Abstract
Objective
To examine the effects of diet macronutrient composition on insulin sensitivity, fasting glucose, and β-cell response to glucose.
Materials/Methods
Participants were 42 normal glucose tolerant (NGT, fasting glucose <100 mg/dL) and 27 impaired fasting glucose (IFG) healthy, overweight/obese (BMI 32.5 ±4.2 kg/m2), men and women. For 8 weeks, participants were provided with eucaloric diets, either higher-carbohydrate/lower-fat (55% carbohydrate, 18% protein, 27% fat) or lower- carbohydrate/higher-fat (43:18:39). Insulin sensitivity and β-cell response to glucose (basal, PhiB; dynamic, PhiD; and static, PhiS) were calculated by mathematical modeling using glucose, insulin, and C-peptide data obtained during a liquid meal tolerance test.
Results
After 8 weeks, NGT on the higher-carbohydrate/lower-fat diet had higher insulin sensitivity than NGT on the lower-carbohydrate/higher fat diet; this pattern was not observed among IFG. After 8 weeks, IFG on the higher-carbohydrate/lower-fat diet had lower fasting glucose and higher PhiD than IFG on the lower-carbohydrate/higher-fat diet; this pattern was not observed among NGT. Within IFG, fasting glucose at baseline and the change in fasting glucose over the intervention were inversely associated with baseline PhiD (−0.40, P<0.05) and the change in PhiD (−0.42, P<0.05), respectively.
Conclusions
Eight weeks of a higher-carbohydrate/lower-fat diet resulted in higher insulin sensitivity in healthy NGT overweight/obese individuals, and lower fasting glucose and greater glucose-stimulated insulin secretion in individuals with IFG. If confirmed, these results may have an impact on dietary recommendations for overweight individuals with and without IFG.
Keywords: insulin secretion, impaired fasting glucose, nutrition
Introduction
Type 2 diabetes (T2D) affects 9.3% of US adults aged 20 y and older [1], and is projected to affect 366 million individuals worldwide by 2030 [2]. The disease results from impairment in insulin action and/or β-cell function. Decline in both of these processes can be detected years before T2D is manifest [3]. Identifying individuals at risk for T2D, and intervening to prevent disease progression among these individuals could potentially have a major impact on human health worldwide.
Two groups of individuals characteristically at risk for type 2 diabetes are those who are overweight/obese and those with impaired fasting glucose (IFG; glucose ≥ 100 mg/dL). Data from the Nurses’ Health Study indicated that the relative risk for women acquiring T2D within 16 yr was ~5–7 in the overweight range, and ~11–17 in the obese range [4]. Among men and women enrolled in the Framingham Offspring Study, the odds ratio for developing T2D within 7 yr was 2.35 (1.39–3.96) for overweight, and 6.41(3.85–10.65) for obesity [5]. Epidemiological data likewise have indicated that ~5% to 64% of individuals with IFG convert to T2D, depending on the population studied and the length of the follow-up period [6].
The source of the elevated risk for T2D in these individuals may derive in part from insulin resistance. Obesity is associated with impaired insulin action, and individuals with IFG are likewise characterized by relatively low insulin sensitivity [7;8]. However, it is also possible that elevated fasting glucose plays a key role in disease development. Although elevated fasting glucose is the defining characteristic of individuals with IFG, obesity also is associated with elevated glucose, albeit within the “normal range” [9]. Cross-sectional data in humans have shown a correlation between elevated fasting glucose and reduced glucose-stimulated insulin secretion, and studies in animal models have shown that experimental elevation of fasting glucose leads to a decline in glucose-stimulated insulin secretion [10–13]. Despite these observations, the cause-and-effect nature of the relationship between fasting glucose and β-cell function has not been widely evaluated in clinical studies. Data are needed to probe whether reductions in fasting glucose lead to improvement in β-cell function using direct and physiologically relevant tests and measures.
Modification of dietary macronutrient composition may reduce risk for T2D in overweight/obese and IFG individuals by improving insulin sensitivity and/or β-cell function. Based on experimental data, both fat and carbohydrate potentially can affect these processes. Administration of exogenous fatty acids results in an acute decrease in insulin sensitivity [14]. Free fatty acids also affect β-cell function through a variety of mechanisms including uncoupling protein-2 expression, endoplasmic reticulum stress, and reactive oxygen species [15]. Carbohydrate consumption increases demand on the β-cell for insulin secretion, which may lead to endoplasmic reticulum stress [16] and oxidative stress [15], both of which can result in β-cell damage and/or dysfunction. In a recent study, a lower glycemic load diet had beneficial effects on β-cell function [17]. Thus, it is reasonable to hypothesize that reductions in either dietary fat or carbohydrate could be beneficial for glucose control and β-cell function. Current literature on this topic reflects controversy concerning whether lower-fat or lower-carbohydrate diets should be recommended for individuals at risk for T2D [18].
