with a binomial distribution with a log link, were used to estimate PRs and associated 95% CI [27]. A copy macro was employed to account for model restrictions on the parameter space [28]. Final models include variables whose univariate associations had p-values of < 0.25. However, the final model was adjusted for age, obesity, and smoking status, regardless of p-values. SAS version 9.1 (Cary, N.C.) was used for all analyses.
RESULTS
Study participants
Of the 956 CCSS survivor participants diagnosed with soft tissue sarcoma, osteosarcoma, Ewing sarcoma, or other bone tumor of the lower extremity or pelvis, 610 were eligible for the current analysis by completing both the 2003 questionnaire and questions about physical activity (Figure 1). Among the 956 members of the baseline cohort, 68.9% of females and 62.3% of males (p=0.03) completed the 2003 questionnaire. The follow up questionnaire was also completed more frequently by those who reported race/ethnicity as white (68%) versus those who reported their race as non-white (50%) (p < 0.001). The analysis cohort’s median age at diagnosis was 14 years (range 0–20); and 37 years (range 19–53) when reporting physical activity. Their median time since diagnosis was 23 years (range 16–34), and just over half (50.3%) were male. Members of the sibling comparison group were a median age of 34 years (range 18–58) when reporting physical activity, and included 1,298 males (46.3%) and 1,507 (53.7%) females.
Additional characteristics of the study population are shown in Table 1. Most reported their race/ethnicity as white, and over half had a primary diagnosis of osteosarcoma. Over half of the members of this cohort were treated with amputation; a minority were treated with radiation, more than half had chemotherapy that included a vinca alkaloid, and over 70% were treated with an anthracycline. Less than 25% received bleomycin or platinum as part of their treatment regimen. Annual household income less than $40,000 per year, cancer related pain, and obesity were prevalent among approximately one/fourth of participants. Of the cancer survivors 14.1% reported experiencing cancer related anxiety and 16.4% reported smoking. Of the 610 participants with complete physical activity data, 49 were missing exposure information in household income, persistent cancer pain, anxiety, depression, smoking status, or BMI (Table 1). Comparisons between participants included in the model, and participants with missing physical activity or exposure data revealed no differences by sex, race, age at diagnosis, age at evaluation, diagnosis group, radiation exposure, surgical intervention, anthracycline exposure or vincristine exposure. Platinum exposure was higher among those with missing data (38.8% vs. 20.4%, p < 0.001).
Table 1.
Characteristics of the survivor population
| N | % | |
|---|---|---|
| Sex | ||
| Female | 303 | 49·7 |
| Male | 307 | 50·3 |
| Race/ethnicity | ||
| White | 543 | 89·0 |
| Black | 22 | 3·6 |
| Hispanic | 24 | 3·9 |
| Other/unknown | 21 | 3·4 |
| Diagnosis | ||
| Soft tissue sarcoma | 145 | 23·8 |
| Ewing sarcoma | 103 | 16·9 |
| Osteosarcoma | 346 | 56·7 |
| Other bone tumors | 16 | 2·6 |
| Lower extremity surgery | ||
| Hemipelvectomy | 15 | 2·5 |
| Transfemoral amputation | 264 | 43·3 |
| Transtibial amputation | 36 | 5·9 |
| Limb sparing surgery | 214 | 35·1 |
| No bone surgery | 81 | 13·3 |
| Lung surgery (excluding biopsy) | ||
| No | 519 | 85·1 |
| Yes | 91 | 14·9 |
| Chest or lung radiation | ||
| No | 574 | 94·1 |
| Yes | 36 | 5·9 |
| Local radiation | ||
| No | 455 | 74·6 |
| Yes | 155 | 25·4 |
| Vinca alkaloid | ||
| No | 257 | 42·1 |
| Yes | 353 | 57·9 |
| Anthracycline | ||
| No | 174 | 28·5 |
| Yes | 436 | 71·5 |
| Bleomycin | ||
| No | 474 | 77·7 |
| Yes | 136 | 22·3 |
