Abstract
Brain derived neurotrophic factor (BDNF) signaling through its receptor, TrkB is known to regulate GABAergic function and glutamic acid decarboxylase (GAD) 67 expression in neurons. Alterations in BDNF signaling have been implicated in the pathophysiology of schizophrenia and as a result, they are a potential therapeutic target. Interestingly, heterozygous reeler mice (HRM) have decreased GAD67 expression in the frontal cortex and hippocampus and they exhibit many behavioral and neurochemical abnormalities similar to schizophrenia. In the present study, we evaluated the potential of cysteamine, a neuroprotective compound to improve the deficits in GAD67 expression and cognitive function in HRM. We found that cysteamine administration (150 mg/kg/day, through drinking water) for 30 days significantly ameliorated the decreases in GAD67, mature BDNF and full-length TrkB protein levels found in frontal cortex and hippocampus of HRM. A significant attenuation of the increased levels of truncated BDNF in frontal cortex and hippocampus, as well as truncated TrkB in frontal cortex of HRM was also observed following cysteamine treatment. In behavioral studies, HRM were impaired in a Y-maze spatial recognition memory task, but not in a spontaneous alternation task or a sensorimotor, prepulse inhibition (PPI) procedure. Cysteamine improved Y-maze spatial recognition in HRM to the level of wide-type controls and it improved PPI in both wild-type and HRM. Finally, mice deficient in TrkB, showed a reduced response to cysteamine in GAD67 expression suggesting that TrkB signaling plays an important role in GAD67 regulation by cysteamine.
Keywords: GAD67, BDNF, TrkB, cysteamine, cognition, reeler, mice
Introduction
There is growing interest in the potential role of the gamma amino butyric acid (GABA) neurotransmitter system in the pathophysiology and behavioral abnormalities of schizophrenia. GABA has also been implicated in multiple processes of neural development (Owens and Kriegstein, 2002) such as cell proliferation (LoTurco et al 1995), neurite growth (Spoerri, 1988) and adult neurogenesis (Ge et al 2006). A number of studies have reported decrease in mRNA and protein levels of GAD67, a rate limiting enzyme in GABA synthesis in prefrontal cortex as well as other brain areas of schizophrenia subjects (Akbarian and Huang, 2006). In animal studies, GABA levels were reduced in adult heterozygotes of GAD67 knockout mice (Asada et al, 1997). Studies also suggest that the alterations in GABAergic system appear to have an important role in the cognitive abnormalities found in schizophrenia (Lewis and Moghaddam, 2006).
BDNF signaling through TrkB has been shown to be a key regulator in GAD67 expression and GABA function (Arenas et al 1996; Mizuno et al 1994). TrkB levels were found significantly lower in prefrontal cortex and hippocampus of schizophrenia subjects (Takahashi et al 2000; Thompson Ray et al 2011; Weickert et al 2005). In addition, a significant correlation between GAD67 and TrkB mRNA levels were found in the prefrontal cortex of schizophrenia subjects (Hashimoto et al 2005). The relationship between GAD67 and TrkB was further supported by the evidence that TrkB hypomorphic mice, but not BDNF knockout mice have reduced GAD67 expression in prefrontal cortex (Hashimoto et al 2005). These studies suggest that TrkB signaling is a possible molecular target to regulate GAD67 expression in cortex.
Heterozygous reeler mice (HRM) have many neuropathological and behavioral abnormalities homologous to schizophrenia, such as increased neuronal packing density and decreased spine density with GABAergic defect as reflected in downregulation of GAD67 expression in frontal cortex (Liu et al 2001) and hippocampus (Nullmeier et al 2011), as well as deficits in prepulse inhibition (PPI) of startle (Tueting et al 1999). Reelin is a large extracellular matrix protein that plays a critical role during brain development, but also continues to be expressed in adult cortex and hippocampus (D'Arcangelo et al 1995). In the adult brain, reelin is secreted by GABAergic interneurons into the extracellular space surrounding dendrites, dendritic spines and axon boutons (Alcantara et al 1998; Pappas et al 2001). It has been shown that reelin expression is down-regulated in GABAergic neurons of the prefrontal (Brodmann areas 10 and 46) temporal and parietal cortices, hippocampus, caudate nucleus, and glutamatergic cerebellar neurons of schizophrenia patients (Guidotti et al 2000). Our recent experiments have shown that HRM have decreased TrkB signaling in frontal cortex (Pillai and Mahadik, 2008).
Cysteamine, the FDA-approved drug currently prescribed for cystinosis, has anti-oxidant and antiapoptotic properties (Lesort et al 2003; Oliverio et al 1999), and it increases brain as well as serum BDNF levels in rodents (Borrell-Pages et al 2006; Pillai et al 2008). In the present study, we evaluated the potential of cysteamine to improve the deficits in GAD67 expression and cognitive function in HRM. Since TrkB plays an important role in the regulation of GAD67 expression, we investigated whether TrkB is necessary for cysteamine-induced effects on GAD67 expression using TrkB knockout mice.
