Abstract
Primary trophoblasts, placental explants, and cell line cultures are commonly used to investigate placental development, physiology, and pathology, particularly in relation to pregnancy outcomes. Organotypic slice cultures are increasingly used in other systems because they maintain the normal three-dimensional tissue architecture and have all cell types represented. Herein, we demonstrate the utility of the precision-cut placental slice culture model for studying trophoblastic diseases.
Keywords: Placenta, slice culture, ethanol, methodology
Introduction
Organotypic precision-cut slice cultures (PCSC) have been generated from various isolated organs, including lung, liver, intestines, and brain [1–5]. PCSCs are increasingly used for basic science, pharmacology, metabolism, and toxicology research because tissues and organs can be sectioned along specific axes while fully retaining the normal three-dimensional architecture and heterogeneous populations of cells [1–3]. Moreover, slice cultures are more representative of the in vivo state, and they circumvent experimental and interpretation pitfalls associated with the use of continuous transformed cell lines.
Typically, a mechanical chopper is used to generate precision-cut slices of tissues or organs. Depending on the morphological interest, tissue can be sliced along any axis in a uniform manner. Optimum slice thickness varies with tissue requirements for diffusion of oxygen and nutrients and elimination of cellular waste [1]. At the same time, section thickness must be controlled to minimize mechanical injury to cells [6], and render the cultures suitable and standardizable for biological, pharmacological, or environmental toxin exposure studies. PCSCs mimic in vivo conditions because the cell-to-cell and matrix interactions are maintained ex vivo [1].
Current research on the mechanisms of placenta-related diseases is hampered by the lack of feasible models that enable both analysis of disease pathogenesis or high throughput testing of therapeutic compounds. Previously, the options were limited mainly to the use of primary trophoblast cultures and cell lines, but those approaches are now regarded as less relevant to in vivo biological systems due to lack of epithelial-stromal interactions. However, as demonstrated with other organs and tissues, PCSCs have the advantage of retaining the normal three-dimensional tissue architecture along with the diverse cell types that characterize each organ and tissue. Consequently, PCSCs closely mimic the in vivo state and offer a convenient alternative or supplementation to whole animal experiments.
Herein, we demonstrate the feasibility and utility of placental PCSCs for conducting biological research. This method produces ex vivo explants of placental tissue with reproducible, well-defined thickness and provides a convenient alternative to traditional explant cultures. We used acute ethanol exposure as the experimental model, and analyzed the tissues using cytotoxicity and viability assays, histopathological and immunohistochemical staining, protein, and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analyses.
Materials and methods
Adult female Long Evans rats were mated, and pregnancy was monitored as described [7]. Gestation-day (GD) 0 was assigned when sperm was present in the vagina at metestrous stage. On GD19, under general anesthesia and sterile conditions, gestational sacs were separated to harvest placental tissue [8]. Our protocol was approved by the Institutional Animal Care and Use Committee at Lifespan-Rhode Island Hospital, and conforms to the guidelines set by the National Institutes of Health.
This entire procedure was performed in a laminar flow tissue culture hood using standard sterile technique. Placental discs were placed on ice in sterile Petri dishes containing Hank’s balanced salt solution (HBSS) pre-chilled to 4°C. Prior to slicing, placental discs were washed twice in HBSS pre-chilled to 4°C, placed briefly on sterile filter paper to absorb excess HBSS, and then transferred to the center of the plastic mounting tray of a McIlwain Tissue Chopper (Ted Pella, Inc., Redding, CA). The placental disc was positioned to the left of the blade and just beneath the edge of the fully raised blade. Optimum slicing was achieved by positioning the placental disc on its basal plate.
Systematic studies revealed the optimum slice thickness for placenta to be between 150 and 200 µm. In contrast, slices thinner than 150 µm had poor tissue integrity, while slices thicker than 200 µm exhibited highly variable degrees of viability. Further investigations were used to optimize blade force and chopping speed. We found that a slow-to-moderate chopping speed allowed the chilled placental disc to remain set in place and maintain its orientation. Most important was the need to adjust the blade force (dial at 12 o’clock position) such that the slicing was achieved without crushing the tissue.