| Platinum | ||
| No | 477 | 78·2 |
| Yes | 133 | 21·8 |
| Annual household income at follow-up | ||
| <$40,000 | 145 | 23·8 |
| $40,000+ | 439 | 72·0 |
| Not indicated | 26 | 4·3 |
| Persistent cancer related pain at follow-up | ||
| None or mild | 452 | 74·1 |
| Moderate or greater | 156 | 25·6 |
| Not indicated | 2 | 0·3 |
| Anxiety as a result of cancer at follow-up | ||
| None or mild | 521 | 85·4 |
| Moderate or greater | 86 | 14·1 |
| Not indicated | 3 | 0·5 |
| Depression at baseline | ||
| T-score < 63 on Brief Symptom Inventory | 558 | 91·5 |
| T-score 63+ on Brief Symptom Inventory | 46 | 7·5 |
| Not indicated | 6 | 1·0 |
| Current smoker at follow-up | ||
| Yes | 100 | 16·4 |
| No | 508 | 83·3 |
| Not indicated | 2 | 0·3 |
| Body mass index at follow-up | ||
| < 30 kilograms per meter squared | 457 | 74·9 |
| 30+ kilograms per meter squared | 135 | 22·1 |
| Not indicated | 18 | 3·0 |
Participation in physical activity
The percentage of survivors, siblings, and population comparison group members who were physically inactive are shown in Figure 3. After adjusting for age, sex, and intrafamily correlation, sarcoma survivors were 1.20 (95% CI 1.11–1.30) times more likely than siblings and 1.12 (95% CI 1.10–1.15) times more likely than the general population to be physically inactive.
Figure 3.
Percentage of sarcoma survivors, siblings and members of the general population who completed the 2003 Behavioral Risk Factor Surveillance System (BRFSS) survey who did not meet the Centers for Disease Control and Prevention physical activity guidelines. Prevalence ratios (PR) and 95% confidence intervals (CI) were adjusted for age, sex, and in the sibling comparison adjusted for intrafamily correlation.
Risk factors for inactivity
The percentages of survivors who were physically inactive are shown in Table 2 by demographic, treatment status and personal variables. Treatments involving radiation, bleomycin, anthracycline, and lung surgery and self reported cancer related pain or anxiety were not predictors of physical activity nor did they improve the model fits and thus, were not included in the final models. Chemotherapy was not associated with level of physical activity in a dose-dependent manner, so platinum and vinca alkaloids were included as dichotomous variables. In adjusted models, female sex, having undergone a hemipelvectomy, treatment with platinum, and treatment with vinca-alkaloids increased the risk for inactivity. We found no associations between smoking status, obesity, depression or income and whether or not a survivor was physically inactive.
Table 2.
Number of sarcoma survivors who were physically inactive by demographic, treatment, and personal variables adjusted for age, with prevalence ratios and 95 percent confidence intervals·
| Did not meet CDC Guidelines for Physical Activity (Physically inactive) (N=359) |
|||||
|---|---|---|---|---|---|
| No· | N | Row % | PR* | 95% CI | |
| Sex | |||||
| Female | 303 | 191 | 63·0 | 1·15 | 1·01–1·32 |
| Male (referent) | 307 | 168 | 54·7 | 1·00 | |
| Surgery for primary disease | |||||
| Hemipelvectomy | 15 | 13 | 86·7 | 1·43 | 1·05–1·84 |
| Above Knee Amputation | 264 | 168 | 63·6 | 1·04 | 0·86–1·31 |
| Below Knee Amputation | 36 | 21 | 58·3 | 1·04 | 0·70–1·44 |
| Limb Sparing Surgery | 214 | 110 | 51·4 | 0·90 | 0·72–1·15 |
| No Bone Surgery (referent) | 81 | 47 | 58·0 | 1·00 | |
| Platinum included in chemotherapy | |||||
| Yes | 133 | 88 | 66·2 | 1·27 | 1·07–1·48 |
| No (referent) | 477 | 271 | 56·8 | 1·00 | |
| Vinca-alkaloid included in chemotherapy | |||||
| Yes | 353 | 218 | 61·8 | 1·14 | 0·99–1·31 |
| No (referent) | 257 | 141 | 54·9 | 1·00 | |
| Current Smoker | |||||
| Yes | 100 | 64 | 64·0 | 1·10 | 0·91–1·27 |
| No (referent) | 508 | 294 | 57·9 | 1·00 | |