Materials and Methods
Animals and Housing
Male HRM (B6C3Fe reln) and background strain wild-type (WT) mice, 2–3 months of age, were obtained from Jackson Laboratories (Bar Harbor, ME). TrkB knockout (TrkB−/−) were provided by Dr. Barbara Rohrer, Medical University of South Carolina, Charleston, SC and the colony was maintained in our animal housing facility at the Georgia Health Sciences University. The generation of mice lacking the TrkB has been described previously (Rohrer et al 2004). Male WT and TrkB−/− mice used in a given experiment, originated from the same breeding series and were matched for age and weight (age=2–3 months; weight=25–30 g). Animals were housed 4 mice per cage with water and food available ad libitum. Mice were maintained on a 12-h light–dark cycle with the lights on at 0700 hours. All experimental procedures were performed during the light cycle. Animal use procedures were performed after being reviewed and approved by Medical College of Georgia, Committee on Animal Use for Research and Veterans Affairs Medical Center Subcommittee on Animal Use. Procedures were consistent with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines as per Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Drug Treatment
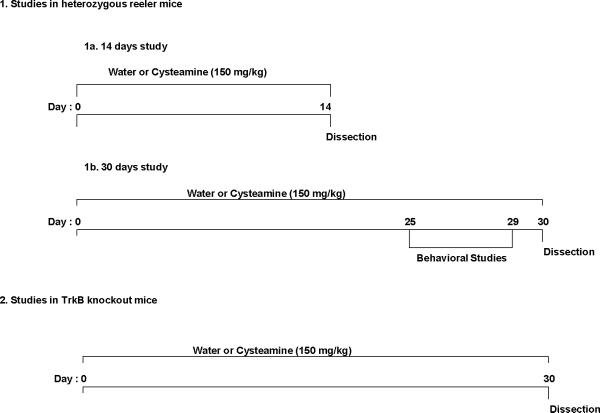
Fig. 1 describes the experimental design used for the drug treatment. Cysteamine (Sigma, St Louis, MO) was dissolved in water and was delivered ad libitum in the drinking water (150 mg/kg body wt/day). Cysteamine dose was selected based on earlier studies where this concentration was found non-toxic, but showed neuroprotective effects (Borrell-Pages et al. 2006; Pillai et al., 2008). The amount of drug intake was measured daily, and adjustments were made depending upon the fluid consumed and weight of the animals. Tap water was used for control group to assure that unanticipated effect of the vehicle was not present. All animals were monitored for change in body weight and food intake as possible adverse effects of the treatment.
Fig 1.
Experimental designs used for testing the behavioral and biochemical responses to cysteamine treatment in mice. (1a) Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to heterozygous reeler mice (HRM) and wild-type (WT) mice for 14 days. On day 14, mice were killed and brains removed for biochemical analyses. (1b) Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and WT mice for 30 days. In order to study the behavioral responses to cysteamine or vehicle treatment, animals were tested for behavioral studies from day 25 to day 29. On day 30, mice were killed and brains removed for biochemical analyses. (2) Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to TrkB knockout and WT mice for 30 days. On day 30, mice were killed and brains removed for biochemical analyses.
Behavioral Studies
All behavioral experiments were conducted in rooms equipped with white noise generators (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB, and ambient lighting of approximately 25–30 Lux (lumen/m2). Animals were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Three behavioral tests were carried out during the last week of drug treatment conducted in the following order, Y-Maze spontaneous alternation, Y-maze two trial recognition memory test, and prepulse inhibition. All behavioral tests were conducted between 0900 and 1700 hours.
Y-Maze Tests
To assess the effects of genotype and cysteamine on memory-related behaviors, two Y maze tasks were used, spontaneous alternation and the two-trial recognition test.
Apparatus
The Y-maze test apparatus consisted of three arms made of acrylic glass (Plexiglas) (painted black) and joined in the middle to form a symmetrical “Y” shape. The arms of the apparatus are 40 cm long × 12 cm wide. The walls of the arms are 35 cm high, allowing the mouse to see distal spatial landmarks while the inside of the arms are identical, providing no intramaze cues.
Spontaneous Alternation test
In this task, test subjects were placed in the Y maze (with all arms open) for 10 min and all arm entries were sequentially scored. The dependent variables were defined as the number of arms entered, and percent alternation, calculated as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (i.e., the number of arms entered minus 2) and multiplied by 100.
Two-trial recognition memory test
With one arm of the Y maze blocked, subjects were placed in one of the arms (start arm) and allowed to explore the 2 open arms for 10 minutes. The subject was then returned to the maze two hours later with all arms open and scored for 5 minutes. The amount of time spent in each arm, and the number of entries into each arm was recorded. The time spent and entries into the previously unexplored arm were assessed as measures of recognition memory.
Prepulse Inhibition
To assess the effects of genotype and cysteamine on sensorimotor gating a PPI procedure (modified for mice) was conducted as described previously (Terry et al 2005). Four startle chambers (San Diego Instruments, San Diego, CA) were used that consist of a Plexiglas tube (diameter 2.8 cm, length 8.9 cm) placed in a sound–attenuating chamber, in which the mice are individually placed. The tube is mounted on a plastic frame, under which a piezoelectric accelerometer is mounted, which records and transduces the motion of the tube. Two days before PPI testing the experimental subjects were each placed in one of the startle test chambers for a period of 10 minutes (without any startle stimuli) as an initial period of acclimation to the apparatus. One day before PPI testing, the animals were again placed in the test chamber and then exposed to 12 startle stimuli and to each prepulse level 3 times. This procedure is done to reduce the highly variable responses to the initial exposures to the startle stimuli as well as to ensure that the prepulse stimuli (alone) have no significant effect on the startle response. On the day of PPI testing, experimental subjects were transported to the startle chamber room and left undisturbed for at least 30 min. Afterwards, the mice were placed in the chamber, and then allowed to habituate for a period of 5 min, during which a 70 dB background white noise was present. After this period, the mice received 12 startle trials, 12 no-stimulus trials, and 12 trials of each of the prepulse/startle trials for a total of 60 trials. The intertrial interval ranged from 10 to 30 s, and the total session lasted about 25–30 min. The startle trials consisted of single 120 dB white noise bursts lasting 20 ms.