After slicing through the entire specimen (<1 minute), the tray holding the sliced tissue was removed from the apparatus and held over a 100-mm2 Petri dish containing ice cold full medium (Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% glutamine, 1% non-essential amino acids, 120 IU/mL penicillin and 120 µg/mL streptomycin). Using a Pasteur pipette, we gently flushed culture medium around the base of the tissue to dislodge and transfer the slices to the dish. Under a dissecting microscope, the slices were completely separated from the chorionic plate and transferred to 6-well culture plates (3 slices/well) containing full medium. Cultures were maintained at 37°C in a standard CO2 cell culture incubator. Media was changed every 24 hours. The tissue chopper was maintained by cleaning the plastic mounting tray and blade with sterile cotton swabs soaked in 70% ethanol and then air-dried between samples.
For comparison with the PCSC method, we generated manually cut slice cultures (MCSC). For the MCSCs, we used the same platform and orientation of the placental discs indicated for the PCSCs. With the guide of a sterile ruler, we cut the placental disc into 1 mm in thickness slices using surgical blades. The slices were separated under a dissecting microscope and cultured according to the protocol described for the PCSCs.
Cytotoxicity was assessed at baseline (initially) and at 24-hour intervals after establishing the PCSCs or MCSCs, by measuring glucose 6-phosphate dehydrogenase (G6PD) release into culture supernatant using a commercial assay (Molecular Probes, Inc., Eugene, OR). Assays were performed with 50 µL samples of medium removed at each time point. Fresh full medium was added daily. G6PD assay results were normalized to tissue protein content in the well measured after 4 days in culture. Tissue protein content was measured using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). We also performed a modified fluorescence-based LIVE/DEAD® Viability/Cytotoxicity Assay (Molecular Probes, Inc., Eugene, OR) to evaluate viability of PCSCs and MCSCs at the 24- and 96-hour time points. This assay simultaneously reveals live and dead cells using Calcein AM (viable cells) and ethidium homodimer-1 (dead cells), which mark recognizable parameters of cell viability and death, respectively. After optimization of the assay for PCSCs and MCSCs, we determined that labeling the cultures with 5 µM calcein AM and 2.5 µM ethidium homodimer-1 for 30 minutes at room temperature provided the clearest signals. The results were evaluated by fluorescence microscopy. Photomicrographic images were merged using ImageJ 44o software [National Institutes of Health].
After 1–4 days in culture, tissues were harvested and either immersion fixed in Histochoice (Amresco Corp., Solon, OH) and embedded in paraffin for histologic sectioning, or they were immediately homogenized in either radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors for protein studies, or Qiazol reagent (Qiagen Inc., Valencia, CA) for mRNA studies. The samples were homogenized in a TissueLyser II apparatus (Qiagen Inc., Valencia, CA).
For histological studies, the fixed samples were embedded in paraffin on edge to generate perpendicular sections of the PCSCs and MCSCs. The objective was to evaluate the tissue integrity and viability through the full thickness of the slices. In addition, PCSCs were embedded en face to study tissue morphology. Adjacent en face sections of the PCSCs (4 µm thick) were immunostained with monoclonal antibodies to cytokeratin (clone MNF-116, Dako), smooth muscle actin (clone 1A4, Dako) and CD31 (clone JC70A, Dako). Antibody binding was detected with EnVisionTM FLEX/HRP reagents, consisting of secondary antibodies covalently linked to a dextran backbone to which a large number of horseradish peroxidase (HRP) molecules were coupled. Diaminobenzidine tetrahydrochloride (DAB) was used as the chromogen. The sections were counterstained lightly with hematoxylin. The immunohistochemical staining reactions were performed using the Dako Autostainer (Dako, Carpenteria, CA).
Protein homogenates were clarified by centrifuging the samples at 14,000 × g for 10 minutes at 4°C. The supernatant fractions we re used to measure protein concentrations with the BCA assay. The protein homogenates were used to measure aspartyl-asparaginyl β-hydroxylase (AAH) immunoreactivity by Western analysis [9], because AAH is abundantly expressed in trophoblasts and it mediates placentation [10, 11]. In brief, for Western blotting, 40 µg protein samples fractionated by sodium dodecyl sulfate, polyacrylamide gel electrophoresis (SDS-PAGE) along with molecular weight standards [12], were transferred to Polyvinylidene Difluoride (PVDF) membranes. Non-specific binding sites were adsorbed with Super-Block-TBS (Pierce, Rockford, IL). The membranes were incubated overnight at 4°C with the FB50 monoclonal antibody to AAH (0.5–1 µg/ml) [12] diluted in Tris-buffered saline (TBS) containing 1% bovine serum albumin, 0.05% Tween-20 (TBST-BSA), and 0.025% NaN3. After rinsing in TBST, the blots were incubated for 1 hour at room temperature with HRP-conjugated anti-mouse IgG (1:30,000) diluted in TBST + 0.5% Casein. Immunoreactivity was revealed using SuperSignal enhanced chemiluminescence reagents (ECL, Pierce Chemical Company, Rockford, IL) and quantified by digital imaging with the Kodak Digital Science Imaging Station (NEN Life Sciences, Boston, MA). To assess sample loading, the blots were stripped and re-probed with polyclonal antibody to p85 subunit of PI3 kinase [13].