This study was conducted to examine the effects of two eucaloric diets differing in concentration of fat and CHO on insulin sensitivity, fasting glucose, and β-cell response in healthy overweight/obese individuals, over one-third of whom demonstrated IFG.
Methods
Participants
A total of 69 men and women aged 21–50 yr were enrolled in the study. Inclusion criteria were BMI 25–45 kg/m2, weight less than 136 kg, age 21–50 yr, non-diabetic, and no weight change greater than 2.3 kg over the past 6 mo. All women were required to be premenopausal, as evidenced by regular menstrual cycles. Exclusion criteria included diagnosis of polycystic ovary syndrome, regular exercise >2 hours per week, pregnancy, current breastfeeding, any disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including oral contraceptives, cholesterol medications, and blood pressure medications), current use of tobacco, use of illegal drugs in last 6 months, history of hypoglycemic episodes, major food allergies or food dislikes, and a medical history that contra-indicated inclusion in the study. Participants were evaluated for glucose tolerance using a 2-h oral glucose tolerance test, and only those who had 2-h glucose in the normal or mildly impaired range (≤155 mg/dL) were eligible for the study. Participants were informed of the experimental design, and oral and written consent were obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Protocol
For 3 days prior to baseline testing, all participants were provided with a standardized diet calculated to be eucaloric using the Harris-Benedict formula [19] with an activity factor of 1.35 for females and 1.5 for males. Comprehensive metabolic testing (described in detail below) was conducted at baseline and after the 8-week intervention. For the first 30 participants, testing was conducted on an inpatient basis; for the remaining participants, testing was conducted on an outpatient basis. This change was necessitated due to a change in availability of inpatient services at the General Clinical Research Center (GCRC). Distribution of participants with respect to the intervention was similar before vs after this transition.
After completing baseline testing, participants were assigned to one of two diets. The two diets were designed to ensure that both glycemic load and fat content were sufficiently different (between diets) to potentially affect metabolic outcomes. At the same time, the diets were developed with practicality in mind for the ultimate translation to clinical practice. Thus, macronutrient composition was within normal ranges, and the foods selected were those we knew to be commonly consumed, popular, foods within our usual research study participant population. The two diets differed in % of energy from carbohydrate (CHO; 55 or 43%) and fat (27 or 39%), with both having 18% protein. Both diets were modified from diets previously used in our research [20]. Details regarding the carbohydrate, fiber, and fatty acid composition of the diets are provided in Table 1. Glycemic load was determined relative to glucose using Nutrition Data System for Research (NDSR) software version 2006 [Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN]. The glycemic load of the higher-CHO/lower-fat diet was ≥75 points/1000 kcal, and that of the lower-CHO/higher-fat diet was ≤45 points/1000 kcal. The glycemic load was calculated per 1000 kcal to ensure that all subjects within one diet group received a proportionate glycemic load per calorie level. The lower-CHO/higher-fat diet tended to emphasize CHO-containing foods such as whole-wheat bread, fruits, and high-fiber vegetables, whereas the higher-CHO/lower-fat diet included such foods as white bread, mashed potatoes, and rice. As a result, the lower-CHO/higher-fat diet had a higher level of dietary fiber. Protein origin was similar between the two diets. For example, both diets included milk/yogurt and eggs, but little red meat, instead emphasizing poultry. Both diets contained some pork, nuts, nut butters, and meat substitutes (vegetable protein patties). Sample menus were provided in a previous publication [21]. Diets were developed using NDSR, and were calculated to be eucaloric, as described above. For 8 weeks, participants reported to the GCRC each weekday morning where they ate breakfast and collected food for their remaining meals. On Fridays, participants picked up food for Saturday and Sunday to consume at home. Participants were asked to maintain their usual physical activity level throughout the intervention.
Table 1.