The prepulse inhibition trials consisted of a prepulse (20 ms burst of white noise with intensities of 75, 80, or 85 dB) followed, 100 ms later, by a startle stimulus (120 dB, 20 ms white noise). During the no-stimulus trial, no startle noise was presented, but the movement of the mouse was recorded. This represents a control trial for detecting differences in overall activity. The 60 different trials were presented pseudorandomly, ensuring that each trial was presented 12 times and that no two consecutive trials were identical. The resulting movement of the mouse in the startle chamber was measured during 100 ms after startle stimulus onset (sampling frequency 1 kHz), rectified, amplified, and fed into a computer that calculates the maximal response over the 100-ms period. Basal startle amplitude was determined as the mean amplitude of the 12 startle trials. Prepulse inhibition was calculated according to the formula 100–100% × (PPx/P120), in which PPx is the mean of the 12 prepulse inhibition trials (i.e., for each individual prepulse intensity), and p120 is the basal startle amplitude.
Tissue sample preparation
Animals were sacrificed by cervical dislocation, and frontal cortex and hippocampus samples from vehicle as well as drug-treated mice were collected according to a mouse brain atlas (Paxinos and Franklin, 2001). Tissue homogenates were made from frozen tissue sonicated in radioimmune precipitation assay (RIPA) buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 1% sodium deoxycholate) for western blotting. This buffer was supplemented with a protease inhibitor cocktail (Sigma). After 15 min incubation on ice, the extracts were clarified by centrifugation at 16,000 g for 15 min at 4°C and stored at −70°C. Protein concentration was determined by the bicinchoninic acid method (BCA Protein Assay Kit, Sigma).
Western blot analysis
Equal amounts of protein were resolved in SDS–polyacrylamide gels and transferred electrophoretically onto a nitrocellulose membrane. The membrane was blocked for 1 h in Phosphate Buffered Saline (PBS) solution with the detergent Tween 20 (PBST; 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween 20, pH 7.4.) and 5% non-fat milk or 5% BSA. The membranes were incubated overnight with the indicated primary antibodies. The primary antibodies used were anti-BDNF (1:500; #sc-546; Santa Cruz Biotechnology, Santa Cruz, CA), anti-TrkB (1:300; #4603; Cell Signaling, Danvers, MA), anti-TrkB.TK- (1:500; #sc-119; Santa Cruz Biotechnology), anti-GAD67 (1:500; #sc-5602; Santa Cruz Biotechnology), and anti-β-actin (1:1000; #A5316; Sigma, St. Louis, MO). The membranes were washed with PBST then incubated with secondary antibody for 1 h. Proteins were visualized by enhanced chemiluminescence. The films were subsequently scanned, and band intensity was quantified by densitometry software (Image J, NIH).
Statistical Analysis
Results are expressed as the mean±SE. The significance of differences was determined by one-way analysis of variance (ANOVA) in experiments analyzing protein levels and Y-maze analysis. Repeated measures ANOVA was used in PPI response measurement followed by the post hoc Bonnferroni's test. p-values less than 0.05 were regarded as statistically significant.
Results
Behavioral studies were carried out only in mice treated with vehicle or cysteamine for 30 days where as biochemical analyses were performed in both 14 and 30 days treatment groups. There were no differences in relative body weight gain or water intake in mice during the treatment.
Effect of cysteamine on GAD67 protein levels in heterozygous reeler mice
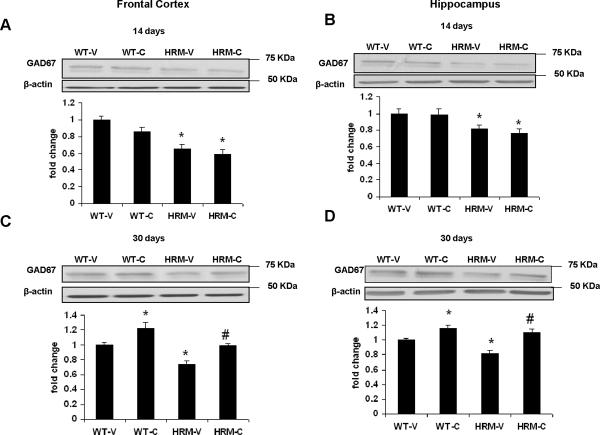
Fig. 2 shows the GAD67 protein levels in prefrontal cortex and hippocampus determined after 14 or 30 days of cysteamine administration. One way ANOVA showed a significant group effect in GAD67 protein levels in frontal cortex [F(3, 16)=16.06, p<0.0001] and hippocampus [F(3, 16)=6.5, p=0.0045]. Subsequent comparisons by Bonferroni's Multiple Comparison test indicated significant reduction in GAD67 protein levels in both frontal cortex (Fig 2A) and hippocampus (Fig 2B) of HRM as compared to WT mice (p<0.05). A comparison between vehicle and cysteamine treated animals after 14 days of treatment revealed no significant difference in the levels of GAD67 protein in the frontal cortex (Fig 2A) and hippocampus (Fig 2B). Mice treated with cysteamine or vehicle for 30 days showed a statistically significant group effect in GAD67 protein levels in frontal cortex [F(3, 16)=13.88, p=0.0001] and hippocampus [F(3, 16)=12.24, p=0.0002]. Subsequent analysis showed that WT mice treated with cysteamine for 30 days showed significant increases in GAD67 protein levels in frontal cortex (Fig 2C) and hippocampus (Fig 2D). The increases in GAD67 levels were also found in the frontal cortex (Fig 2C) and hippocampus (Fig 2D) of HRM (p<0.05).