Total RNA was isolated using the EZ1 RNA universal tissue kit and the BIO Robot EZ1 (Qiagen Inc., Valencia, CA). RNA was reverse transcribed using random oligodeoxynucleotide primers and the AMV First Strand cDNA synthesis kit. We used quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) assays to measure mRNA expression of ATP-binding cassette transporter G2 (ABCG2) because trophoblasts normally express high levels of ABCG2 [14, 15]. The objective was to assess the stability of ABCG2 expression in PCSCs over the course of study. Results were normalized to 18S ribosomal RNA measured in the same samples [16].
We next conducted studies to determine if placental PCSCs could be used to study disease mechanisms, particularly in relation to fetal alcohol spectrum disorders. Previous studies showed that prenatal ethanol exposure impairs placentation [17] and trophoblastic cell survival and function [18]. Herein, we examined the effects of acute ethanol exposure on cell survival mechanisms. After 24-hours in culture, PCSCs were exposed to 0 mM or 100 mM ethanol for 48 hours in sealed humidified chambers [19]. Proteins were extracted from the tissues as described earlier, and the samples were used to measure Bcl2, Bax, and Bak immunoreactivities by direct binding enzyme-linked immunosorbant assays (ELISAs) with results normalized to protein content in the wells [18].
In brief, samples containing ~50 ng of protein in 100 µl TBS were adsorbed to the flat surfaces of opaque white Maxisorp polystyrene 96-well plates (Nunc ThermoScientific, Rochester, NY) by overnight incubation at 4°C. Non-specific binding sites were blocked by a 3-hour, room temperature incubation with TBST-BSA. Samples were incubated with 0.5–1.0 µg/ml polyclonal antibodies to Bcl-2 (N-19), Bax (P-19), or Bak (G-23) (all from Santa Cruz, Santa Cruz, CA) for 1 h at 37°C. Antibody binding was detected with HRP-conjugated secondary antibody (1:10,000; Pierce, Rockford, IL) and the Amplex UltraRed soluble fluorophore (Molecular Probes, Eugene, OR) [20]. Immunoreactivity was measured (Ex 530/Em 590) in a SpectraMax M5 microplate reader (Molecular Devices Corp., Sunnyvale, CA). The results were normalized to protein content in each well as quantified using the NanoOrange® Protein Quantitation Kit (Molecular Probes, Eugene, OR). Negative control reactions included omission of protein sample, or the primary antibody, secondary antibody, or both antibodies. Between steps, the wells were washed 3 times with TBST using a Nunc Immunowash apparatus (Nunc, Rochester, NY). In addition to the ELISAs, histological sections of the PCSCs were immunostained to detect Bcl-2 and Bax.
Data depicted in the graphs represent mean ± S.E.M. corresponding to immunoreactivity or mRNA expression. Inter-group comparisons were made using Student t-tests or two-way analysis of variance (ANOVA) with the Bonferroni post hoc test of significance. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, CA). Significant P-values are indicated over the graphs.
Results and discussion
Pregnant Long Evans dams typically harbor 10 gestational sacs with each placenta weighing approximately 0.5 g on GD19. The PCSCs enable us to generate eight to ten high quality tissue slices per placenta, and five 6-well cultures per rat. All the experiments were repeated three times with consistent results.
