Abstract
Symmetrical N,N’-diarylureas: 1,3-bis(3,4-dichlorophenyl)-, 1,3-bis[4-chloro-3(trifluoromethyl) phenyl]- and 1,3-bis[3,5-bis(trifluoromethyl)phenyl]urea, were identified as potent activators of the eIF2α kinase heme regulated inhibitor. They reduce the abundance of the eIF2·GTP·tRNAiMet ternary complex and inhibit cancer cell proliferation. An optimization process was undertaken to improve their solubility while preserving their biological activity. Non-symmetrical hybrid ureas were generated by combining one of the hydrophobic phenyl moieties present in the symmetrical ureas with the polar 3-hydroxy-tolyl moiety. O-alkylation of the later added potentially solubilizing charge bearing groups. The new non-symmetrical N,N’-diarylureas were characterized by ternary complex reporter gene and cell proliferation assays, demonstrating good bioactivities. A representative sample of these compounds potently induced phosphorylation of eIF2α and expression of CHOP at the protein and mRNA levels. These inhibitors of translation initiation may become leads for the development of potent, non-toxic, and target specific anti-cancer agents.
Keywords: SAR study; N,N’-diarylureas; inhibition of translation initiation; ternary complex; phosphorylation of eIF2α; anti-cancer drugs
Development of mechanism-based non-cytotoxic anti-cancer drugs remains an unmet need. Therefore, targeting cellular processes critical for the malignant transformation and/or maintenance of the transformed phenotype may provide a novel paradigm for the development of potent and safe anti-cancer drugs. Translation initiation, which plays a central role in cellular proliferation, differentiation, and survival, is tightly regulated. Unrestricted translation initiation causes malignant transformation in vitro and may play a causative role in the genesis of many human cancers1–4 while restricting translation initiation reverses transformed phenotypes.5 A critical step in the translation initiation cascade is the assembly of a ternary complex (TC) between eukaryotic translation initiation factor 2 (eIF2), GTP, and initiator methionine tRNA (tRNAiMet). Recognition of the AUG start codon by the 48S translation initiation complex involves the hydrolysis of GTP in the TC to GDP and precedes the joining of the 60S ribosomal subunit that becomes part of the translation competent ribosome. The exchange of GDP in the eIF2·GDP complex for GTP by the guanine nucleotide exchange factor eIF2B must take place for enabling a new round of translation initiation. Phosphorylation of eIF2α by eF2α kinases such as heme regulated inhibitor (HRI) inhibits this exchange reaction thereby reducing the abundance of the TC and inhibiting translation initiation.6
In normal physiology, a tight regulation of the amount of the TC is critical for controlling cell proliferation. Forced expression of eIF2α-S51A, a nonphosphorylable eIF2α mutant, increases the amount of the ternary complex, renders the translation initiation unrestricted, and causes transformation of normal cells.7,8 Similarly, the overexpression of tRNAiMet also causes cellular transformation.9 In contrast, restricting the amount of eIF2·GTP·tRNAiMet ternary complex inhibits proliferation of cancer cells in vitro and tumors in vivo.10–12 These findings indicate that restricting the amount of the TC could serve as a viable approach to cancer therapy. The promise that inhibition of translation initiation in general and of TC formation in particular will offer a new paradigm in anti-cancer therapy is based on the dependence of the malignant transformation and maintenance of malignant phenotypes on the overexpression of oncogenes and growth factors. Although inhibition of translation initiation will affect the overall rate of translation, it has a much greater impact on the translation of a subset of mRNAs that includes a disproportionate number of mRNA encoding for growth factors and oncogenic proteins13,14 thus inhibiting the cancer progression with minimal effect on normal cells.15
In a cell-based high throughput screening (HTS) campaign we have identified members of N,N’-diarylureas as agents that activate HRI, induce eIF2α phosphorylation, reduce the abundance of eIF2·GTP·tRNAiMet TC, and inhibit translation initiation.6 As such, these agents potently inhibited cancer cell proliferation in vitro and repressed growth of mammary tumors in a mouse model of human breast cancer. The wide diversity of cellular and molecular targets that can be interrogated by judiciously modified N,N’-diarylureas suggests them to be a privileged scaffold. For example, various N,N’-diarylureas were reported to effectively and specifically inhibit expression of enzymes such as cyclooxygenase COX-2,16 diacyl glycerol-acyltransferase DGAT-1,17 β-secretase BACE-1,18 acyl-CoA:cholesterol O-acyltransferase ACAT,19 p38 mitogen-activated protein kinase,20 and cyclophilin A.21 In addition, some antagonize specifically receptors such as homomeric kainate receptor subtype GluR522 and CXCR2 chemokine receptor,23,24 while others activate the insulin receptor β-subunit tyrosine kinase.25 In a recent study, 1-[3-(4-bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea (Nelotanserin) was discovered as a potent inverse-agonist of the 5-hydroxy-trypamine-2A (5-HT2A) serotonin receptor.26 This drug was advanced into clinical trials for the treatment of insomnia. In the field of cancer therapy, series of bis(morpholino-1,3,5-triazine) diarylurea derivatives27 and 4-morpholinopyrrolopyrimidine diarylurea derivatives28 were found as potent inhibitors of a promising target: the phosphatidylinositol-3-kinases (PI3Ks). Moreover, recent studies reported the effective interaction of N,N’-diarylureas with molecular targets involved in malignant transformation and maintenance, such as the vascular endothelial growth factor receptor (VEGFR) tyrosine kinases,29–32 checkpoint kinase 1,33–35 insulin like growth factor receptor IGF-1R36,37 and raf kinase.38,39
Herein, we report our efforts to carry out a structure-activity relationship study aimed at improving the physicochemical properties of N,N’-diarylureas that inhibit translation initiation as the first step in a hit-to-lead optimization. To this end, we modified the structure of selected symmetrical N,N’-diarylureas in order to improve their solubility without compromising their potency. The previously reported non-symmetrical N,N’-diaryureas had an N-phenyl substituted by electron withdrawing groups and the other one N-aryl was either benzo[1,2,3]thiadiazol-6-yl or a benzo[1,2,5] oxadiazol-5-yl moiety, and were less potent than the active parent symmetrical N,N’-diarylureas 1–3 both in the dual luciferase TC and transcription factor C/EBP homologous protein (CHOP) expression assays.6 Nevertheless, combination of fragments originating from 1–3 such as N-(3,4-dichloro)-, N-(3-trifluoromethyl-4-chloro)- or N-(3,5-bis(trifluoromethyl))-phenyl with an N’-(3-hydroxyl-tolyl)- fragment generated active non-symmetrical N,N’-diarylureas and introduced a potential derivatization site. This site was subsequently used to introduce polar and solubilizing substitutions aimed at improving the physicochemical properties. The resultant focused library of new non-symmetrical N,N’-diarylureas was characterized for its inhibition of both formation of eIF2·GTP·tRNAiMet TC and cancer cell proliferation.