| Not indicated# | 2 | 1 | 50·0 | NE | |
| BMI 30+ kg/m2 | |||||
| Yes | 135 | 87 | 64·4 | 1·13 | 0·98–1·31 |
| No (referent) | 457 | 272 | 57·3 | 1·00 | |
| Not indicated# | 18 | 9 | 50·0 | NE | |
| Depression | |||||
| Yes | 46 | 30 | 65·2 | 1·07 | 0·82–1·28 |
| No (referent) | 558 | 326 | 58·4 | 1·00 | |
| Not indicated# | 6 | 3 | 50·0 | NE | |
| Household income | |||||
| <$40,000 per year | 145 | 89 | 61·4 | 1·0 | 0·87–1·17 |
| $40,000+ per year (referent) | 439 | 255 | 58·1 | 1·0 | |
| Not indicated# | 26 | 15 | 57·7 | NE | |
Adjusted for age,
Not included in estimate
CDC=Centers for Disease control and Prevention, N=number, No·=total number, %=percent, PR=prevalence ratio, 95% CI=95 percent confidence interval, NE=not estimated, BMI=body mass index, kg/m2=kilograms per meter squared
DISCUSSION
While we have previously described that adult sarcoma survivors are less active than their siblings [12], our current analysis allowed us to 1) describe their activity patterns compared to the general population and 2) specifically characterize a phenotype of the sarcoma survivor at greatest risk for a poor activity outcome in this cohort. The prevalence of inactivity in our study (58.9%) is higher than the 50.1% among individuals with impaired mobility status and lower than the 75% among individuals with diabetes reported by investigators who used data from the National Health Interview Survey [29,30]. In our analysis, being female, having a hemipelvectomy, and having received platinum or vinca alkaloid chemotherapy were associated with increased risk for physical inactivity. Demographic and personal factors (smoking, obesity depression, cancer pain, anxiety, or low income) were not associated with inactivity in our analysis.
Some treatment factors were predictors of poor activity behaviors. An important finding in this study is that there is an association between two classes of chemotherapeutic agents, vinca alkaloids and platinums, and physical inactivity. Both vinca alkaloids and platinum chemotherapeutic agents are known for their neurotoxic impact on the peripheral nervous system. The resulting chemotherapy-induced peripheral neuropathy (CIPN) has been reported in patients during and after treatment for many childhood cancers, including adolescents and young adults treated for sarcomas [8]. Peripheral neuropathies typically present during treatment, and although they may diminish over time, such deficits are likely to persist in many survivors [31–33]. In adolescents and young adults previously treated for sarcoma, evidence of persistent symptoms of neurotoxicity from cisplatin exposure have been reported, including decreased deep tendon reflexes and increased vibration sensation thresholds [8]. Persistent neuropathy in lower extremities may account for the balance deficits and decreased levels of activity that have been reported in survivors of childhood cancer [34–35].
The fact that besides female sex, we found no associations between demographic and personal factors and inactivity in our analysis is interesting and important. In this population, it appears that interventions targeted specifically at females and treatment related outcomes, like managing exercise with a missing or prosthetic limb, or when less than optimal sensation or motor function is available, may be more important for this population than other factors like lower household income, depression, and obesity. Sarcoma survivors may benefit from working with skilled providers who are knowledgeable and who have experience with prosthetics and movement retraining, such as physical therapists, to help them optimize physical activity levels among lower extremity sarcoma survivors before they can reintegrate into community wellness and exercise programs.