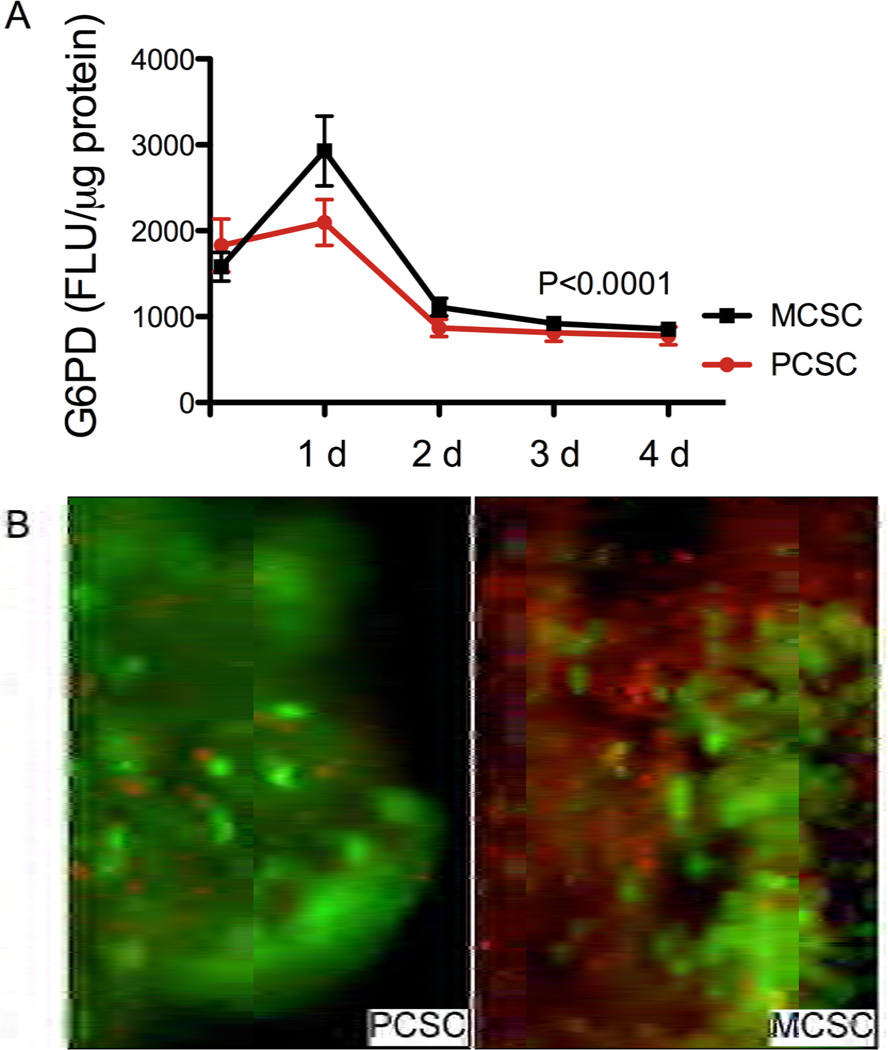
Cytotoxicity assays demonstrated that G6PD release was highest 24 hours after establishing the cultures and the levels were significantly higher in MCSC than PCSC (P<0.05). Over the next 24 hours, G6PD release declined significantly, and thereafter, the levels stabilized and remained significantly below the maximum (P<0.0001) regardless of the slicing method (Fig. 1A). Most likely, the G6PD release that occurred during the first 24 hours in culture reflected injury caused by the tissue slicing. Corresponding with the G6PD assay results, the tissue viability assay demonstrated large numbers of dead cells (bright red fluorescence) in 1-day-old PCSCs and MCSCs, and many fewer dead cells in older cultures (data not shown). Of note is that on culture Day 4, the proportions of live cells (green fluorescence) in the PCSCs were conspicuously greater than in the MCSCs (Fig. 1B). Together, these results suggest that experiments using PCSCs or MCSCs should be conducted with cultures that are at least 48 hours old.
Figure 1.