The design of this focused library builds on the basic observation that the symmetrical N,N’-diarylureas 1–3 are hydrophobic and suffer from poor solubility in physiological fluids. We therefore altered the physicochemical properties of these agents by modifying the substituents on one of the two aryl rings. In addition to lowering the calculated Log P (CLog P) of the molecules (see Table S1 in the Supplementary Data), the substitution with a hydroxyl- (compounds 4–6) and methoxy group (compounds 7–9) were undertaken to introduce a function amenable to simple chemical modification and to test the impact of a simple O-methylation on the biological activities of interest, respectively. The phenol function was substituted with polar and potentially charged groups such as N,N-dimethylaminoethylene group (compounds 11–13), morpholinoethylene group (compounds 15–17) and piperazinoethylene group (compounds 23–25), which are widely used in medicinal chemistry to enhance aqueous solubility.40 Alternatively, we have attempted to extend the putative pharmacophore by the use of 2-amino-3-hydroxypyridine (compounds 26–28) as one of the N-aryl moieties comprising the non-symmetrical N,N’-diarylureas. Similar introduction of a 3-hydroxypyridin-2-yl ring into N,N’-diarylureas was previously reported for inhibitors of insulin-like growth factor I receptor and resulted in ureas with good biological activity.20,36
All the N,N’-diarylureas were prepared in good yields from the corresponding aromatic amines and phenyl isocyanates in 1,4-dioxane at 55 °C (Schemes 1–3). All the employed phenyl isocyantes were commercially available and were used without further purification. N,N’-diarylureas 1–9 and 26–28 were obtained from commercially available anilines and 2-amino-3-hydroxypyridine, respectively (Schemes 1 and 3). N,N’-diarylureas 11–13, 15–17 and 23–25 were obtained from the corresponding 3-alkoxy-4-methyl anilines that were generated from a common commercially available precursor 2-methyl-5-nitrophenol (Scheme 2). The 3-alkoxy-4-methyl anilines were obtained by a two step synthesis which included O-alkylation of the phenol by different N,N-disubstituted ethylamines employing the Mitsunobu reaction41 leading to the corresponding 2-methyl-5-nitrophenoxyalkyls 10 and 14 followed by SnCl2-mediated reduction in ethanol at 90 °C of the nitro- to the amino-function.42 The anilines, which were isolated from the extract of the alkaline aqueous phase of the quenched reaction mixture, were used directly without further purification. Similar strategy was employed to introduce the piperazine moiety but it required protection of the secondary amine by benzyloxycarbonyl group prior the Mitsunobu reaction generating the benzyl 4-(2-(2-methyl-5-nitrophenoxy)ethyl)piperazine-1-carboxylate (compound 19). Removal of the benzyloxycarbonyl group from the protected N,N’-diarylureas 20–22 by Pd/C-mediated hydrogenolysis yielded the final 23–25. All the synthetic procedures are described in detail in the Supplementary Data. The purity of the N,N’-diarylureas exceeded 95% as determined by RP-HPLC (see Table S2 in the Supplementary Data) and the structural integrity was confirmed by 1H- and 13C-NMR, and HR-MS (see Supplementary Data).
Scheme 1.
Reagents and conditions: (i) anilines, 1,4-dioxane, 55 °C.
Scheme 3.
Reagents and conditions: (i) 2-amino-3-hydroxypyridine, dioxane 55 °C.
Scheme 2.
Reagents and conditions: (i) N,N-dimethylethanolamine, PPh3, DEAD, THF, 0 °C; (ii) 4-(2-hydroxyethyl)morpholine, PPh3, DEAD, THF, 0 °C; (iii) SnCl2, EtOH, 90 °C; (iv) phenylisocyanates, dioxane, 55 °C; (v) benzylchloroformate, NaOH 4N, CH3CN/H2O; (vi) PPh3, DEAD, THF, 0 °C; (vii) H2, Pd-C, MeOH, 1 atm; (viii) HCl 4N, dioxane.