Fig 2.
Cysteamine treatment ameliorates the reduction in GAD67 expression in frontal cortex and hippocampus of heterozygous reeler mice (HRM). Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 14 (Figs 2A and 2B) or 30 (Figs 2C and 2D) days. GAD67 expression was determined by western blot analysis in frontal cortex (Figs 2A and 2C) and hippocampus (Figs 2B and 2D). WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Data represent mean±SE (n =5 per group) expressed as fold change in GAD67 protein levels as compared to WT-V (* P < 0.05 vs WT-V; #P < 0.05 vs HRM-V; Bonferroni's test). β-actin is the loading control.
Effect of cysteamine on BDNF protein levels in heterozygous reeler mice
Our immunoblot analysis with BDNF antibody showed proBDNF (~32 KDa), truncated BDNF (~27 KDa) and mature BDNF (~14 KDa). One-way ANOVA showed a significant group effect in BDNF protein levels in frontal cortex (mature BDNF [F(3, 16)=34.55, p<0.0001]; truncated BDNF [F(3, 16)=108.44, p<0.0001]) and hippocampus (mature BDNF [F(3, 16)=53.81, p<0.0001]; truncated BDNF [F(3, 16)=7.115, p<0.003]) of mice treated with vehicle or cysteamine for 14 days. Post hoc analysis revealed a significant decrease in mature BDNF protein and increase in truncated BDNF levels in frontal cortex and hippocampus of HRM as compared to WT mice (p<0.05). Cysteamine treatment for 14 days did not result in any significant change in BDNF protein levels in any of the brain regions examined (Fig 3A and 3B). Next, we evaluated the effects of 30 days of cysteamine treatment on BDNF proteins in frontal cortex and hippocampus of HRM and WT mice. One-way ANOVA showed a significant group effect in BDNF protein levels in frontal cortex (mature BDNF [F(3, 16)=15.84, p<0.0001]; truncated BDNF [F(3, 16)=17.59, p<0.0001]) and hippocampus (mature BDNF [F(3, 16)=45.54, p<0.0001]; truncated BDNF [F(3, 16)=8.895, p=0.0011]) of mice treated with vehicle or cysteamine for 30 days. Post hoc analysis showed that cysteamine treatment could significantly attenuate the changes in mature BDNF and truncated BDNF protein levels in frontal cortex of HRM (p < 0.05; Fig 3C). In addition, we found a significant decrease in truncated BDNF levels in hippocampus following cysteamine treatment whereas mature BDNF levels were significantly increased following cysteamine treatment in this brain region of HRM (p<0.05; Fig 3D). We did not find any change in proBDNF levels in frontal cortex and hippocampus between HRM and WT mice (Fig 3A and 3B). Also, cysteamine treatment did not have any effect on proBDNF levels in HRM and WT mice in the above brain regions.
Fig 3.
Cysteamine treatment ameliorates the changes in BDNF expression in frontal cortex and hippocampus of heterozygous reeler mice (HRM). Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 14 (Figs 3A and 3B) or 30 (Figs 3C and 3D) days. Mature BDNF (m-BDNF), truncated BDNF (trunc-BDNF) and pro-BDNF were determined by western blot analysis in frontal cortex (Figs 3A and 3C) and hippocampus (Figs 3B and 3D). WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Solid bars represent m-BDNF, open bars represent pro-BDNF and stripped bars represent trunc-BDNF. Data represent mean±SE (n = 5 per group) expressed as fold change in BDNF protein levels as compared to WT-V (* P < 0.05 vs WT-V; #P < 0.05 vs HRM-V; Bonferroni's test). β-actin is the loading control.
Effect of cysteamine on TrkB protein levels in heterozygous reeler mice
TrkB proteins exist as full length (~148 KDa) and truncated (~98 KDa) forms. We used two separate antibodies to detect these two different TrkB forms in our samples. One-way ANOVA showed a significant group effect in full length TrkB protein levels in frontal cortex [F(3, 16)=17.05, p<0.0001] and hippocampus [F(3, 16)=24.77, p<0.0001] of mice treated with vehicle or cysteamine for 14 days. Post hoc analysis showed a significant decrease in full length TrkB protein levels in frontal cortex and hippocampus of HRM as compared to WT mice (p < 0.05; Fig 4). No significant change in full length TrkB protein levels was observed following 14 days of cysteamine treatment (Figs 4A and 4B). Mice treated with cysteamine or vehicle for 30 days showed a statistically significant group effect in TrkB protein levels in frontal cortex [F(3, 16)=39.77, p < 0.0001] and hippocampus [F(3, 16)=40.11, p < 0.0001]. Full length TrkB levels were significantly increased in frontal cortex and hippocampus of WT mice following 30 days of cysteamine treatment (p < 0.05). Full length TrkB levels were increased also in the frontal cortex and hippocampus of HRM (p < 0.05).
Fig 4.
Cysteamine treatment ameliorates the reduction in TrkB expression in frontal cortex and hippocampus of heterozygous reeler mice (HRM). Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 14 (Figs 4A and 4B) or 30 (Figs 4C and 4D) days. TrkB expression was determined by western blot analysis in frontal cortex (Figs 4A and 4C) and hippocampus (Figs 4B and 4D). WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Data represent mean±SE (n = 5 per group) expressed as fold change in TrkB protein levels as compared to WT-V (* P < 0.05 vs WT-V; #P < 0.05 vs HRM-V; Bonferroni's test). β-actin is the loading control.