Cytotoxicity and viability monitoring in placental slice cultures: A) G6PD release cytotoxicity assay. Precision-cut slice cultures (PCSCs) and manually cut slice cultures (MCSCs) were monitored daily for G6PD release into the culture supernatant over the course of 4 days in vitro. Results were normalized to protein content in the tissue slices. Graphed data depict the mean ± S.E.M. of G6PD levels. Intergroup comparisons were made using 2-way ANOVA with the Bonferroni post-tests. B) LIVE/DEAD® Viability/Cytotoxicity Assay was used to monitor changes in culture viability (Calcein AM labeling) and cytotoxity (ethidium homodimer-1 membrane permeability) in PCSCs and MCSCs. Cultures were examined an photographed by fluorescence microscopy and images were merged using ImageJ software. [Original magnification X400]
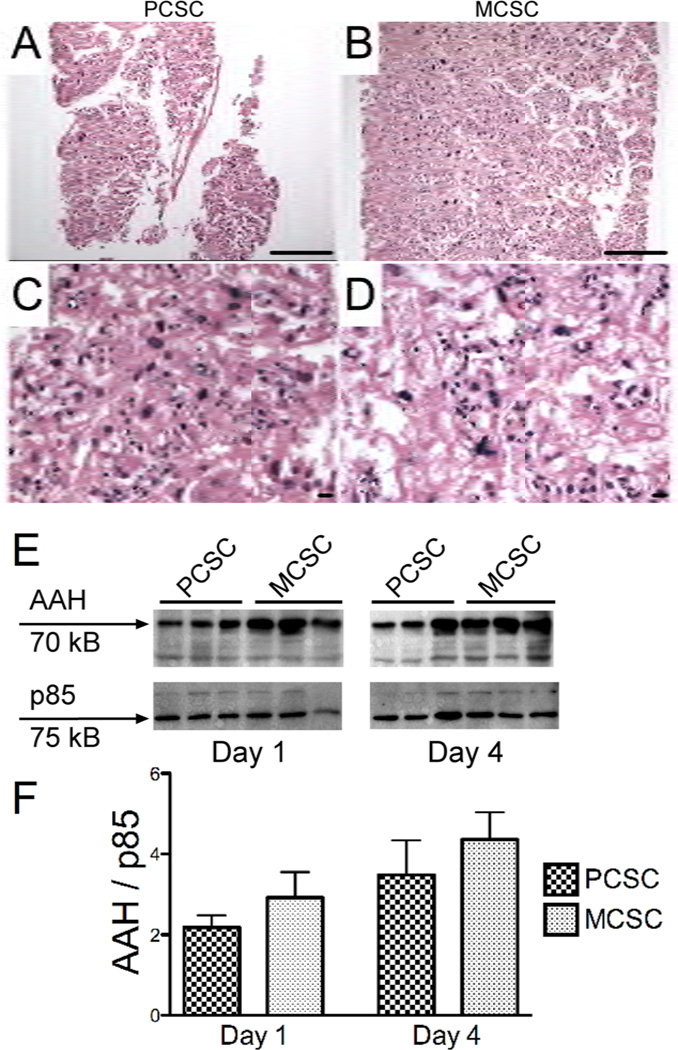
As expected, the PCSCs were consistently and uniformly thinner (Fig. 2A) than the MCSCs (Fig. 2B). Moreover, perpendicular sections through the tissue slices revealed more consistent preservation of the full thickness architecture in PCSCs (Fig. 2C), and conspicuous foci of necrosis and parenchymal loss at the centers of the MCSCs (Fig. 2D). Western blot analysis revealed a trend toward increasing AAH immunoreactivity over time in culture, with the highest levels measured at the 96-h time point for both PCSCs and MCSCs (Figs. 2E and 2F). Although AAH protein levels were generally higher in the MCSCs, the inter-group differences did not reach statistical significance. Continued expression of AAH throughout the period of culture most likely reflects preserved viability of trophoblastic cells in both PCSCs and MCSCs. The finding of higher levels of AAH protein at later time points in culture corresponds with our previous observation that AAH expression increases with duration of pregnancy and placental maturation [9].
Figure 2.
Structural integrity and protein expression in PCSCs and MCSCs. A–D) H&E stained perpendicular plane sections of placental (A) PCSCs and (B) MCSCs demonstrating full-thickness slices of 4-day old cultures. [magnification bar= 25 µm] H&E stained labyrinthine region of (C) PCSCs and (D) MCSCs depicting the central regions of the slices. [magnification bar= 25 µm] E) Western blot analysis of aspartyl-asparaginyl β-hydroxylase (AAH) in placental PCSC and MCSC homogenates harvested after 1 or 4 days in culture. The blots were stripped and re-probed with polyclonal antibody to the p85 subunit of PI3 kinase as a sample loading control. F) Immunoreactivity was quantified by digital imaging and the mean ± S.E.M. AAH/p85 ratios are depicted graphically.