There are several limitations to this study. First, our analysis was limited to the data provided by the CCSS questionnaires and medical record abstractions. Differential participation in the 2003 questionnaire by sex and race were present. If females or those who were non-white were more likely to be inactive completed the questionnaire, our estimates of the prevalence of inactivity are inflated. Conversely, if those more likely to be inactive avoided completing the questionnaire, our estimates of the prevalence of inactivity are too low. Because our analyses were focused on discovery of personal and treatment related risks for inactivity, we did not evaluate comorbid conditions, for example, diabetes and cardiac disease, also likely to be associated with poor activity outcomes. In addition, there are potentially additional psychosocial predictors of inactivity, such as self efficacy, self motivation, or social support [22], that were not part of the data set. Also, the study relied on self-report of physical activity. While self-report methods have been criticized, Strath et al have reported an 80% agreement between objective and self-report measures of meeting moderate and vigorous activity guidelines [36]. Finally, these data describe individual characteristics of individuals who are at greater risk of physical inactivity but they do not provide information about perceived barriers to participation in physical activity. Such information would provide additional insights into the design of successful interventions for this population of survivors.
In summary, childhood lower-extremity sarcoma survivors are at risk for being physically inactive. This study has identified sex and treatment factors associated with these behaviors including being female, hemipelvectomy surgery, and treatment with platinum, or vinca-alkaloid chemotherapy. Interventions tailored to address these identified factors need to be developed and should consider the new CDC guidelines for children which are even more rigorous (60 minutes 7 days a week of moderate to vigorous activity - adults 30 minutes 5 times per week in addition to 2 days a week strengthening exercises) than the guidelines we used to identify outcomes in this analysis [37]. In addition, physical activity programs should address the unique needs of lower extremity sarcoma survivors such as poor balance, peripheral neuropathy, and prosthetic or missing limbs. We hypothesize that programs designed and implemented specifically for this population of cancer survivors will be more likely to meet with success, less frustrating, and more successful in sustaining changes in physical activity levels. Such interventions will become increasingly more important as medical care for sarcoma continues to improve, resulting in more adult survivors.
ACKNOWLEDGMENTS
Funding for this study was provided by the National Cancer Institute Grant No. CA 55727 (L.L. Robison, P.I.), with additional support provided to St Jude Children’s Research Hospital by the National Cancer Institute Cancer Center Support (CORE) grant CA 21765 and by the American Lebanese Syrian Associated Charities (ALSAC). We would also like to acknowledge the American Physical Therapy Association (APTA) Oncology Section for their assistance and support in the development of the manuscript.
REFERENCES
- 1.Heare T, Hensley MA, Dell'Orfano S. Bone tumors: osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr. 2009;21:365–372. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. based on November 2008 SEER data submission. [updated 2009; cited 2011 January 17]; Available from: http://seer.cancer.gov/csr/1975_2006/. [Google Scholar]
- 3.Hosalkar HS, Dormans JP. Limb sparing surgery for pediatric musculoskeletal tumors. Pediatr Blood Cancer. 2004;42:295–310. doi: 10.1002/pbc.10406. [DOI] [PubMed] [Google Scholar]
- 4.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9:422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 5.Carty CP, Dickinson IC, Watts MC, Crawford RW, Steadman P. Impairment and disability following limb salvage procedures for bone sarcoma. Knee. 2009;16:405–408. doi: 10.1016/j.knee.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, Kaste S, Meacham LR, Mahajan A, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26:3038–3045. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc Toxicol. 2007;7:122–128. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 8.Earl HM, Connolly S, Latoufis C, Eagle K, Ash CM, Fowler C, et al. Long-term neurotoxicity of chemotherapy in adolescents and young adults treated for bone and soft tissue sarcomas. Sarcoma. 1998;2:97–105. doi: 10.1080/13577149878055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaste SC, Ahn H, Liu T, Liu W, Krasin MJ, Hudson MM, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2008;50:1032–1038. doi: 10.1002/pbc.21281. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 11.Meacham LR, Chow EJ, Ness KK, Kamdar KY, Chen Y, Yasui Y, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, et al. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005;29:147–153. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- 15.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Secretariat / WHO International Association of Cancer Registries. International Classification of Diseases for Oncology. 3rd Edition. Lyon Cedex, France: World Health Organization; 2000. (ICD-O-3) [Google Scholar]
- 19.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) [accessed 2001 May 14];Behavioral Risk Factor Surveillance System Survey Questionnaire. 2003 Available from: http://www.cdc.gov/brfss/questionnaires/pdf-ques/2003brfss.pdf.