Composition of the test diets. Data are shown for two representative energy levels
| Total CHO (g) |
Total Sugar (g)1 |
Added Sugar (g)2 |
Starch (g) |
Total Fiber (g) |
Soluble Fiber (g) |
Insoluble Fiber (g) |
SFA (%) (g) |
MUFA (%) y(g) |
PUFA (%) (g) |
ω3 (g) |
Oleic acid (g) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 55:18:27 (%energy from CHO:protein:fat) | ||||||||||||
| 1800 kcal/d |
250.4 | 109.7 | 58.8 | 112.5 | 17.3 | 5.6 | 11.8 | 8.62 17 |
9.67 19 |
6.6% 13 |
0.8 6 |
18.08 |
| 2500 kcal/d |
347.8 | 164 | 94 | 144.8 | 23.2 | 6.8 | 16.5 | 7.86 23 |
9.33 28 |
8.24 22 |
1.3 2 |
24.34 |
| 43:18:39 (%energy from CHO:protein:fat) | ||||||||||||
| 1800 kcal/d |
187.2 | 77.1 | 14.9 | 76.7 | 22.2 | 6.1 | 16.1 | 11.93 22 |
14.2 26 |
11.09 23 |
1.5 4 |
26.49 |
| 2500 kcal/d |
259.3 | 117.9 | 35 | 100.3 | 29.8 | 7.7 | 22 | 12.9 kcal/d 35 |
14.48 kcal/d 40 |
9.71 kcal/d 27 |
2.1 kcal/d 6 |
37.54 |
(1800 and 2500 kcal/d).
Abbreviations; CHO=carbohydrate; SFA=saturated fatty acids; MUFA=monounsaturated fatty acids; PUFA=polyunsaturated fatty acids; ω3=omega-3 fatty acids.
The sum of glucose, fructose, galactose, sucrose, lactose, and maltose.
Sugars and syrups added to foods during food preparation or commercial food processing.
Liquid meal tolerance test
Insulin sensitivity and β-cell responsivity were determined using glucose, insulin, and C-peptide data obtained during a liquid meal tolerance test. Participants were required to fast for 12 hours prior to the test, which was performed starting at between 0700 and 0800 h. To perform the test, a flexible intravenous catheter was placed in the antecubital space of one arm. At time “zero”, a liquid meal was provided (Carnation Instant Breakfast and whole milk). The meal was calculated to provide 7 kcal/kg of body weight as 24% fat, 58.6% CHO, and 17.4% protein (mean glucose ingested was 57g; range 36 – 78g). Participants were required to consume the meal within 5 min. Blood was drawn at −15 and −5 min before initiation of meal consumption (time “zero”); every 5 min from time zero to 30 min; every 10 min from 30 to 180 min; and at 210 and 240 min. Sera were stored at −85°C.
The insulin sensitivity index (SI) was calculated using a formula based on the insulin and glucose values over the course of the meal test [22]. A higher SI index indicates greater insulin sensitivity, and is considered metabolically favorable. Glucose and C-peptide values were analyzed for measures of β-cell function [22]. Model output from this procedure includes basal (PhiB), dynamic (PhiD), and static (PhiS) β-cell response to glucose. A detailed description of these processes has been published [23]. Briefly, PhiB reflects the amount of insulin secreted for a given amount of glucose during basal (fasted) conditions. PhiB is often elevated in obesity and insulin resistance, and is associated with elevated fasting insulin. PhiD reflects the amount of insulin secreted in response to an increase in blood glucose. PhiD is somewhat analogous to first-phase insulin secretion, which is the process that is deficient among individuals who are progressing towards T2D. PhiS reflects the amount of insulin secreted for a given amount of glucose during non-fasted (above basal) conditions.
Analysis of glucose and hormones
Concentrations of glucose, insulin, and C-peptide were analyzed in the Core Laboratory of the GCRC, Nutrition Obesity Research Center and Diabetes Research and Training Center. Glucose was measured in 3 μL sera using the Glucose oxidase method (Stanbio Laboratory, Boerne, TX). This analysis had an intra-assay coefficient of variation (CV) of 1.2% and inter-assay CV of 3.1%. Insulin was assayed in 50 μL aliquots using immunofluorescence technology on a TOSOH AIA-II analyzer (TOSOH Corp., South San Francisco, CA). This analysis had an intra-assay CV of 1.5% and inter-assay CV of 4.4%. Thirteen hemolyzed samples were omitted due to low insulin values. C-peptide was assayed in 20 μL aliquots using the TOSOH analyzer (intra-assay CV of 1.7% and inter-assay CV of 2.6%).
Statistical Analysis
Descriptive statistics were calculated for all variables of interest, and compared between NGT and IFG using ANOVA. Fasting glucose, SI, and the β-cell response measures were log-10 transformed to ensure a normal distribution. P<0.05 was considered statistically significant. Phi values were compared between groups both unadjusted and adjusted for SI to examine the ability of the β-cell to compensate for insulin resistance. All analyses were performed using SAS (version 9.2; SAS Institute, Inc., Cary, NC).
