Abstract
Central Sensitization (CS) has been proposed as a common pathophysiological mechanism to explain related syndromes for which no specific organic cause can be found. The term Central Sensitivity Syndrome (CSS) has been proposed to describe these poorly understood disorders related to CS. The goal of this investigation was to develop the Central Sensitization Inventory (CSI), which identifies key symptoms associated with CSSs, and quantifies the degree of these symptoms. The utility of the CSI, to differentiate among different types of chronic pain patients that presumably have different levels of CS impairment, was then evaluated. Study 1 demonstrated strong psychometric properties (test-retest reliability = 0.817; Cronbach's alpha = 0.879) of the CSI in a cohort of normative subjects. A factor analysis (including both normative and chronic pain subjects) yielded 4 major factors (all related to somatic and emotional symptoms), accounting for 53.4% of the variance in the dataset. In Study 2, the CSI was administered to four groups: fibromyalgia (FM); chronic widespread pain (CWP) without FM; work-related regional chronic low back pain (CLBP); and normative control group. Analyses revealed that the FM patients reported the highest CSI scores, and the normative population the lowest (p<.05). Analyses also demonstrated that the prevalence of previously diagnosed CSSs and related disorders was highest in the FM group and lowest in the normative group (p<.001). Taken together, these two studies demonstrate the psychometric strength, clinical utility, and the initial construct validity of the CSI in evaluating CS-related clinical symptoms in chronic pain populations.
Keywords: Central Sensitization Inventory (CSI), Central Sensitization, Central Sensitivity Syndrome, chronic pain, factor analysis, reliability, Fibromyalgia, work-related lumbar pain
Introduction
Physical symptoms that are unexplained by organic cause are a relatively common phenomenon. It is estimated that modern diagnostic testing can find no organic explanation for 10% of reported and persisting physical symptoms 1. Furthermore, the occurrence of multiple somatic symptoms is associated with higher rates of psychopathology and predict poorer treatment outcomes and higher health care utilization 2-4,5. Such non-organic symptom complaints have traditionally been grouped into separate syndromes, such as fibromyalgia (FM), chronic fatigue, irritable bowel (IBS), and temporomandibular joint (TMJ) disorders. Historically, different terms have been used to group these types of somatization disorders, including “Functional Syndromes” 6, 7, “medically unexplained symptoms” 8, 9, and “Bodily Distress Syndrome” 10. These syndromes share many common features, including pain, fatigue, poor sleep, cognitive deficits, headaches, anxiety and depression, suggesting that they may share a common etiology 9. Recent evidence has found that Central Sensitization (CS), which involves an abnormal and intense enhancement of pain by mechanisms in the central nervous system, may be the common link between these disorders 11. Yunus 12 has proposed the term “Central Sensitivity Syndrome” (CSS) for non-organic disorders that are presumed to share this CS etiology. Unlike previous terminology for these non-organic disorders, which propose no common pathophysiological mechanism, the CSS model provides a very appealing theoretical construct for categorizing these related syndromes. Within this model, symptom presentations that have previously been viewed as individual somatoform disorders can now be viewed as many forms of a common CSS, with CS as a root cause 13-15.
CS is characterized by allodynia (painful sensation to a normally non-painful stimulus, such as touch), hyperalgesia (excessive sensitivity to a normally painful stimulus, such as pressure), expansion of the receptive field (pain that extends beyond the area of peripheral nerve supply), and unusually prolonged pain after the stimulus has been removed (usually burning, throbbing, tingling, or numbness). A number of explanations have been proposed to explain the development of CS, including dysregulation in both ascending and descending central nervous system pathways due to physical trauma and sustained pain impulses, and the chronic release of pro-inflammatory cytokines by the immune system, as a result of physical trauma or viral infection 16. Other non-CS biological mechanisms have also been associated with CSS disorders, such as a dysfunction of the stress system, including the hypothalamic-pituitary-adrenal axis 17. In addition, it is well-recognized that psychiatric disorders, including anxiety, panic and depression, are often associated with CSSs 18-21. Due to the interaction between psychosocial factors and biological mechanisms, it has been recommended that CSSs be viewed within a biopsychosocial model 22.
Evidence for CS in many CSSs, including FM, TMJ, and IBS, has been demonstrated by reduced subjective pain thresholds in patients vs. pain-free controls to various stimuli, such as electrical, pressure, cold, and heat. Objective measures of CS (not relying on subjective self-report) have been demonstrated with brain imaging and with nocioceptive spinal reflex tests 22, 23. However, evidence of CS has not been found in all disorders in the proposed CSS family 11. The reduced pain threshold phenomenon, which corresponds to CS, has also been found in patients outside of the CSS family including regional low back pain. It has been suggested that changes in central pain processing in some individuals with regional pain can result in the later development of chronic widespread pain (CWP) 24. In fact, longitudinal studies have found that about one quarter of patients with chronic neck or back pain will develop CWP 25.
A variety of self-report measures have been developed to evaluate individual symptom manifestations of CSS, including: FM 26-34; chronic fatigue syndrome 35, 36; and IBS 37, 38. Other instruments are available for measuring comorbid medical conditions, but none of which are focused on CSS 39-41. A single self-report instrument that identifies key comorbid symptoms associated CS and CSSs, and which quantifies the degree of these symptoms, has not previously appeared in the literature and is therefore likely to be a useful clinical screening tool.