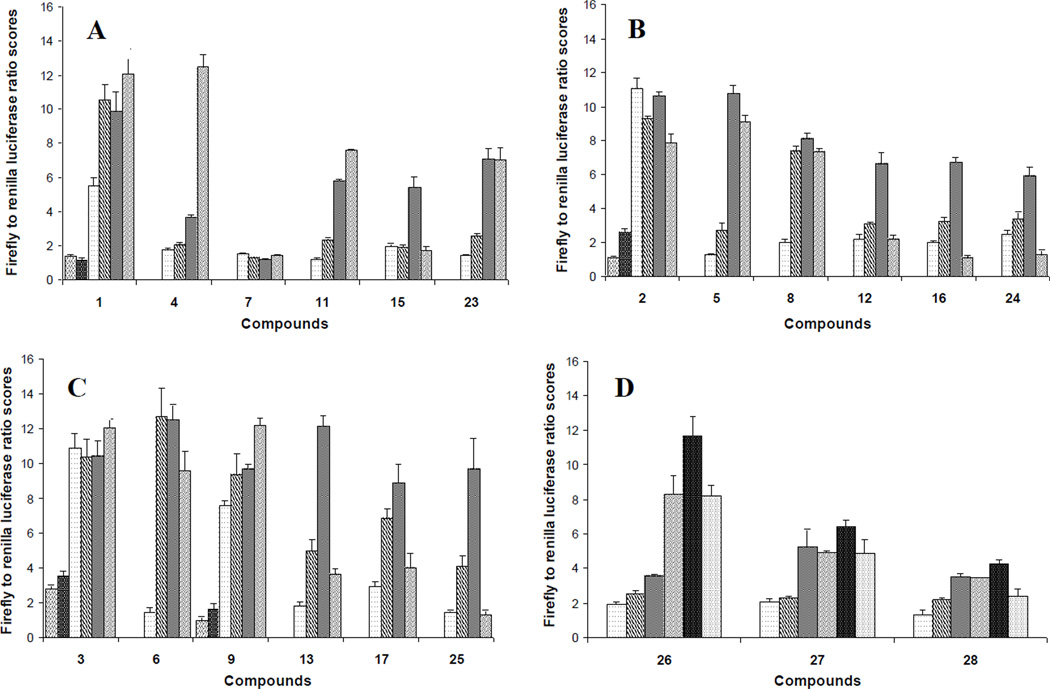
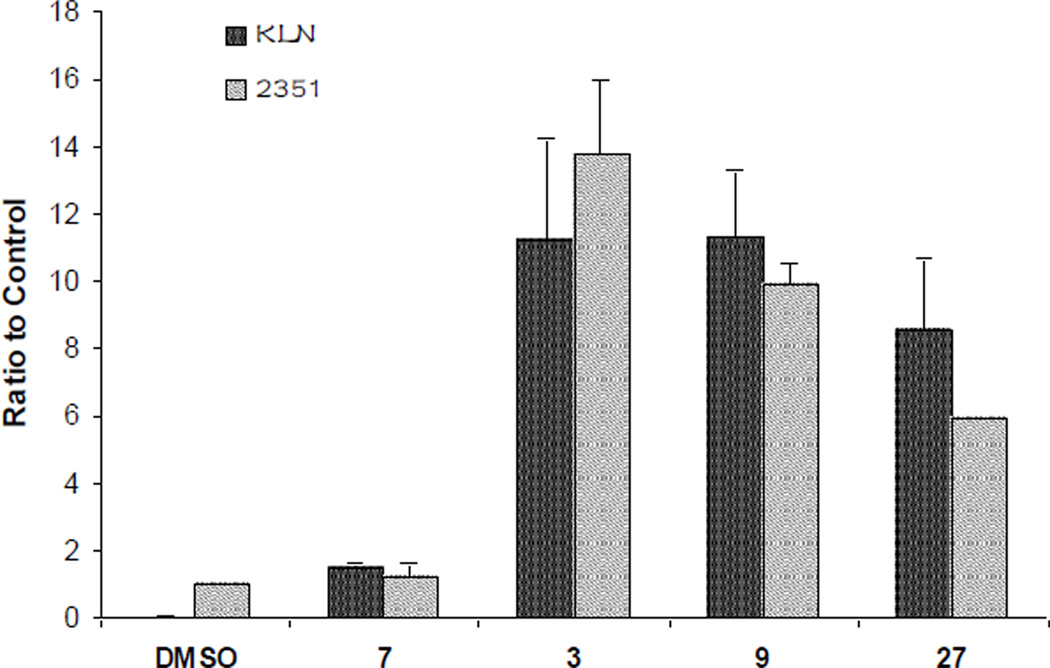
The non-symmetical N,N’-diarylureas in this SAR study were characterized for the depletion of the TC by the Dual-Glo Luciferase assay kit (Promega) in transiently transfected human CRL-2351 breast cancer cells (Figure 1) and in stably transfected murine KLN-205 lung squamous cell carcinoma (Figure S1 in the Supplementary Data).43 Although the overall activity profiles in KLN and CRL-2351 cells were similar the former were somewhat less sensitive to the ureas. Therefore, we focused our attention on results obtained with the most responsive cells, the CRL-2351. The details of assay development and validation have been described elsewhere.6,45 The non-symmetrical N,N’-diarylureas were also evaluated for growth inhibition in the KLN-205, CRL-2351, and human CRL-2813 melanoma cells using the sulforhoadamine B (SRB) assay (Table 1).11 These cell lines were chosen because they represent prevalent cancers and in preliminary studies they were used by us to validate the TC assay. Next, we selected representative N,N’-diarylureas and tested their activity in secondary mechanistic assays, namely eIF2α phosphorylation and CHOP expression assays that are directly related to the mechanism of action of these agents.
Figure 1.
Ternary complex assays on transiently tTA pBISA-DL(ATF-4) transfected CRL-2351 cell line. Activity of compounds was measured by the firefly/renilla luciferase ratio (F/R) compared to vehicle treated cells, and expressed as a function of the concentration (A, B, and C:  0.625 μM,
0.625 μM,  1.25 μM,
1.25 μM,  2.5 μM,
2.5 μM,  5 μM,
5 μM,  10 μM,
10 μM,  20 μM, and D:
20 μM, and D:  2.5 μM,
2.5 μM,  5 μM,
5 μM,  10 μM,
10 μM,  20 μM,
20 μM,  40 μM,
40 μM,  80 μM). N,N’-diarylureas were sorted into four groups based on the nature of their common aryl substituent: (A) ureas substituted by 3,4-dichlorophenyl, (B) ureas substituted by 3-trifluoromethyl-4-chloropenyl, (C) ureas substituted by 3,5-bis(trifluoromethyl)phenyl, and (D) ureas comprised of a 3-hydroxypyridin-2-yl ring. Each F/R value was obtained from averaging three independent experiments each conducted on a different day in triplicate and taking the F/R ratio score in vehicle wells in the same plates as 1.
80 μM). N,N’-diarylureas were sorted into four groups based on the nature of their common aryl substituent: (A) ureas substituted by 3,4-dichlorophenyl, (B) ureas substituted by 3-trifluoromethyl-4-chloropenyl, (C) ureas substituted by 3,5-bis(trifluoromethyl)phenyl, and (D) ureas comprised of a 3-hydroxypyridin-2-yl ring. Each F/R value was obtained from averaging three independent experiments each conducted on a different day in triplicate and taking the F/R ratio score in vehicle wells in the same plates as 1.
Table 1.