In order to determine whether fasting glucose concentration was inversely associated with β-cell function in this sample, and whether the relationship differed with glucose tolerance status, Pearson correlation coefficients were generated using baseline data. Pearson correlation analysis also was used to examine the association between the change in fasting glucose and the change in PhiD over the 8-week intervention period. Analyses were conducted within the NGT and IFG subgroups.
To examine the effect of the diet intervention on fasting glucose, insulin sensitivity, and β-cell response measures, multiple linear regression analysis was used. The dependent variable in all cases was the 8-week (post-intervention) value. Independent variables were the corresponding baseline (pre-intervention) value, gender, and weight change. Despite provision of food at an energy level calculated to maintain baseline body weight, most subjects showed some change in body weight over the study period (mean change −1.64 ± 2.01 kg). Thus, weight change was included in the statistical models to account for the possible influence of energy balance. Preliminary analyses indicated that inclusion of ethnicity did not alter results, so to maximize statistical power, this term was not included. Residuals were examined for their distribution, and PhiD data from one subject were eliminated based on a studentized residual >2. Analyses were conducted within the NGT and IFG subgroups. For graphical presentation of fasting glucose, SI, and PhiD data, analysis of covariance (ANCOVA) subsequently was used to generate adjusted means and standard errors.
Results
Descriptive information is shown in Table 2 by glucose tolerance status (42 participants were NGT, 27 were IFG). Participants were 46% men, 54% women; 53% European-American, 47% African-American; 29% overweight (BMI 25–29.9 kg/m2), 71% obese (BMI >30 kg/m2). Twelve (44%) of the IFG subjects were African-American. IFG subjects were older and heavier than NGT subjects. IFG subjects had higher fasting concentrations of insulin and glucose, a higher PhiB, and a lower SI. After adjusting for SI, PhiB did not differ, PhiD was lower, and PhiS tended to be lower (P=0.094) in IFG vs NGT.
Table 2.
Baseline characteristics of the study participants by glucose tolerance status (mean ± SD).
| NGT n=42 |
IFG n=27 |
|
|---|---|---|
| Sex (M/F) | 12/30 | 19/8 |
| Ethnicity (EA/AA) | 21/21 | 15/12 |
| Age (yr) | 32.9 ±8.3 | 38.6 ±7.2* |
| Weight (kg) | 93.8 ±17.8 | 107.0 ±17.2* |
| BMI (kg/m2) | 31.7 ±4.2 | 33.7 ±4.0 |
| Fasting glucose (mg/dL) | 92.0 ±5.2 | 108.7 ±5.5* |
| Fasting insulin (μIU/ml) | 10.8 ±4.9 | 14.6 ±8.0* |
| Insulin sensitivity index (SI) | 3.99 ±0.45 | 2.43 ±0.35* |
| PhiB (109 min−1) | 9.1 ±3.6 | 11.6 ±4.7* |
| PhiB (109 min−1; adjusted for SI)1 | 9.2 ±0.4 | 9.9 ±0.6 |
| PhiD (109) | 563.2 ±249.9 | 481.3 ±230.7 |
| PhiD (109; adjusted for SI)1 | 512.9 ±45.6 | 380.2 ±42.5* |
| PhiS (109 min−1) | 69.4 ±35.6 | 70.2 ±37.1 |
| PhiS (109 min−1; adjusted for SI)1 | 66.1 ±5.9 | 52.0 ±5.8 |
NGT=normal glucose tolerant; IFG=impaired fasting glucose
P<0.05 vs. NGT
adjusted mean ± sem
Six subjects dropped out of the study before the end of the intervention leaving a final sample size of 63. Weight change over the course of the intervention averaged −1.64 kg, and ranged from −6.4 kg to +4.7 kg.