Next, we examined the truncated TrkB levels in HRM and WT mice. A statistically significant group effect was revealed in one-way ANOVA in truncated TrkB levels in frontal cortex [F(3, 16)=10.06, p = 0.0006], but not in hippocampus [F(3, 16)=0.1987, p = 0.8958] of mice treated with vehicle or cysteamine for 14 days. Post hoc analysis showed a significant increase in truncated TrkB protein levels in frontal cortex of HRM as compared to WT mice (p < 0.05; Fig 5A). We did not find any significant change in truncated TrkB protein levels in hippocampus of HRM (Fig 5B). Mice treated with cysteamine or vehicle for 30 days showed a statistically significant group effect in truncated TrkB protein levels in frontal cortex [F(3, 16)=8.12, p = 0.0016], but not in hippocampus F(3, 16)=0.557, p = 0.651]. No significant effect on truncated TrkB levels was found following cysteamine treatment for 14 or 30 days (Fig 5A and 5D). Taken together, these results indicate that cysteamine treatment could significantly attenuate the change in full length TrkB levels, but not in truncated TrkB levels in HRM.
Fig 5.
Cysteamine treatment has no effect on truncated TrkB expression in frontal cortex of heterozygous reeler mice (HRM). Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 14 (Figs 5A and 5B) or 30 (Figs 5C and 5D) days. Truncated TrkB (trunc-TrkB) expression was determined by western blot analysis in frontal cortex (Figs 5A and 5C) and hippocampus (Figs 5B and 5D). WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Data represent mean±SE (n = 5 per group) expressed as fold change in trunc-TrkB protein levels as compared to WT-V (* P < 0.05 vs WT-V; Bonferroni's test). β-actin is the loading control.
Effect of cysteamine on GAD67 protein levels in TrkB knockout mice
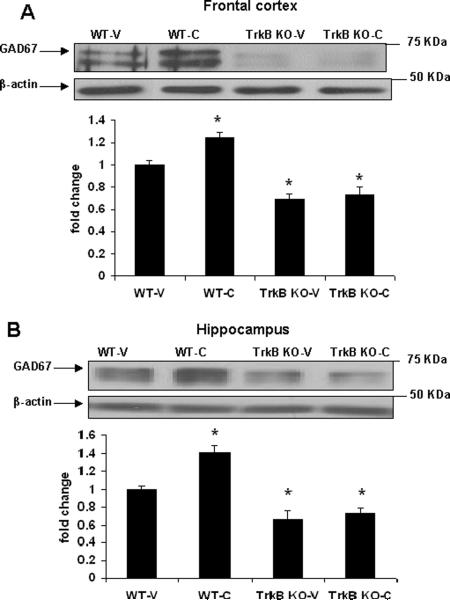
Since we found a significant increase in GAD67 and TrkB protein levels following cysteamine treatment and TrkB is a known regulator of GAD67 expression we next examined whether TrkB is necessary for cysteamine-induced effects on GAD67 expression. A statistically significant group effect was revealed in one-way ANOVA in GAD67 protein levels in frontal cortex [F(3, 15)=22.84, p < 0.0001] and hippocampus [F(3, 15)=28.18, p < 0.0001] of mice treated with vehicle or cysteamine. We found a significant reduction in GAD67 protein levels in frontal cortex and hippocampus of TrkB−/− mice as compared to WT mice (p < 0.05; Fig 6). Next, we tested the effect of cysteamine on GAD67 protein levels in TrkB−/− and WT mice. Cysteamine treatment for 30 days increased GAD67 protein levels in WT mice (p < 0.05). However, in the knockout mice, cysteamine failed to increase GAD67 protein levels. The above data indicate that TrkB is necessary for cysteamine-mediated effects on GAD67 expression.
Fig 6.
Cysteamine treatment has no effect on GAD67 expression in frontal cortex and hippocampus of TrkB knockout (TrkB−/−) mice. Cysteamine (150 mg/kg/day) or water (vehicle) was administered through drinking water to TrkB−/− and wild-type (WT) mice for 30 days. GAD67 expression was determined by western blot analysis in (A) frontal cortex and (B) hippocampus. WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; TrkB−/−-V: TrkB knockout mice treated with vehicle; TrkB−/−-C: TrkB knockout mice treated with cysteamine. Data represent mean±SE (n = 4 for WT-V and n = 5 each for WT-C, TrkB−/−-V and TrkB−/−-C) expressed as fold change in GAD67 protein levels as compared to WT-V (* P < 0.05 vs WT-V; Bonferroni's test). β-actin is the loading control.
Effect of cysteamine on PPI and Y-maze in heterozygous reeler mice
We used a combination of auditory-evoked startle (120 dB) and three levels of prepulses (75, 80, and 85 dB). A repeated-measures ANOVA revealed a significant effect of prepulse [F(2, 60)=111.9, p < 0.0001], but no significant prepulse × treatment interaction [F(2,60)=0.643, N.S.] or main effect of treatment [F(1, 30)=0.02, N.S.]. We found a significant decrease in %PPI in HRM as compared to WT mice at 75dB level (p < 0.05; Fig 7A). Next, we examined the effect on %PPI of cysteamine treatment for 30 days in HRM and WT mice, in comparison with vehicle treated mice. Individual planned comparisons at each level of prepulse intensity revealed significant differences in PPI between vehicle and cysteamine at all prepulse levels in the WT mice whereas only at the 75 dB and 85 dB prepulse levels in the HRM (p <0.05; Fig 7A). We found a significant difference in startle response to 120-dB stimuli following cysteamine treatment [F(3, 28)=6.69, p = 0.0015]. Post hoc analysis showed that cysteamine treatment for 30 days increased startle response in both HRM and WT mice (p < 0.05; Fig 7B).