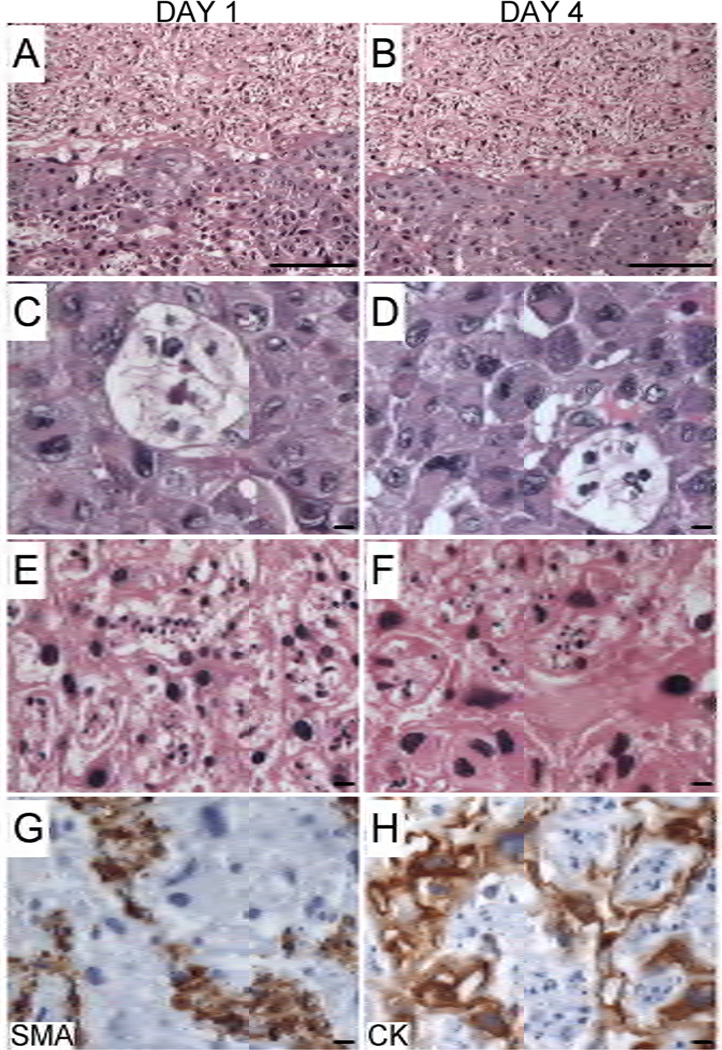
Histological studies showed that tissue architecture is preserved (Figs. 3A and 3B) and trophoblastic cells, including glycogen cells, spongiotrophoblasts, and trophoblastic giant cells located at the junctional zone remained cytologically intact and viable throughout the period in culture (Figs. 3C and 3D). Although trophoblastic cells within the labyrinthine region were also cytologically intact, fetal vascular elements exhibited nuclear fragmentation reflecting degeneration, suggesting greater vulnerability to traumatic injury from the slicing procedure or sensitivity to the culture conditions (Figs. 3E and 3F).
Figure 3.
Structural integrity and cell types represented in placental PCSCs over time in culture. (A–B) H&E stained paraffin-embedded en face plane sections of PCSCs demonstrating preservation of the architectural integrity (A) 1 or (B) 4 days after establishing the cultures [magnification bar 25 µm]. (C–F) H&E stained sections of the junctional zone (C-Day 1, D–Day 4) and labyrinthine region (E–Day 1, F–Day 4). Adjacent sections were immunostained for (G) smooth muscle actin (SMA) to detect vascular elements, or (H) cytokeratin (CK) to label trophoblasts. Immunoreactivity was revealed with HRP-tagged, polymer conjugated secondary antibody and DAB substrate. Sections were counterstained lightly with Hematoxylin. [magnification bar 25 µm]
Enface paraffin-embedded sections of the PCSCs were immunostained to detect smooth muscle actin (Fig. 3G), cytokeratin (Fig. 3H), and CD31. Numerous intact cells exhibited abundant intra-cytoplasmic cytokeratin immunoreactivity, confirming their trophoblastic nature (Fig. 3H). In contrast, many necrotic cells had SMA immunoreactivity, but were CD31- and cytokeratin-negative, indicating that they most likely represented damaged vascular structures (Fig. 3G), consistent with the histological findings noted earlier. As a further assessment of trophoblastic cell function, we measured ABCG2 mRNA levels by qRT-PCR analysis. Those studies demonstrated sustained levels of ABCG2 mRNA transcripts from Day 1 (1.84 ± 0.11) to Day 4 (1.67 ± 0.08) (data correspond to the mean mRNA/18S ratio ± S.E.M.)