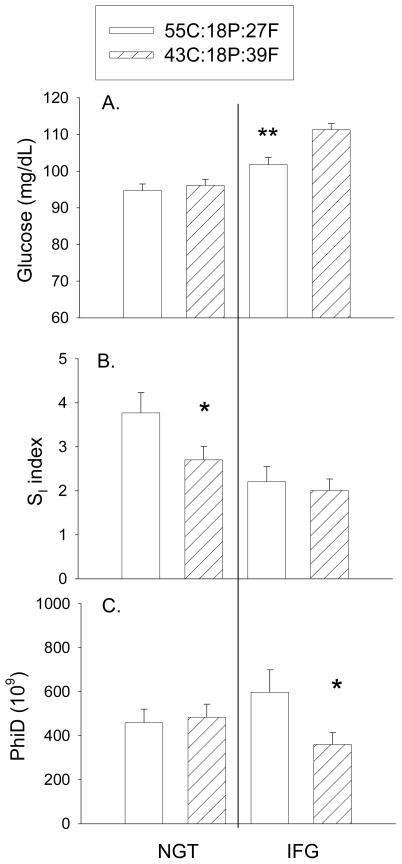
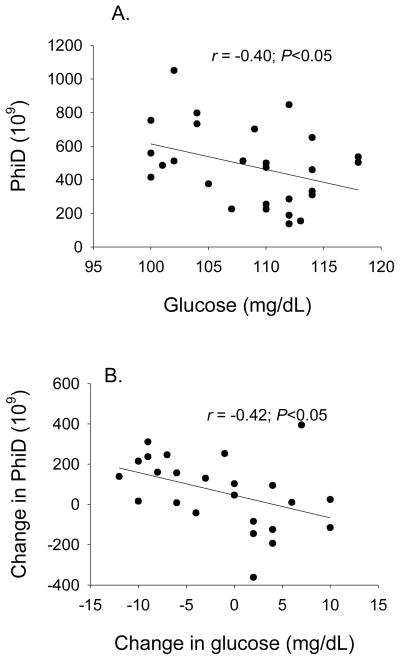
After the intervention, fasting glucose did not differ with diet among participants within NGT (Fig. 1; adjusted for baseline glucose, gender, weight change). However within IFG, the higher-CHO/lower-fat diet was associated with lower fasting glucose (P<0.01). Diet was independently associated with 8-week SI within the NGT subjects (P<0.05), but not the IFG subjects, with participants on the higher-CHO/lower-fat diet having higher SI (adjusted for baseline SI, gender, weight change). Within IFG, PhiD at 8 weeks (adjusted for baseline PhiD, gender, and weight change) was significantly higher among subjects who consumed the higher-CHO/lower-fat diet vs the lower-CHO/higher-fat diet; no diet effect was observed within NGT. At baseline, fasting glucose was inversely associated with PhiD in subjects with IFG (r = −0.40, P<0.05, Fig. 2A) but not NGT (r = 0.06, P=0.695). Within IFG, the change in PhiD over the intervention was associated with the change in fasting glucose (−0.43, P<0.05; Fig. 2B). Diet effects were not observed for PhiB or PhiS in either subgroup. We previously have reported higher PhiS in all subjects combined in response to the higher-CHO/lower-fat diet [21].
Fig. 1.
Effects of the 8-week diet intervention on A. fasting glucose, B. insulin sensitivity, and C. PhiD. The higher-CHO/lower-fat diet (open bars) was associated with lower fasting glucose (P<0.01) and higher PhiD (P<0.05) within IFG participants (right side), and higher SI (P<0.05) within NGT participants (left side), relative to the lower-CHO/higher-fat diet (hatched bars).
Data adjusted for baseline outcome, gender, and weight change.
Fig. 2.
Within IFG, fasting glucose concentration was associated with PhiD both at baseline (A. cross-sectionally; −0.40, P<0.05), and over the course of the intervention (B. change over 8 weeks; −0.42, P<0.05).
Conclusions
It seems increasingly clear that the primary precipitating defect in the etiology of T2D may differ among individuals. Obesity-mediated insulin resistance may be a factor in a large number of cases. However, in these cases, weight loss can be effective in preventing and/or reversing T2D in the early stages, given that β-cell function has remained intact. In contrast, a sub-set of individuals may develop T2D in response primarily to β-cell insufficiency, the causes of which are likely diverse. Reversal of T2D in these cases may be difficult, an observation that emphasizes the importance of identifying individuals with this phenotype, and aggressively instituting preventive measures. Individuals with IFG show signs of early β-cell insufficiency, and may therefore be an important group to target for preventive strategies.
We report here that 8 weeks of a higher-CHO/lower-fat diet had beneficial effects for overweight/obese individuals with and without IFG. The 55:18:27 (%energy from CHO:protein:fat) diet improved insulin sensitivity in individuals who were NGT, and both reduced fasting glucose concentration and improved β-cell response in individuals with IFG. These results suggest that the diet had pleiotropic actions, affecting insulin sensitivity among individuals with NGT, and affecting fasting glucose and β-cell function (but not insulin sensitivity) among participants with IFG. From a clinical care perspective, these results suggest that the lower-fat diet may help prevent progression towards T2D in overweight/obese individuals, both those who are NGT and those who are IFG.