Inhibition of cancer cell proliferation by N,N’-diarylureas as determined by the SRB assaya
| Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| KLN | 2813 | 2351 | ||
| 1 | >20 | 1.0±0.3 | 2.4±0.5 | |
| 2 | 1.3±0.5 | 1.8±0.1 | 2.4±0.6 | |
| 3 | 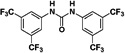 |
4.2±0.8 | 0.3±0.1 | 1.8±0.7 |
| 4 | >20 | 2.3±0.7 | 7.5±0.5 | |
| 5 | >20 | 1.6±0.6 | 9.5±0.2 | |
| 6 | 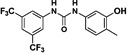 |
15.7±3.2 | 1.2±0.6 | 2.8±0.7 |
| 7 | >20 | 0.5±0.7 | 0.8±0.4 | |
| 8 | 18.6±2.5 | 2.8±1.1 | 2.3±0.8 | |
| 9 | 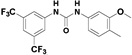 |
6.6±2.7 | 0.5±0.4 | 1.8±0.8 |
| 11 | 14.7±2.3 | 5.8±1.2 | 4.1±2.2 | |
| 12 | 13.6±2.4 | 2.3±1.1 | 2.4±0.1 | |
| 13 | 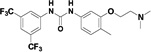 |
8.9±3.4 | 7.6±3.1 | 2.4±0.4 |
| 15 | >20 | 12±0.1 | 2.4±0.1 | |
| 16 | >20 | 1.1±0.2 | 2.6±0.1 | |
| 17 | 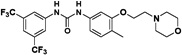 |
18.8±0.3 | 0.8±0.04 | 2.3±0.1 |
| 23 | >20 | 5.2±0.1 | 5.0±0.6 | |
| 24 | >20 | 2.5±1.6 | 1.8±0.4 | |
| 25 | 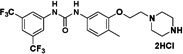 |
>20 | 3.2±0.5 | 4.2±0.8 |
| 26 | >20 | 1.6±0.6 | 1.4±0.9 | |
| 27 | 15.4±1.5 | 0.6±0.4 | 0.8±0.6 | |
| 28 | 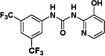 |
14.8±1.4 | 0.6±0.3 | 0.7±0.1 |
Each IC50 determination was performed at four concentrations (0.54, 1.8, 6 and 20 µM) on three different cell lines (KLN, CRL-2813 and CRL-2351) and each assay point was determined by triplicate in the SRB - (sulforhodamine B) assay.
In the dual luciferase TC assay we engineered cancer cell lines that express firefly (F) and renilla (R) luciferase open reading frame (ORF) under the control of a bi-directional promoter/enhancer complex.44 This assay was elaborated in our laboratory in order to identify compounds that reduce the availability of the eIF2·GTP·tRNAiMet TC. The premise of this assay is based on the fact that reduced availability of the TC inhibits translation of most mRNAs but paradoxically increases translation of some mRNAs that contain multiple tandem upstream ORF in their 5’-untranslated regions (5’UTRs). In our assay, firefly luciferase mRNA is fused to 5’UTR of activating transcription factor 4 (ATF-4) mRNA that has multiple tandem uORFs while renilla luciferase mRNA is fused to a 5’UTR lacking any uORFs. Compounds that reduce the availability of the TC would increase firefly luciferase expression while decreasing the renilla luciferase expression, resulting in an increased firefly/renilla luciferase ratio (F/R). To calculate the activity scores of the N,N’-diarylureas, the F/R ratios in compound treated wells were normalized to vehicle (DMSO) treated wells (F/R = 1). Figure 1 shows the F/R ratio scores for the different N,N’-diarylureas obtained from at least four-point dose-response studies carried out in triplicates in CRL-2351 cells. The N,N’-diarylureas were organized in four groups based on the nature of their substituents. In group A, all the compounds possess a common aryl moiety which is 3,4-dichlorophenyl while in group B and C the common aryl moieties are 3-trifluoromethyl-4-chlorophenyl and 3,5-bis(trifluoromethyl)-phenyl, respectively. As for group D, all the ureas possess the same 3-hydroxypyridin-2-yl moiety. The three parent symmetrical N,N’-diarylureas 1–3 indicate marked inhibition of TC formation as revealed by the marked increase of F/R ratio score over control. A F/R~11 was obtained at 2.5 μM for ureas 2 and 3 and at 5 μM for urea 1. Evidently, poor solubility in aqueous solutions that originates from their high hydrophobicity as reflected by their relatively high CLogP values (see Table S1 in the Supplementary Data) is a serious limitation in their development as potential therapeutics.
Therefore, we designed non-symmetrical N,N’-diarylureas 4–9 and 26–28 in which one of the phenyl moieties present in symmetrical N,N’-diarylureas 1–3 was preserved and introduced the phenyl 3-hydroxy-p-tolyl (as in ureas 4–6), 3-methoxy-p-tolyl (as in ureas 7–9), or 3-hydroxypyridin-2-yl (as in ureas 26–28) moieties that will carry a polar substituent (see CLogP values in Table S1) and at the same time provide a diversification site for further structural elaboration. As anticipated, the non-symmetrical N,N’-diarylureas 4–6 were able to maintain their activity in the TC assay with F/R ratio scores around 11 but at higher concentrations (20 μM for urea 4, 10 μM for urea 5 and 5 μM for urea 6). While the non-symmetrical urea 6 was almost as potent as its symmetrical parent urea 3, non-symmetrical ureas 4 and 5 were 4-fold less potent than their corresponding symmetrical ureas 1 and 2. The introduction of 3-hydroxypyridin-2-yl group resulted in lowering the F/R ratio score even more than the introduction of the 3-hydroxy-p-tolyl moiety (Figure 1, panel D). Indeed, urea 26, the most potent 3-hydroxypyridin-2-yl containing urea with a maximal F/R ratio score of 12 at 40 μM was still less potent than 4, the least active 3-hydroxy-p-tolyl-containing urea presenting the same maximal F/R ratio score but at 20 μM. Hence, we selected the non-symmetrical N,N’-diarylureas 4–6 for further SAR studies. As a prelude to potential decorations of the 3-hydroxyl function with polar and potentially charged substituents we carried out O-methylations generating 3-methoxy-p-tolyl-containing ureas 7–9 and characterized their ability to inhibit TC formation. Among these O-methylated N,N’-diarylureas, urea 7 was the least potent (maximal F/R ratio score < 2) while ureas 8 and 9 reached maximal F/R ratio scores of ~8 and ~9 at 5 μM, respectively. O-methylated ureas 8 and 9 were equipotent with the corresponding non-methylated ureas 5 and 6 in inhibiting TC formation. These encouraging results led to the design and synthesis of the next series of N,N’-diarylureas 11–13, 15–17, and 23–25 that were O-substituted with N,N-dialkylaminoethyl moieties (Scheme 2). The elaboration of the O-alkylating group by polar and basic moieties was quite rewarding. The improved solubility of non-symmetrical N,N’-diarylureas comprised of 3-(2-(dimethyl-amino) ethoxy)-4-methylphenyl (ureas 11–13), 4-methyl-3-(2-morpholinoethoxy)phenyl (ureas 15–17), and 4-methyl-3-(2-(piperazin-1-yl)ethoxy)phenyl (ureas 23–25) as indicated by CLogP, polarizability, and polar surface area (see Table S1 in the Supplementary Data) was accompanied by some loss of activity in the TC assay as compared to the corresponding phenols. At 10 μM the F/R ratio scores for these ureas ranged from ~6 to almost ~12. Regardless the nature of the N-{3-[2-(N,N-dialkylamino)ethoxy]-p-tolyl} moiety, the most potent inhibitors of TC formation were ureas 13, 17 and 25 that were N-substituted by the 3,5-bis(trifluoromethyl)phenyl moiety (at 10 μM max. F/R ratio scores were 9- to 12-fold of the control). Interestingly, O-alkylation of urea 4 with the various N,N-dialkylaminoethyl moieties resulted in ureas 11, 15, and 23 that were markedly more potent in the TC assay as compared with the O-methylated urea 7 (Figure 1, panel A).