Fig 7.
Effects of Cysteamine on Prepulse inhibition (PPI) of the auditory startle response in heterozygous reeler mice (HRM). Cysteamine (150 mg/kg/day) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 30 days. WTV: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Data represent mean±SE (n = 8 per group) expressed as (A) %PPI and (B) startle response (* P < 0.05 vs WT-V; #P < 0.05 vs HRM-V; Bonferroni's test).
The data from Y-maze test analyzed by one-way ANOVA showed a significant group effect in spatial memory function [F(3, 29)= 16.41; p < 0.001; Fig 8]. Post hoc analysis showed that HRM have impaired spatial memory, spending less time in the novel arm than WT mice (p<0.05). Cysteamine treated HRM spent significantly more time in novel arms than vehicle treated HRM (p < 0.05). Working memory was studied by measuring spontaneous alternation in the Y-maze. There was no significant effect of genotype or treatment on the percentage of alternation (data not shown). This suggests that although hippocampus-dependent spatial memory is changed following cysteamine treatment in HRM, nonhippocampal working memory is intact with cysteamine treatment in both WT and HRM.
Fig 8.
Effects of Cysteamine on spatial recognition memory in heterozygous reeler mice (HRM). Cysteamine (150 mg/kg) or water (vehicle) was administered through drinking water to HRM and wild-type (WT) mice for 30 days. WT-V: wild-type treated with vehicle (water); WT-C: wild-type treated with cysteamine; HRM-V: Heterozygous reeler mice treated with vehicle; HRM-C: Heterozygous reeler mice treated with cysteamine. Data represent mean±SE (n = 9 for WT-V and n = 8 each for WT-C, HRM-V and HRMC) expressed as % Time in novel arm (* P < 0.05 vs WT-V; #P < 0.05 vs HRM-V; Bonferroni's test).
Discussion
In this study, we evaluated the effects of cysteamine treatment in HRM on the levels of GAD67 and BDNF/TrkB proteins in two different brain regions (frontal cortex and hippocampus) that have been implicated in schizophrenia. We also evaluated the drug for its effects on domains of cognition known to be adversely affected in schizophrenia (information processing and spatial memory). In the neurochemical studies, we observed increases in GAD67 and TrkB protein levels upon exposure of cysteamine in frontal cortex and hippocampus of HRM. Other studies have shown alterations in TrkB signaling (Pillai and Mahadik, 2008) and GAD67 expression (Liu et al 2001; Nullmeier et al 2011) in HRM. The involvement of TrkB in cysteamine-induced GAD67 expression is strengthened by our data demonstrating that cysteamine failed to increase GAD67 expression in TrkB−/− mice.
TrkB signaling has been shown to induce GABA function and GAD67 expression in neurons (Arenas et al 1996; Mizuno et al 1994).. TrkB exists in two forms, full length TrkB and truncated TrkB (which lacks tyrosine kinase domain). We have earlier reported a significant increase in truncated TrkB levels in the frontal cortex of HRM (Pillai and Mahadik, 2008). In the present study, we found a significant increase in truncated BDNF levels in both frontal cortex and hippocampus of HRM. The 28 KDa form of BDNF has been shown to be a cleaved form of the proBDNF in both humans and animals (Carlino et al 2011; Seidah et al 1999). However, the biological role of truncated BDNF is not known. Further studies are needed to elucidate the role of truncated BDNF in the regulation of TrkB signaling as well as GAD67 expression in view of the fact that we did not observe significant changes in pro-BDNF levels in HRM.
The major finding of the present study is the potential of cysteamine to normalize GAD67 expression in HRM. It has been suggested that reelin through DAB1 signaling selectively regulates GAD67, but not GAD65 expression in interneurons (Guidotti et al 2000). Interestingly, we found a significant increase in TrkB protein levels following cysteamine treatment in both frontal cortex and hippocampus. Our data show that the cysteamine treatment induces GAD67 expression in the frontal cortex and hippocampus of WT mice, but no change in GAD67 protein levels was found in the TrkB−/− mice following cysteamine exposure. Proper development and regulation of GABAergic interneurons are important for normal cognitive function of the adult brain (Woo and Lu, 2006). In addition, significant positive correlations between TrkB and GAD67 mRNAs were observed in the dorsolateral prefrontal cortex of schizophrenia subjects (Hashimoto et al 2005). Together, the above data indicate that TrkB might be a molecular target for cysteamine action on GABAergic system.
To assess the effects of genotype and cysteamine on behaviors that have relevance to schizophrenia we utilized a prepulse inhibition (PPI) procedure and two Y-maze tasks. The rationale for selecting the PPI procedure was based on several factors: 1) The test is quite useful for identifying sensory information-processing deficits, a common feature in several neuropsychiatric conditions including schizophrenia. Auditory (sensory) gating deficits in schizophrenia are in fact thought to contribute to the deficits in attention, cognitive impairment, and even hallucinations (Adler et al 1998); 2) PPI of the acoustic startle reflex is a cross-species phenomenon (identical in all mammals studies to date) and thus it is easily studied in animals as well as humans (Braff et al 1992). 3) The PPI model in mice has been found to be particularly useful for studying the effects of gene alterations on sensorimotor gating (Geyer et al 2002).