We noted significant improvement in SI with the higher CHO/lower fat diet in the NGT group but not in the IFG group. The reason for the lack of effect in the IFG group is not clear. The meal SI index captures both skeletal muscle and hepatic insulin sensitivity. It is possible that the relative contribution of these components differs with subgroup. In this case, if the diet preferentially affected insulin sensitivity at one site vs. the other, results may differ with subgroup. Insulin resistance in IFG is thought to reside at the liver [7]; thus it is possible that our diet affected skeletal muscle. It is also possible that individuals with IFG have a more severe insulin resistance that is not easily reversed with diet. In this study, IFG individuals had, on average, 39% lower SI than did NGT participants (P<0.01; Table 2), a difference that was not eliminated by adjustment for BMI (adjusted SI was 30% lower; P<0.05). Finally, it is possible that the meal SI index better reflects insulin sensitivity in NGT vs. IFG subjects.
The higher CHO/lower-fat diet was associated with higher PhiD within IFG but not NGT subjects. PhiD is the response of the β-cell to an increase in glucose concentration, and therefore reflects the response to the increase in glucose that occurs with ingestion of the liquid meal. In this study, the IFG subjects had lower PhiD than the NGT individuals, a difference that was significant when considered relative to SI, suggesting that their β-cell function was compromised. We cannot determine, in this study, the precise mechanism through which the diet altered PhiD, or why the response was specific to the IGF group. However, a clue to both of these issues lies in the observation that fasting glucose also improved (decreased) in the IFG subjects, and the improvement in fasting glucose was significantly associated with the improvement in PhiD. Animal model, in vitro, and human data have suggested that even a mild, persistent elevation in glucose can impair β-cell function [10–13]. On a cross-sectional basis, in the San Antonio Metabolism Study, impairment in early insulin secretion was apparent among subjects with fasting glucose concentrations ≥ 5.6 mmol/l (101 mg/dL) [13]. A similar study in the UK identified 5.0−5.4 mmol/l (90–97 mg/dL) as the range of fasting glucose within which impairment in first-phase insulin secretion was first apparent [10]. Thus, results from the present study support the hypothesis that the higher-CHO/lower-fat diet acted primarily to lower fasting glucose, which in turn promoted an increase in PhiD. To our knowledge, this is the first study in human subjects to demonstrate a longitudinal association between a decrease in fasting glucose and an increase in β-cell responsiveness.
The mechanism for the diet’s effect on fasting glucose is not clear. Evidence exists that elevated fasting glucose in non-diabetic individuals is due to a decrease in non-insulin-dependent glucose clearance, perhaps at skeletal muscle [9]. Thus, the diet may have increased the ability of glucose to mediate its own disposal. Sex hormone binding globulin (SHBG) has been implicated in the regulation of fasting glucose, and is reduced by exposure to liver fat [24]. It is possible that our higher-CHO/lower-fat diet allowed for depletion of liver fat and increased SHBG production. It also has been reported that fasting glucose and gluconeogenic flux are associated with visceral fat [25]. Perhaps the diet affected either the amount or the metabolic characteristics of visceral fat. Further research is needed to identify the mechanism through which the higher-CHO/lower-fat diet lowered fasting glucose.
Due to the design of the study, we cannot determine which component of the higher-CHO/lower-fat diet was responsible for the improved metabolic profile. However it seems more likely that the lower fat content (27% vs 39%) was beneficial. Experimental, in vitro, and animal model studies have uniformly yielded results indicating adverse effects of fatty acids on insulin sensitivity and β-cell function [26;27]. Infusion of free fatty acids into healthy humans results in a decrease in insulin-stimulated glucose uptake [14]. Fatty acid metabolites such as diacylglycerol and ceramides impair insulin signaling by stimulating expression of protein kinase C [28]. Fatty acids may impair β-cell function by increasing uncoupling protein expression, which acts to decrease production of the ATP required for insulin secretion. Fatty acids also may lead to production of reactive oxygen species, which impair insulin signaling [15]. Further, existing epidemiological and clinical trial data support a beneficial role for low dietary fat in the prevention of type 2 diabetes [29;30]. Thus, although it is reasonable to speculate that the lower-fat aspect of the higher-CHO/lower-fat diet was responsible for the observed beneficial effects, further research is needed to verify this hypothesis.