Structure-activity relationship analysis of the N,N’-diarylureas consistently shows that ureas containing at least one 3,5-bis(trifluoromethyl)phenyl moiety were always more active in the TC assay than the analogous ureas containing either the 3,4-dichlorophenyl or the 3-trifluoromethyl-4-chlorophenyl moieties (Figure 1, panels A, B and C). In general, our two-step structure optimization strategy aimed at improving the physicochemical properties and at the same time preserving the F/R ratio scores proved to be quite successful. In the first step, we generated N,N’-diarylurea hybrids comprised of one aryl moiety equipped with electron withdrawing substituents and a second aryl moiety carrying an hydroxyl function. The former aryl moiety, hydrophobic in nature, seems to contribute most of the TC related activity, while the latter aryl moiety, more polar and hydrophilic in nature, contributes the favorable physicochemical properties. In the second step, O-alkylation of the hydroxyl by the N,N-dialkylaminoethyl moieties caused only a slight loss of activity in the TC assay but continue to improve the physicochemical properties.
The reduction of TC abundance was confirmed in two secondary mechanistic assays that included phosphorylation of eIF2α and expression of CHOP, a downstream effector. To this end we selected four N,N’-diarylureas: the active symmetrical 3, non-symmetrical 9 and 27, and the inactive 7, which served as a negative control. Phosphorylation of eIF2α was measured in CRL-2351 and KLN cells by the Western blot assay46 in which the total and the phosphorylated eIF2α (eIF2α-P) were analyzed using specific antibodies (Figure 2). Similarly expression of CHOP protein, a propapoptotic transcription factor, and CHOP mRNA levels, which are transcriptionally controlled by ATF-4, were determined respectively by Western blot and real-time PCR in the same cell lines (Figures 2 and 3). As anticipated, in both KLN and CRL-2351 cells, all the N,N’-diarylureas that were active in the TC assay (3, 9 and 27) also caused phosphorylation of eIF2α and increased the expression of CHOP protein without affecting the level of total-eIF2α or β-actin, a housekeeping protein. Indeed, the inactive urea 7 had minimal effect on the phosphorylation of eIF2α and did not induce expression of CHOP protein in either cell line. Consistently ureas 3, 9, and 27 significantly increased the expression of endogenous CHOP mRNA while urea 7 had minimal effect. These data showed a direct correlation between the activity of the compounds in the TC assay and their ability to induce the phosphorylation of eIF2α and the expression of endogenous CHOP protein and CHOP mRNA. Taken together, we conclude that these novel N,N’-diarlyureas inhibit translation initiation through phosphorylation of eIF2α, which in turn reduces the abundance of the TC.
Figure 2.
Phosphorylation of eIF2α and expression of CHOP protein in KLN and CRL-2351 cell lines. Murine KLN cells (A) were incubated with 20 μM while human CLR-2351 cells (B) were incubated with 5 μM of each compound. Expression of total and eIF2α-P (a) and β-actin and CHOP (b) were determined by Western blot analysis.
Figure 3.
Endogenous CHOP mRNA expression in KLN and CRL-2351 cell lines. Cells were incubated with 20 μM (for KLN) or 5 μM (for CRL-2351) of each compound and expression of endogenous CHOP mRNA was determined by real time PCR analysis. Each value was determined by triplicate.
Inhibition of cancer cells proliferation of the above mentioned focused library of N,N’-diarylureas was measured by the cell growth inhibition assay47 in one murine (KLN) and two human (CRL-2813 and CRL-2351) cancer cell lines employing the SRB assay (Table 1). In general, human melanoma CRL-2813 and human breast CRL-2351 cancer cell lines were the most sensitive to the ureas as compared to the KLN murine lung squamous carcinoma cell line. This is consistent with the higher activity of these compounds in the TC assay in CRL-2351 compared to KLN cells (Figures 1 and S1). Unlike the F/R ratio scores obtained in the TC assay, the dynamic range of IC50 values in the cell proliferation assay was smaller and less sensitive to the significant structural modifications. For example, low micromolar ureas 9 and 17 that possess the same common 3,5-bis(trifluoromethyl)phenyl moiety but differ in the substituents on the other phenyl moiety where the 3-hydroxyl function is substituted by either a methyl (urea 9) or a 2-(morpholin-4-yl)ethyl (urea 17), present similar IC50 values (IC50 = 0.5 and 1.8 μM for 9 and IC50 = 0.8 and 2.3 μM for 17 in CRL-2813 and CRL-2351 cells, respectively). In contrast, these ureas differ more in the concentration at which maximal F/R ratio score was obtained (a F/R ratio score of 10 at 5 μM and at 10 μM for ureas 9 and 17, respectively). More strikingly, urea 7 which was inactive in the TC assay is an effective inhibitor of cell proliferation with an IC50 = 0.5 and 0.8 μM in CRL-2813 and CRL-2351 cells, respectively. Interestingly, the 3-hydroxy-pyridin-2-yl containing N,N’-diarylureas 26–28 that were moderately potent in the TC assay inhibited potently cancer cell proliferation with IC50 = 1.6 and 1.4 μM for 26, IC50 = 0.6 and 0.8 μM for 27, and IC50 = 0.6 and 0.7 μM for 28 in CRL-2813 and CRL-2351 cells, respectively. The complexity underlining the cell proliferation assay originates from the systemic nature of the effects and the different time courses of this assay compared to the mechanistic assays (5 days for the cell proliferation assay vs. 16 hours for the TC assay and 5 hours for the eIF2α-P and CHOP expression assays). The systemic nature of compounds’ effect on cell proliferation also incorporates their off-target activity. This may explain, at least in part, why compound 7 was highly active in the cell proliferation assay despite being only marginally active in the mechanistic assays. Also, the decrease in F/R ratio score observed for N,N’-diarylureas such as 13, 17, and 25 at the highest tested concentration may be due to some off-target cytotoxicity.