In the present study, we used a combination of auditory-evoked startle (120 dB) and three levels of prepulses (75, 80, and 85 dB). We found a significant decrease in %PPI in HRM as compared to WT mice only at 75dB level. The literature show contradictory data on PPI deficits in HRM. The study by Tueting et al 1999 found alterations in PPI in HRM, but the above data was not replicated in two other studies (Podhorna and Didriksen, 2004; Salinger et al 2003). However, Qiu et al 2006 found a significant diminution of the force of a 120 dB startle response for HRM with the application of an acoustic stimulation of 82 dB intensity, but not of other intensities (70, 76 or 88). These inconsistencies between data from different laboratories may result from differences in the age, behavioral training, rearing conditions, genetic background and interpretation, all of which can influence the outcome of behavioral tests (Wahlsten et al 2003). Regardless, cysteamine treatment could increase %PPI in both HRM and WT mice. In addition, a significant increase in startle response to 120-dB stimuli was found in cysteamine treated mice as compared to vehicle-treated mice. Our data on the potential of cysteamine to increase %PPI is in agreement with an earlier report that cysteamine blocks amphetamine-induced decreases in PPI (Feifel and Minor, 1997). We found increases in both % PPI and startle response following cysteamine treatment. It has been suggested that the effects of centrally active drugs on startle response and PPI in mice tend to be dissociated (Geyer et al 2002), reflecting the difference in the nature of complexity in cellular mechanisms between the startle response and PPI. It has been reported that increased startle reactivity is not necessarily be associated with decreased PPI (Paylor and Crawley 1997). The data from the above study is supported by the findings that the nicotine treatment increases both startle reactivity and PPI (Acri et al 1994; Schreiber et al 2002). Furthermore, haloperidol increased PPI, but did not alter the increased startle reactivity in the NMDA receptor knock out mice (Duncan et al 2006). The above data suggest that there does not appear to be a predictable relationship between startle amplitude and PPI.
For memory-related assessments we employed a Y-maze and two tasks, spontaneous alternation and the two-trial recognition test. Both tasks make use of the normal navigation and exploratory behaviors of rodents, do not require rule learning, and, other than the potential fear-related response associated with the exposure to a novel environment, they do not have significant aversive components (i.e., food restriction or water immersion). In addition, both tasks are thought to rely on short-term spatial memory processes (Hughes, 2004). In the continuous or free-running version of the task, (i.e., spontaneous alternation) the tendency for rodents to alternate their nonreinforced choices of the maze arms on successive opportunities is employed and working memory is required. As the name implies, the two-trial recognition memory test is thought to employ spatial recognition memory. Each of the Y-maze tests described here has been shown to be sensitive to hippocampal damage, gene manipulation, and pharmacological interventions (Conrad et al 1997; Dellu et al 1992, 2000; Hughes, 2004; Martin et al 2003).
In the spatial memory test in Y maze, HRM spent less time in the novel arm than WT mice. However, we did not find any difference in spontaneous alternation behavior, a working memory test between HRM and WT mice. Our data on the absence of working memory deficits in HRM are in agreement with previous studies (Krueger et al 2006; Sallinger et al 2003). Cysteamine treatment could increase spatial recognition memory in both HRM as well as WT mice.
Our study has a few limitations. BDNF signaling through TrkB has been shown to promote inhibitory synaptogenesis, the development of GABAergic interneurons and induces the expression of GAD67 (Huang et al., 1999; Yamada et al., 2002). It is known that GAD67 abnormalities affect parvalbumin-containing GABA neurons involved in gamma oscillations (Gonzalez-Burgos G, Lewis DA, 2008). The gamma band oscillations are associated with information transmission and processing (Herrmann et al., 2010). Moreover, schizophrenia is associated with impaired performance and reduced frontal γ activity in individuals during cognitive tasks (Cho et al., 2006) and pharmacologically enhanced GABAAR activity simultaneously improves cognitive performance and frontal γ band power in individuals with schizophrenia (Lewis et al., 2008). It is possible that increase in GAD67 expression following cysteamine treatment improves GABAergic neurotransmission and thereby cognitive function, but further studies are warranted. It is also possible that decreases in GAD67 in TrkB knockout mice are due to a loss of interneurons, as reported by reductions in the number of GABAergic synapses and GAD65 expression (Carmona et al., 2006). In that case, the interneurons involved in the cysteamine response in the HRM may no longer be rescuable in the TrkB knockout mice due to an entirely cysteamine-independent mechanism. It is also important to determine whether there is an overall loss of interneurons in HRM. Such an experiment would be helpful to know whether the changes in GAD67 expression observed with cysteamine results from a specific increase in the remaining “resistant” cells, not just a change in all GAD67-positive cells.
BDNF/TrkB signaling is an important mediator of neuronal development and function, and numerous studies suggest that abnormal BDNF/TrkB signaling may be involved in pathophysiology of neuropsychiatric disorders including schizophrenia. Identification of potent modulators of TrkB signaling provides tools for further evaluating the role of BDNF/TrkB signaling in these disease states. Furthermore, the finding that cysteamine can modulate GAD67 expression through TrkB suggests that TrkB signaling could be a possible therapeutic target in schizophrenia. Compounds like cysteamine, which can increase TrkB signaling could be possible adjunctive therapy for treating this disorder.
Acknowledgements
The authors are thankful to Dr. Barbara Rohrer for providing TrkB knockout mice. This study was supported by a grant from NIH/NIMH (MH083215-02) to Anilkumar Pillai.