Our intervention was designed to assess the role of gross macronutrient composition on insulin sensitivity and secretion. As such, we did not consider more detailed aspects of diet, such as fatty acid composition or CHO type, which may have affected glucose and insulin outcomes. For example, higher free-living intake of whole-grain foods was associated with lower fasting glucose and insulin concentrations [31]. Thus, it is possible that our higher-CHO diet could be rendered even more beneficial by increasing the proportion of whole grain. It is also possible that the relatively poor metabolic effects of our higher-fat diet were due to the fatty acid composition rather than the total fat content. Increased monounsaturated fatty acid (MUFA) content, relative to saturated fat, has been observed to ameliorate the acute detrimental effects of a high-fat diet on insulin sensitivity and elevated postprandial insulin concentration in certain populations [32]. Future studies are needed to tease apart the influence of total fat and fat quality on insulin sensitivity and secretion under carefully controlled conditions.
It is also important to note that our study was designed to maintain body weight. Results may have differed under weight loss conditions. Indeed, lower-CHO diets have been reported to have potentially beneficial effects on metabolic outcomes under weight loss conditions [18]. It is also possible that a lower-CHO diet would be beneficial for weight loss per se, with indirect effects on metabolic outcomes.
Results from this study should be considered in the context of existing published guidelines for diabetes prevention. The American Diabetes Association recommends that individuals at risk for diabetes reduce intake of dietary fat and meet the USDA recommendation for dietary fiber (14 g fiber per 1000 kcal per day) and whole-grains (half of grain intake should be whole-grain) [33;34]. Those with T2D should consume less than 7% of total calories as saturated fat and minimize intake of trans fats. The DASH diet (Dietary Approaches to Stop Hypertension) was originally developed to combat hypertension [35], but also has been observed to minimize risk for T2D, particularly in overweight and obese individuals [36]. This diet is comprised of 55% CHO, 18% protein, and 27% fat [35], which is identical to the lower-fat diet used in this study. Our results cannot be extrapolated to populations other than overweight/obese men and women who are NGT or IFG. However, our results support the recommendations of the ADA in minimizing consumption of dietary fat for the prevention of T2D.
Strengths of this study were rigorous control of food intake by provision of all meals and snacks, and use of robust measures of insulin sensitivity and β-cell function. The study participants were approximately 50% African-American, a group that is at disproportionate risk for T2D. A limitation of the study was that the diets differed with respect to both fat and CHO and did not consider the type of fat and CHO. Further study will be required to identify the specific components of the diets that had beneficial or adverse effects.
In conclusion, consumption of a eucaloric 55:18:27 (% energy from CHO:protein:fat) diet vs. a eucaloric 43:18:39 diet for 8 weeks had beneficial effects on CHO metabolism among overweight individuals. However, results varied with glucose tolerance status. Among NGT individuals, the higher-CHO/lower-fat diet resulted in higher insulin sensitivity, whereas among participants with IFG, the diet resulted in lower fasting glucose and higher dynamic β-cell response to glucose. Among IFG participants, linear relationships were observed between fasting glucose and PhiD both at baseline and over the course of the intervention, suggesting that the reduction in fasting glucose was permissive to an increase in β-cell function. These results support existing dietary recommendations for prevention of T2D by demonstrating beneficial effects of a lower-fat diet intervention on fasting glucose, insulin sensitivity, and β-cell function. Importantly, these results suggest that diet modification can successfully reduce risk for T2D in at-risk individuals without weight loss. Additional research is needed both to identify the specific components of the higher-CHO/lower-fat diet responsible for the beneficial effects, and to further optimize the diet.
Acknowledgments
The authors gratefully acknowledge the help of Maryellen Williams and Cindy Zeng of the UAB Metabolism Core Laboratory (Nutrition Obesity Research Center, Diabetes Research and Training Center, Center for Clinical and Translational Science) with laboratory analyses, and of Betty Darnell and Suzanne Choquette of the UAB Center for Clinical and Translational Science with experimental design and diet development. The authors have no relevant conflict of interest to disclose.
Funding
This work was supported by R01DK67538, M01-RR-00032, UL1RR025777, P30-DK56336, P60DK079626.
List of abbreviations
- T2D
type 2 diabetes
- PhiB
basal β-cell response to glucose
- PhiD
dynamic β-cell response to glucose
- PhiS
static β-cell response to glucose
- NGT
normal glucose tolerant
- IFG
impaired fasting glucose
- CHO
carbohydrate
- SI
insulin sensitivity index
Footnotes
Disclosure statement: the authors have nothing to disclose.