Importantly, the sub- to low-micromolar IC50 values observed for most of the N,N’-diarylureas presented in this study and the good correlation between the sensitivities of cell lines to inhibition of cell proliferation and the F/R ratio scores in the TC assay are very encouraging and indicate that this class of compounds warrant further development as promising target specific anti-cancer agents.
Hit-to-lead optimization of highly hydrophobic symmetrical N,N’-diarylureas substituted by electron withdrawing groups has led to a novel series of more polar and potentially charged non-symmetrical N,N’-diarylureas that are lowering the abundance of the TC and thus leading to the inhibition of translation initiation. These agents activate HRI and phosphorylate eIF2α causing depletion of eIF2·GTP·tRNAiMet TC. We demonstrate that representative non-symmetrical N,N’-diarylureas that deplete the TC and inhibit cancer cell proliferation also cause phosphorylation of eIF2α and induce expression of CHOP at the protein and mRNA levels. In the absence of a high resolution structure of N,N’-diarylurea-target complex we could not apply target-guided structural optimization. The two step strategy for the improvement in physicochemical properties was accompanied by an acceptable reduction in their potency to lower the abundance of the TC. Nevertheless, reduced hydrophobicity and increased polarity together with their ability to achieve similar potency in reducing the abundance of the TC albeit at somewhat higher concentrations than the highly hydrophobic and symmetrical N,N’-diarylureas, suggest that non-symmetrical N,N’-diarylureas are good candidates for further development as anti-cancer agents. We plan to promote some of the more promising non-symmetrical N,N’-diarylureas described in this study into in vivo tests in animal models of human cancers and assess their potential as novel, non-toxic, and mechanismspecific anti-cancer agents.
Supplementary Material
Acknowledgement
This work was supported by Susan B. Komen Cure for Cancer Foundation research grant BCTR0707713 and NIH grant #R21AG032546 to Bertal H. Aktas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Supplementary Data associated with this article (calculated physicochemical properties of the reported N,N’-diarylureas, TC assays on KLN cell line, HPLC and HR mass spectral data of the N,N’-diarylureas, synthetic procedures, 1H NMR and HPLC analyses of the four selected N,N’-diarylureas (3, 7, 9, and 27) can be found at:
References and Notes
- 1.De Benedetti A, Harris AL. Int. J. Biochem. Cell Biol. 1999;31:59. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 2.Graff JR, Boghaert ER, De Benedetti A, Tudor DL, Zimmer CC, Chan SK, Zimmer SG. Int. J. Cancer. 1995;60:255. doi: 10.1002/ijc.2910600221. [DOI] [PubMed] [Google Scholar]
- 3.Cohen N, Sharma M, Kentsis A, Perez JM, Strudwick S, Borden KL. EMBO J. 2001;20:4547. doi: 10.1093/emboj/20.16.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kentsis A, Dwyer EC, Perez JM, Sharma M, Chen A, Pan ZQ, Borden KL. J. Mol. Biol. 2001;312:609. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 5.Rousseau D, Gingras AC, Pause A, Sonenberg N. Oncogene. 1996;13:2415. [PubMed] [Google Scholar]
- 6.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, He X, Zvereva N, Supko JG, Chorev M, Halperin JA, Aktas BH. Nat. Chem. Biol. 2011;7:610. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berns A. Cell. 2008;133:29. doi: 10.1016/j.cell.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Marshall RA, Dorywalska M, Puglisi JD. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15364. doi: 10.1073/pnas.0805299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dever TE, Yang W, Astrom S, Bystrom AS, Hinnebusch AG. Mol. Cell. Biol. 1995;15:6351. doi: 10.1128/mcb.15.11.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aktas H, Fluckiger R, Acosta JA, Savage JM, Palakurthi SS, Halperin JA. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8280. doi: 10.1073/pnas.95.14.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palakurthi SS, Fluckiger R, Aktas H, Changolkar AK, Shahsafaei A, Harneit S, Kilic E, Halperin JA. Cancer Res. 2000;60:2919. [PubMed] [Google Scholar]
- 12.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Cancer Res. 2001;61:6213. [PubMed] [Google Scholar]
- 13.Kozak M. J. Cell Biol. 1991;115:887. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak M. J. Biol. Chem. 1991;266:19867. [PubMed] [Google Scholar]
- 15.De Benedetti A, Graff JR. Oncogene. 2004;23:3189. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 16.Zarghi A, Kakhgi S, Hadipoor A, Daraee B, Dadrass OG, Hedayati M. Bioorg. Med. Chem. Lett. 2008;18:1336. doi: 10.1016/j.bmcl.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Souers AJ, Voorbach M, Falls HD, Droz B, Brodjian S, Lau YY, Iyengar RR, Gao J, Judd AS, Wagaw SH, Ravn MM, Engstrom KM, Lynch JK, Mulhern MM, Freeman J, Dayton BD, Wang X, Grihalde N, Fry D, Beno DW, Marsh KC, Su Z, Diaz GJ, Collins CA, Sham H, Reilly RM, Brune ME, Kym PR. J. Med. Chem. 2008;51:380. doi: 10.1021/jm7013887. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Luthi U, Kolb P, Edler K, Cecchini M, Audetat S, Barberis A, Caflisch A. J. Med. Chem. 2005;48:5108. doi: 10.1021/jm050499d. [DOI] [PubMed] [Google Scholar]