- 21.Jones DA, Ainsworth BE, Croft JB, Macera CA, Lloyd EE, Yusuf HR. Moderate leisure-time physical activity: who is meeting the public health recommendations? A national cross-sectional study. Arch Fam Med. 1998;7:285–289. doi: 10.1001/archfami.7.3.285. [DOI] [PubMed] [Google Scholar]
- 22.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Warms CA, Belza BL, Whitney JD. Correlates of physical activity in adults with mobility limitations. Fam Community Health. 2007;30:S5–S16. doi: 10.1097/01.FCH.0000264876.42945.e4. [DOI] [PubMed] [Google Scholar]
- 24.Osterkamp LK. Current perspective on assessment of human body proportions of relevance to amputees. J Am Diet Assoc. 1995;95:215–218. doi: 10.1016/S0002-8223(95)00050-X. [DOI] [PubMed] [Google Scholar]
- 25.Katon W, Richardson L, Russo J, McCarty CA, Rockhill C, McCauley E, et al. Depressive symptoms in adolescence: the association with multiple health risk behaviors. Gen Hosp Psychiatry. 2010;32:233–239. doi: 10.1016/j.genhosppsych.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derogatis LR. Brief Symptom Inventory (BSI) 18, Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson, Inc.; 2000. [Google Scholar]
- 27.McCullock C, Searle S. Generalized, Linear, and Mixed Models. Wiley, NY: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 28.Deddens JA, Petersen MR, Lei X, editors. SUGI Proceedings (Paper 270-28) Seattle, Washington: 2003. March 30 – April 2, Estimation of prevalence ratios when PROC GENMOD does not converge. [Google Scholar]
- 29.Egede LE, Poston ME. Racial/ethnic differences in leisure-time physical activity levels among individuals with diabetes. Diabetes Care. 2004;27:2493–2494. doi: 10.2337/diacare.27.10.2493. [DOI] [PubMed] [Google Scholar]
- 30.Jones G, Sinclair L. Multiple health disparities among minority adults with mobility limitations: An application of the ICF framework and codes. Disability & Rehabilitation. 2008;30:901–915. doi: 10.1080/09638280701800392. [DOI] [PubMed] [Google Scholar]
- 31.Gilchrist L, Tanner L, Hooke MC. Measuring chemotherapy-induced peripheral neuropathy in children: development of the Ped-mTNS and pilot study results. Rehab Oncol. 2009;27:7–15. [Google Scholar]
- 32.Ramchandren S, Leonard M, Mody RJ, Donohue JE, Moyer J, Hutchinson R, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14:184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinders-Messelink HA, Van Weerden TW, Fock JM, Gidding CE, Vingerhoets HM, Schoemaker MM, et al. Mild axonal neuropathy of children during treatment for acute lymphoblastic leukaemia. Eur J Paediatr Neurol. 2000;4:225–233. doi: 10.1053/ejpn.1999.0310. [DOI] [PubMed] [Google Scholar]
- 34.Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 35.Wright MJ, Galea V, Barr RD. Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Phys Ther. 2005;85:782–790. [PubMed] [Google Scholar]
- 36.Strath SJ, Bassett DR, Jr, Ham SA, Swartz AM. Assessment of physical activity by telephone interview versus objective monitoring. Med Sci Sports Exerc. 2003;35:2112–2118. doi: 10.1249/01.MSS.0000099091.38917.76. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion. Physical Activity Guidelines for Americans: Children and Adolescents. 2008 [updated 2008; cited 2011 January 17]; Available from: http://www.cdc.gov/healthyyouth/physicalactivity/guidelines.htm.