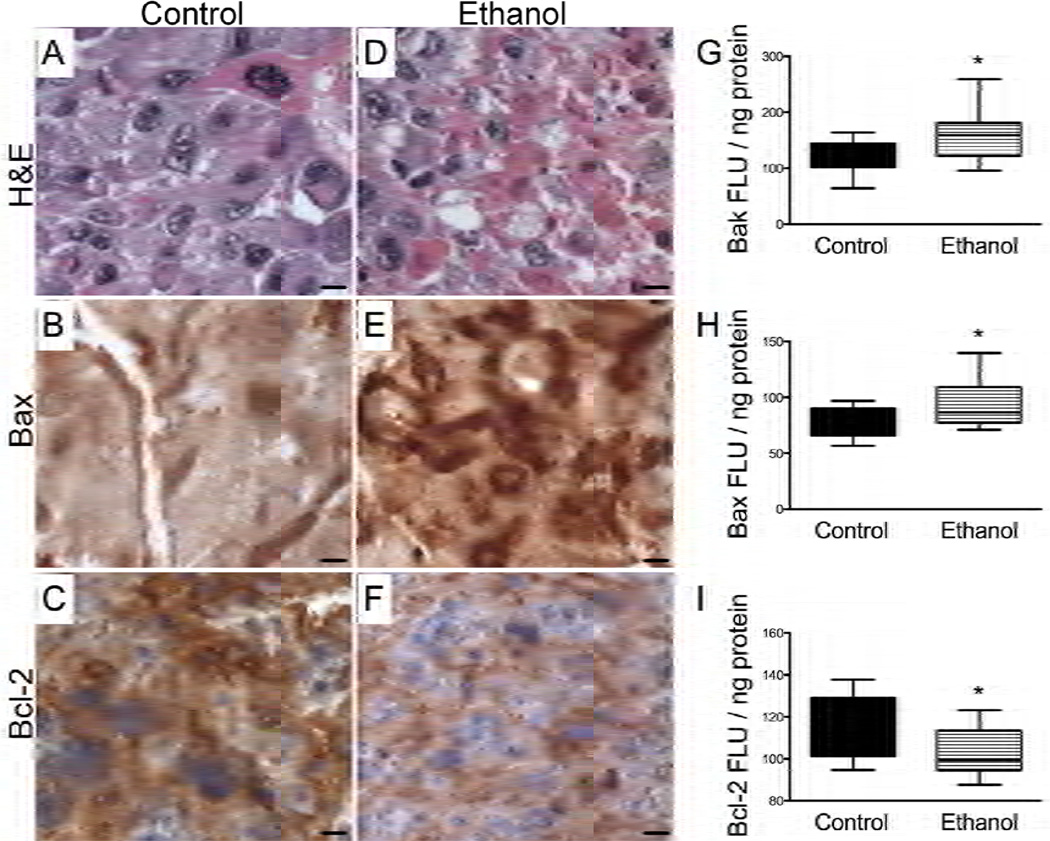
Ethanol exposure resulted in increased tissue fragmentation and necrosis within the junctional zones of the placental PCSCs (Fig. 4D). Correspondingly, Bax immunoreactivity was increased in the spongiotrophoblasts (Fig. 4E), and Bcl2 immunoreactivity was reduced in labyrinthine trophoblasts and spongiotrophoblasts (Fig. 4F), as shown by immunohistochemical staining. These findings were confirmed by ELISA, which demonstrated significantly increased levels of pro-apoptotic Bax and Bak and reduced levels of anti-apoptotic Bcl2 in ethanol-exposed relative to control PCSCs (Fig. 4G–4I), similar to the in vivo findings with a chronic prenatal ethanol exposure model [18]. These findings suggest that placental PCSC experimental models could be used to study disease mechanisms, and may also be suitable for high throughput screening of therapeutic compounds.
Figure 4.
Ethanol-induced necrosis/apoptosis of placental PCSCs. Placental PCSCs were treated with 0 mM or 100 mM ethanol in sealed humidified chambers at 37°C for 2 days, beginning 24 hours after establish ing the cultures. H&E stained paraffin-embedded sections depict (A) control and (D) ethanol exposed placental PCSCs. (B, E) Bax and (C, F) Bcl2 immunoreactivity were detected in trophoblastic cells by immunostaining adjacent sections of (B, C) control and (E, F) ethanol exposed PCSCs. [magnification bar= 25 µm] (G–I) Protein expression levels of (G) Bak, (H) Bax, and (I) Bcl2 were measured by direct binding ELISAs. The graphs depict the mean ± S.E.M. levels of immunoreactivity normalized to protein content in the wells. Intergroup comparisons were made using Student t-tests. Significant p-values (P<0.05) are indicated above the graphs.