- 19.Asano S, Ban H, Kino K, Ioriya K, Muraoka M. Bioorg. Med. Chem. Lett. 2009;19:1062. doi: 10.1016/j.bmcl.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Davidson W, Frego L, Peet GW, Kroe RR, Labadia ME, Lukas SM, Snow RJ, Jakes S, Grygon CA, Pargellis C, Werneburg BG. Biochemistry. 2004;43:11658. doi: 10.1021/bi0495073. [DOI] [PubMed] [Google Scholar]
- 21.Guichou JF, Viaud J, Mettling C, Subra G, Lin YL, Chavanieu A. J. Med. Chem. 2006;49:900. doi: 10.1021/jm050716a. [DOI] [PubMed] [Google Scholar]
- 22.Valgeirsson J, Nielsen EO, Peters D, Mathiesen C, Kristensen AS, Madsen U. J. Med. Chem. 2004;47:6948. doi: 10.1021/jm030638w. [DOI] [PubMed] [Google Scholar]
- 23.Jin Q, Nie H, McCleland BW, Widdowson KL, Palovich MR, Elliott JD, Goodman RM, Burman M, Sarau HM, Ward KW, Nord M, Orr BM, Gorycki PD, Busch-Petersen J. Bioorg. Med. Chem. Lett. 2004;14:4375. doi: 10.1016/j.bmcl.2004.06.097. [DOI] [PubMed] [Google Scholar]
- 24.Widdowson KL, Elliott JD, Veber DF, Nie H, Rutledge MC, McCleland BW, Xiang JN, Jurewicz AJ, Hertzberg RP, Foley JJ, Griswold DE, Martin L, Lee JM, White JR, Sarau HM. J. Med. Chem. 2004;47:1319. doi: 10.1021/jm034248l. [DOI] [PubMed] [Google Scholar]
- 25.Pender C, Goldfine ID, Manchem VP, Evans JL, Spevak WR, Shi S, Rao S, Bajjalieh S, Maddux BA, Youngren JF. J. Biol. Chem. 2002;277:43565. doi: 10.1074/jbc.M202426200. [DOI] [PubMed] [Google Scholar]
- 26.Teegarden BR, Li H, Jayakumar H, Strah-Pleynet S, Dosa PI, Selaya SD, Kato N, Elwell KH, Davidson J, Cheng K, Saldana H, Frazer JM, Whelan K, Foster J, Espitia S, Webb RR, Beeley NR, Thomsen W, Morairty SR, Kilduff TS, Al-Shamma HA. J. Med. Chem. 2010;53:1923. doi: 10.1021/jm9007328. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, Khafizova G, Brooijmans N, Mallon R, Hollander I, Feldberg L, Lucas J, Yu K, Gibbons J, Abraham RT, Chaudhary I, Mansour TS. J. Med. Chem. 2010;53:2636. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Venkatesan AM, Dehnhardt CM, Ayral-Kaloustian S, Brooijmans N, Mallon R, Feldberg L, Hollander I, Lucas J, Yu K, Kong F, Mansour TS. J. Med. Chem. 2010;53:3169. doi: 10.1021/jm901783v. [DOI] [PubMed] [Google Scholar]
- 29.Dai Y, Guo Y, Frey RR, Ji Z, Curtin ML, Ahmed AA, Albert DH, Arnold L, Arries SS, Barlozzari T, Bauch JL, Bouska JJ, Bousquet PF, Cunha GA, Glaser KB, Guo J, Li J, Marcotte PA, Marsh KC, Moskey MD, Pease LJ, Stewart KD, Stoll VS, Tapang P, Wishart N, Davidsen SK, Michaelides MR. J. Med. Chem. 2005;48:6066. doi: 10.1021/jm050458h. [DOI] [PubMed] [Google Scholar]
- 30.Dai Y, Hartandi K, Ji Z, Ahmed AA, Albert DH, Bauch JL, Bouska JJ, Bousquet PF, Cunha GA, Glaser KB, Harris CM, Hickman D, Guo J, Li J, Marcotte PA, Marsh KC, Moskey MD, Martin RL, Olson AM, Osterling DJ, Pease LJ, Soni NB, Stewart KD, Stoll VS, Tapang P, Reuter DR, Davidsen SK, Michaelides MR. J. Med. Chem. 2007;50:1584. doi: 10.1021/jm061280h. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki Y, Tang J, Maeda Y, Nakano M, Wang L, Nolte RT, Sato H, Sugai M, Okamoto Y, Truesdale AT, Hassler DF, Nartey EN, Patrick DR, Ho ML, Ozawa K. Bioorg. Med. Chem. Lett. 2007;17:1773. doi: 10.1016/j.bmcl.2006.12.077. [DOI] [PubMed] [Google Scholar]
- 32.Sun M, Chen J, Wei H, Yin S, Yang Y, Ji M. Chem. Biol. Drug Des. 2009;73:644. doi: 10.1111/j.1747-0285.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 33.Arrington KL, Dudkin VY. ChemMedChem. 2007;2:1571. doi: 10.1002/cmdc.200700131. [DOI] [PubMed] [Google Scholar]
- 34.Tao ZF, Wang L, Stewart KD, Chen Z, Gu W, Bui MH, Merta P, Zhang H, Kovar P, Johnson E, Park C, Judge R, Rosenberg S, Sowin T, Lin NH. J. Med. Chem. 2007;50:1514. doi: 10.1021/jm061247v. [DOI] [PubMed] [Google Scholar]
- 35.Boyle RG, Imogai HJ, Cherry M. PCT Int. Appl. WO 2003101444 A1 20031211. 2003 [Google Scholar]; Chem. Abstr. 2003;140:16750. [Google Scholar]
- 36.Anderson MO, Yu H, Penaranda C, Maddux BA, Goldfine ID, Youngren JF, Guy RK. J. Comb. Chem. 2006;8:784. doi: 10.1021/cc050136z. [DOI] [PubMed] [Google Scholar]
- 37.Gable KL, Maddux BA, Penaranda C, Zavodovskaya M, Campbell MJ, Lobo M, Robinson L, Schow S, Kerner JA, Goldfine ID, Youngren JF. Mol. Cancer Ther. 2006;5:1079. doi: 10.1158/1535-7163.MCT-05-0397. [DOI] [PubMed] [Google Scholar]
- 38.Khire UR, Bankston D, Barbosa J, Brittelli DR, Caringal Y, Carlson R, Dumas J, Gane T, Heald SL, Hibner B, Johnson JS, Katz ME, Kennure N, Kingery-Wood J, Lee W, Liu XG, Lowinger TB, McAlexander I, Monahan MK, Natero R, Renick J, Riedl B, Rong H, Sibley RN, Smith RA, Wolanin D. Bioorg. Med. Chem. Lett. 2004;14:783. doi: 10.1016/j.bmcl.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. Cancer Res. 2004;64:7099. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 40.Penning TD, Chandrakumar NS, Chen BB, Chen HY, Desai BN, Djuric SW, Docter SH, Gasiecki AF, Haack RA, Miyashiro JM, Russell MA, Yu SS, Corley DG, Durley RC, Kilpatrick BF, Parnas BL, Askonas LJ, Gierse JK, Harding EI, Highkin MK, Kachur JF, Kim SH, Krivi GG, Villani-Price D, Pyla EY, Smith WG. J. Med. Chem. 2000;43:721. doi: 10.1021/jm990496z. [DOI] [PubMed] [Google Scholar]
- 41.Zhu GD, Gandhi VB, Gong J, Thomas S, Woods KW, Song X, Li T, Diebold RB, Luo Y, Liu X, Guan R, Klinghofer V, Johnson EF, Bouska J, Olson A, Marsh KC, Stoll VS, Mamo M, Polakowski J, Campbell TJ, Martin RL, Gintant GA, Penning TD, Li Q, Rosenberg SH, Giranda VL. J. Med. Chem. 2007;50:2990. doi: 10.1021/jm0701019. [DOI] [PubMed] [Google Scholar]