Footnotes
Statement of Interest None
References
- Acri JB, Morse DE, Popke EJ, Grunberg NE. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology. 1994;114:369–74. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 epression in schizophrenia and related disorders. Brain Research Reviews. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alcántara S, Ruiz M, D'Arcangelo G, Ezan F, et al. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. Journal of Neuroscience. 1998;18:7779–99. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas E, Akerud P, Wong V, Boylan C, et al. Effects of BDNF and NT-4/5 on striatonigral neuropeptides or nigral GABA neurons in vivo. European Journal of Neuroscience. 1996;8:1707–17. doi: 10.1111/j.1460-9568.1996.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6496–9. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pagès M, Canals JM, Cordelières FP, Parker JA, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. Journal of Clinical Investigation. 2006;116:1410–24. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Grillion C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Carlino D, Leone E, Di Cola F, Baj G, et al. Low serum truncated-BDNF isoform correlates with higher cognitive impairment in schizophrenia. Journal of Psychiatric Research. 2011;45:273–9. doi: 10.1016/j.jpsychires.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Carmona MA, Pozas E, Martinez A, Espinosa-Parrilla JF, et al. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cerebral Cortex. 2006;16:47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Research. 1997;759:76–83. doi: 10.1016/s0006-8993(97)00236-9. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Dellu F, Contarino A, Simon H, Koob GF, et al. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiology of Learning and Memory. 2000;73:31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, et al. A two-trial memory task with automated recording: study in young and aged rats. Brain Research. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacology Biochemistry & Behavior. 2006;85:481–91. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Minor KL. Cysteamine blocks amphetamine-induced deficits in sensorimotor gating. Pharmacology Biochemistry & Behavior. 1997;58:689–93. doi: 10.1016/s0091-3057(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular Psychiatry. 2002;7:1039–53. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of General Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. Journal of Neuroscience. 2005;25:372–83. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neuroscience and Biobehavioral Reviews. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience & Biobehavioral Reviews. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Hebert BF, Olausson P, et al. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology. 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. mplications for the treatment of polyglutamine disorders. Journal of Biological Chemistry. 2003;278:3825–30. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. American Journal of Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Archives of Neurology. 2006;63:1372–6. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, et al. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3477–82. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, et al. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Martin S, Jones M, Simpson E, van den BM. Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport. 2003;14:1979–1982. doi: 10.1097/00001756-200310270-00020. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Developmental Biology. 1994;165:243–56. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Nullmeier S, Panther P, Dobrowolny H, Frotscher M, et al. Region-specific alteration of GABAergic markers in the brain of heterozygous reeler mice. European Journal of Neuroscience. 2011;33:689–98. doi: 10.1111/j.1460-9568.2010.07563.x. [DOI] [PubMed] [Google Scholar]
- Oliverio S, Amendola A, Rodolfo C, Spinedi A, et al. Inhibition of “tissue” transglutaminase increases cell survival by preventing apoptosis. Journal of Biological Chemistry. 1999;274:34123–8. doi: 10.1074/jbc.274.48.34123. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002;36:989–91. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. Journal of Neurocytology. 2001;30:413–25. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd edn. Academic Press; San Diego: 2001. [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997;132:169–80. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP. Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophrenia Research. 2008;100:325–33. doi: 10.1016/j.schres.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Pillai A, Veeranan-Karmegam R, Dhandapani KM, Mahadik SP. Cystamine prevents haloperidol-induced decrease of BDNF/TrkB signaling in mouse frontal cortex. Journal of Neurochemistry. 2008;107:941–51. doi: 10.1111/j.1471-4159.2008.05665.x. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M. The heterozygous reeler mouse: behavioural phenotype. Behavioral Brain Research. 2004;153:43–54. doi: 10.1016/j.bbr.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, et al. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiology of Learning and Memory. 2006;85:228–42. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Blanco R, Marc RE, Lloyd MB, et al. Functionally intact glutamate-mediated signaling in bipolar cells of the TRKB knockout mouse retina. Visual Neuroscience. 2004;21:703–13. doi: 10.1017/S0952523804045055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinger WL, Ladrow P, Wheeler C. Behavioral phenotype of the reeler mutant mouse: effects of RELN gene dosage and social isolation. Behavioral Neuroscience. 2003;117:1257–75. doi: 10.1037/0735-7044.117.6.1257. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Dalmus M, De Vry J. Effects of alpha 4/beta 2- and alpha 7-nicotine acetylcholine receptor agonists on prepulse inhibition of the acoustic startle response in rats and mice. Psychopharmacology. 2002;159:248–57. doi: 10.1007/s00213-001-0927-8. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, et al. Mammalian subtilisin/kexin isozyme SKI-1: A widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1321–6. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerri PE. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse. 1988;2:11–22. doi: 10.1002/syn.890020104. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shirakawa O, Toyooka K, Kitamura N, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of chizophrenic patients. Molecular Psychiatry. 2000;5:293–300. doi: 10.1038/sj.mp.4000718. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Gearhart DA, Mahadik SP, Warsi S, et al. Chronic exposure to typical or atypical antipsychotics in rodents: Temporal Effects on Central alpha 7 Nicotinic Acetylcholine Receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD(67) mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. Journal of Psychiatry and Neuroscience. 2011;36:100048. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueting P, Costa E, Dwivedi Y, Guidotti A, et al. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–34. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, et al. Different data from different labs: lessons from studies of gene- environment interaction. Journal of Neurobiology. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Ligons DL, Romanczyk T, Ungaro G, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Molecular Psychiatry. 2005;10:637–50. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, et al. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. Journal of Neuroscience. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]