Summary and conclusions
Precision cut slice cultures provide a novel tool for studying structure, function, and disease in placental tissue ex vivo, and allow for experimental manipulation for rapid assessment of the effects of various exposures on RNA/protein expression and morphology. This model also has the potential for broad application in the fields of toxicology and therapeutic targeting.
ACKNOWLEDGEMENT
The project described was supported by Award Number K08-AA016783 from the National Institute On Alcohol Abuse and Alcoholism.
Supported by AA-016783 from the National Institutes of Health
Abbreviations
- PCSC
Precision-cut slice culture
- MCSC
Manually cut slice culture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Parrish AR, Gandolfi AJ, Brendel K. Precision-cut tissue slices: applications in pharmacology and toxicology. Life Sci. 1995;57:1887–1901. doi: 10.1016/0024-3205(95)02176-j. [DOI] [PubMed] [Google Scholar]
- 2.Groneberg DA, Grosse-Siestrup C, Fischer A. In vitro models to study hepatotoxicity. Toxicol Pathol. 2002;30:394–399. doi: 10.1080/01926230252929972. [DOI] [PubMed] [Google Scholar]
- 3.Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- 4.de Graaf IA, Olinga P, de Jager MH, Merema MT, de Kanter R, van de Kerkhof EG, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 5:1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- 5.Lake BG, Meredith C, Scott MP, Renwick AB, Price RJ. Use of cultured precision-cut rat lung slices to study the in vitro induction of pulmonary cytochrome P450 forms. Xenobiotica. 2003;33:691–702. doi: 10.1080/00498225031000108306. [DOI] [PubMed] [Google Scholar]
- 6.de Kanter R, Monshouwer M, Meijer DK, Groothuis GM. Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr Drug Metab. 2002;3:39–59. doi: 10.2174/1389200023338071. [DOI] [PubMed] [Google Scholar]
- 7.Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- 8.Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- 9.Lavaissiere L, Jia S, Nishiyama M, de la Monte S, Stern AM, Wands JR, et al. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J Clin Invest. 1996;98:1313–1323. doi: 10.1172/JCI118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundogan FBA, Gilligan J, Lau E, Mark P, De Paepe ME, de la Monte SM. si-RNA inhibition of aspartyl-asparaginyl beta-hydroxylase expression impairs cell motility, Notch signaling, and fetal growth. Pathol Res Pract. 2011;207:545–553. doi: 10.1016/j.prp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gundogan F, Elwood G, Greco D, Rubin LP, Pinar H, Carlson RI, et al. Role of aspartyl-(asparaginyl) beta-hydroxylase in placental implantation: relevance to early pregnancy loss. Hum Pathol. 2007;38:50–59. doi: 10.1016/j.humpath.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Ince N, de la Monte SM, Wands JR. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000;60:1261–1266. [PubMed] [Google Scholar]
- 13.Cantarini MC, de la Monte SM, Pang M, Tong M, D'Errico A, Trevisani F, et al. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 14.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 15.Shiverick K, Ino K, Harada T, Keelan J, Kikkawa F. Placental enzymes and transporters: new functions and genetic polymorphisms--a workshop report. Placenta. 2007;28 Suppl A:S125–S128. doi: 10.1016/j.placenta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 2007;113:659–673. doi: 10.1007/s00401-007-0199-4. [DOI] [PubMed] [Google Scholar]
- 17.Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, et al. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29:148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundogan F, Elwood G, Mark P, Feijoo A, Longato L, Tong M, et al. Ethanol-induced oxidative stress and mitochondrial dysfunction in rat placenta: relevance to pregnancy loss. Alcohol Clin Exp Res. 2010;34:415–423. doi: 10.1111/j.1530-0277.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee K, Mohr L, Wands JR, de la Monte SM. Ethanol inhibition of insulin signaling in hepatocellular carcinoma cells. Alcohol Clin Exp Res. 1998;22:2093–2101. [PubMed] [Google Scholar]
- 20.Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]