- 42.Malkov AV, Figlus M, Stoncius S, Kocovsky P. J. Org. Chem. 2007;72:1315. doi: 10.1021/jo062215i. [DOI] [PubMed] [Google Scholar]
- 43.Stable and transient transfection into KLN-205 and CRL-2351, respectively, utilized in this study are described elsewhere (see ref. 6). Cells were seeded at the density of 105 in 60-mm dish (stable transfection) or 104 cells per well of 96-well plate (transient transfection) and transfected one day later using the Lipofectamine 2000 (Invitrogen). For selection of stable cell lines, transfected cells were transferred to 100-mm plates and selected with appropriate antibiotics.
- 44.Plasmids and Ternary complex assay. Briefly, we modified bi-directional mammalian expression vector pBI (Clontech, CA) to expand the multiple cloning sites (MCSs) and designated it thereafter as pBISA. This vector contains seven copies of the tetracycline-regulated transactivator response element (TRE), which together act as core promoter/enhancer. The TRE is flanked on both sides by minimal human cytomegalovirus (CMV) minimal promoters allowing bi-directional transcription and two MCSs. Firefly and renilla luciferases were subcloned into MCS-I and MCS-II, respectively. This plasmid, designated pBISA-DL, transcribes two mRNAs that contain the 90 nucleotide plasmid derived 5'UTR (same sequence in both mRNAs), and the ORF encoding either firefly or renilla luciferase followed by a polyadenylation sequence (see ref. 45). This plasmid was further modified by inserting the 5'UTR of ATF-4 into MCS-I in front of the firefly luciferase mRNA. Transcription from this direction generates an mRNA that contains the firefly luciferase ORF preceded by a 5'UTR composed of 90 nucleotides derived from the plasmid and 267 nucleotides derived from the 5’UTR of ATF-4 mRNA. Transcription from the other direction generates an mRNA that contains the renilla luciferase ORF proceeded only by the 90-nucleotide plasmid-derived sequence in the 5'UTR. This expression plasmid is called pBISA-DL(ATF-4). In the dual luciferase assay, cells expressing firefly and renilla luciferases were lysed and the extracts assayed with a Dual-Glo Luciferase assay kit, per manufacturer’s instruction (Promega Inc. Madison, WI). The data calculations were carried out as the ratio of firefly to renilla luciferase signal (see ref. 6).
- 45.Ziegeler G, Ming J, Koseki JC, Sevinc S, Chen T, Ergun S, Qin X, Aktas BH. J. Biol. Chem. 2010;285:15408. doi: 10.1074/jbc.M110.113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Western blotting. Cells cultured under recommended media conditions, were plated and maintained in serum-containing media without antibiotics in 14-cm plates (Nunc) until reaching 70% confluency. Cells were then treated with compounds (20 µM for KLN and 5 µM for CRL-2351) for 6 hours, washed with cold PBS once, and lysed with M-PER Mammalian Protein Extraction Reagent (Pierce) for 1 hour on ice. The cell lysates were further centrifuged at 12,000 RPM for 5 min and the supernatants were transferred to fresh tubes and the concentrations were determined by BCA methods. Proteins were denatured by mixing with Laemmli Sample Buffer, heated at 100°C for 5 min and separated by SDS-PAGE and probed with anti-phosphoserine-51-eIF2α (Phos-eIF2α), anti-total eIF2α-specific antibodies (Total-eIF2α) (Biosource International, Hopkinton, MA), anti-CHOP, or anti b-actin (Santa Cruz Biotechnology, CA).
- 47.Cell growth assay. Adherent mouse (KLN) and human solid tumor cells (CRL-2351, and CRL-2813) were plated in 96-well plates and maintained for 5 days in the presence of 0.54 to 6 µM of individual compound, and cell proliferation was measured by the sulforhodamine B (SRB) assay as described in ref. 11: briefly, cells were fixed in 10% cold trichloroacetic acid at 4°C for 1 h, extensively washed with double-distilled H2O and air-dried. Plates were then incubated with 0.4% SRB in 1% acetic acid for 1 h, washed with 1% acetic acid to remove the unbound dye, and air-dried. The bound dye was solubilized by addition of 10 mM Tris (pH 10), and the absorbance was determined in a Titertek Multiscan plate reader at 490 nm. The data calculations were carried out as described (the values for mean ± SD of data from replicate wells are calculated): data are expressed in terms of %T/C [(OD of treated cells/OD of control cells)×100], as a measure of cell viability and survival in the presence of test materials. Calculations are also made for the concentration of test agents giving a T/C value of 50%, or 50% growth inhibition (IC50). With the SRB assay, a measure is made of the cell population density at time 0 (the time at which drugs are added) from two extra reference plates of inoculated cells fixed with TCA just prior to drug addition to the test plates. Thus, we have three measurements: control optical density (C), test optical density (T), and optical density at time zero (T0). The calculation is 100×[(T-T0)/(C-T0)]. If T is less than T0, cell killing has occurred and can be calculated from 100×[(T-T0)/T0]. Thus, for each drug-cell line combination, a dose-response curve is generated and three levels of effect are calculated.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.