Author contributions
B Gower provided study design and oversight, data analysis, and manuscript preparation; LL Goree provided study implementation, data management, and input into manuscript preparation; P Chandler-Laney, A Ellis, and K Casazza helped with study design and implementation and provided input into manuscript preparation; W. Granger conducted all mathematical modeling for insulin sensitivity and secretion, and reviewed the manuscript.
Clinical trials registration number: NA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Rana JS, Li TY, Manson JE, et al. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care. 2007;30:53–58. doi: 10.2337/dc06-1456. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PWF, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 6.Unwin N, Shaw J, et al. Writing committee. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic Medicine. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Tripathy D, DeFronzo R. Contribution of B-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani M, DeFronzo R. Pathogenesis of insulin resistance in skeletal muscle. Journal of Biomedicine and Biotechnology. 2010;2010 doi: 10.1155/2010/476279. 10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jani R, Molina M, Matsuda M, et al. Decreased non–insulin-dependent glucose clearance contributes to the rise in fasting plasma glucose in the nondiabetic range. Diabetes Care. 2008;31:311–315. doi: 10.2337/dc07-1593. [DOI] [PubMed] [Google Scholar]
- 10.Godsland IF, Jeffs JAR, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia. 2004;47:1157–1166. doi: 10.1007/s00125-004-1454-z. [DOI] [PubMed] [Google Scholar]
- 11.Rossetti L, Shulman GI, Zawalich W, et al. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987;80:1037–1044. doi: 10.1172/JCI113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer J, Sturis J, Katschinski M, et al. Acute hyperglycemia alters the ability of the normal beta-cell to sense and respond to glucose. American Journal of Physiology (Endocrinology and Metabolism) 2002;282:E917–E922. doi: 10.1152/ajpendo.00427.2001. [DOI] [PubMed] [Google Scholar]
- 13.Gastaldelli A, Ferrannini E, Miyazaki Y, et al. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolims (SAM) study. Diabetologia. 2004;47:31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- 14.Boden G, Lebed B, Schatz M, et al. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 15.Robertson RP, Harmon J, Tran POT, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 16.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: The thin red line between adaptation and failure. Sci Signal. 2010;3(110):e7. doi: 10.1126/scisignal.3110pe7. [DOI] [PubMed] [Google Scholar]
- 17.Solomon TP, Haus JM, Kelly KR, et al. A low–glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. The American Journal of Clinical Nutrition. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acheson KJ. Carbohydrate for weight and metabolic control: Where do we stand? Nutrition. 2010;26:141–145. doi: 10.1016/j.nut.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington DC: Carnegie Institution; 1919. [Google Scholar]
- 20.Douglas CA, Gower BA, Darnell BE, et al. Role of diet in the treatment of PCOS. Fertil Steril. 2006;85:679–688. doi: 10.1016/j.fertnstert.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goree LL, Chandler-Laney PC, Ellis AC, et al. Dietary macronutrient composition affects beta-cell responsiveness but not insulin sensitivity. Am J Clin Nutr. 2011 doi: 10.3945/ajcn.110.002162. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breda E, Cavaghan MK, Toffolo G, et al. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. doi: 10.2337/diabetes.50.1.150. [DOI] [PubMed] [Google Scholar]
- 23.Cobelli C, Toffolo G, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin senstivity and hepatic extraction, from intravenous and oral glucose tests. American Journal of Physiology (Endocrinology and Metabolism) 2007;293:E1–E15. doi: 10.1152/ajpendo.00421.2006. [DOI] [PubMed] [Google Scholar]
- 24.Peter A, Kantartzis K, Machann J, et al. Relationships of circulating sex hormone–binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 26.McGarry JD. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Boden G. Fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1996;45:3–10. [PubMed] [Google Scholar]
- 28.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Koning L, Fung TT, Liao X, et al. Low-carbohydrate diet scores and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:844–850. doi: 10.3945/ajcn.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nettleton JA, McKeown NM, Kanoni S, et al. Interactions of dietary whole-grain intake with fasting glucose- and insulin-related genetic loci in individuals of European descent. Diabetes Care. 2010;33:2684–2691. doi: 10.2337/dc10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez S, Bermudez B, Pacheco YM, et al. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. The American Journal of Clinical Nutrition. 2008;88:638–644. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Standards of medical care in diabetes - 2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people With diabetes mellitus: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 35.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 36.de Koning L, Chiuve SE, Fung TT, et al. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34:1150–1156. doi: 10.2337/dc10-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]