The purpose of the present study was to develop such a new self-report measure designed to assess key somatic and emotional complaints often associated with CSS. The item pool was created from a literature search of comorbid symptoms and conditions of FM and other CSSs (see Table 1). The clinical goal of this screening instrument, the Central Sensitization Inventory or CSI, is to help better assess symptoms thought to be associated with CS in order to aid physicians and other clinicians in syndrome categorization, sensitivity, severity identification, and treatment planning, to help minimize, or possibly avoid, unnecessary diagnostics and treatment procedures. For this purpose, the psychometric validity and clinical utility of the CSI were evaluated in two separate studies. First, The CSI was evaluated assessing test-retest reliability and internal consistency. Also, a factor analysis was utilized to identify specific items that load onto distinguishable factors. In the second study, validation was established by comparing scores between four subject groups, including FM, chronic widespread pain (CWP), regional chronic low back pain (CLBP), and a normative control group. It was hypothesized that the degree of CSS symptomology, and resulting scores on the CSI, would be highest in the FM group and progressively less in the other three groups.
Table 1. Somatic and Emotional Indices of Central Sensitization and Central Sensitivity Syndromes.
|
Methods
Study 1
This initial component of the present investigation developed the CSI and evaluated its psychometric properties. Somatic and emotional symptoms that have been found to be associated with CS and CSSs, based in part upon a literature search of comorbid symptoms and disorders associated with FM and other CSSs, were used in the development of the CSI items (Table 1).
Instrument Development
An interdisciplinary team that included physicians (psychiatrists and orthopedic surgeons), rehabilitation specialists, clinical psychologists, health psychologists, and psychophysiological specialists, who work exclusively with individuals with chronic pain conditions, developed the items for this Inventory. The resultant Central Sensitization Inventory (CSI) contains a Part A of 25 statements related to current health symptoms. Each of these items are measured on a 5-point temporal Likert scale, with the following numeric rating scale: Never (0), Rarely (1), Sometimes (2), Often (3), and Always (4). A cumulative score ranges from 0-100. Additionally, information is collected in Part B on previously diagnosed CSS and related conditions. The Central Sensitization Inventory is presented in APPENDIX A.
Reliability Study
The participants in the reliability study were characterized as “normal” in that they were from a general population not know to currently be in treatment for chronic pain. These participants included undergraduate students, graduate students, staff and faculty members at The University of Texas at Arlington. The Institutional Review Board at The University of Texas at Arlington approved this reliability study, and all participants provided written informed consent prior to participation. The demographic make-up of the participants in the reliability portion of the study was: 29.4% male; 21.2% Asian, 18.4% Black, 17.0% Hispanic, 36.4% White, and 7.0% Other/Multiracial. The mean age of this sample was 22.4 years (SD=4.7 yrs). A total of 149 participants who completed the first and second administrations of the assessment were used in this portion of the study. The CSI was administered at two time points, approximately five days apart.
Factor Analysis
Completed questionnaires for 359 participants were considered for the factor analysis. These participants included both individuals from a general population and patients with chronic pain conditions. In addition to the normative group, the questionnaires from 210 consecutive patients with chronic disabling occupational musculoskeletal disorders (CDOMD), attending a functional restoration rehabilitation program at the Productive Rehabilitation Institute of Dallas for Ergonomics, Dallas, TX (PRIDE) a regional referral center for chronic pain patients] were used. The CSI was administered to the CDOMD patients upon admission, prior to treatment.
Statistical Analyses
For the test-retest portion of Study 1, correlational comparisons were made for each of the 25 items, along with the overall sum score. Internal consistency for the first assessment was also evaluated. The data were analyzed using SPSS v.18. For the test-retest analysis, a Pearson's correlation (r) was used to determine the item correlation between each administration of the assessment. Cronbach's alpha was used to determine the internal consistency of the responses. The significance level for all analyses was set at alpha = 0.05. For the factor analysis, a Principal Component Analysis was conducted, using a Promax Rotation. Factors were considered for Eigenvalues greater than one. The cut-off for the loadings was set at 0.4.
Study 2
The purpose of this study was to evaluate the utility of the CSI to differentiate among different subject groups of chronic pain patients, presumed to have different degrees of CSS-related symptomology, and a normative control group.
Participants
A total of 105 chronic pain patients and 40 healthy college students were recruited from PRIDE and The University of Texas at Arlington, respectively. The criteria for participation in the PRIDE treatment program were: (1) a minimum of 3 months elapsed between the date of injury and treatment; (2) primary and/or secondary care were unsuccessful or unnecessary; (3) surgery was either not an option or did not produce relief from the injury; (4) severe pain and functional limitations remained; and (5) patients must communicate in English or Spanish.
There were four subsamples of subjects in this study:
FM group: This group consisted of 30 fibromyalgia patients (who also met criteria for chronically disabling occupational musculoskeletal disorders (CDOMD)) who consented to treatment at PRIDE. The criteria for FM diagnoses were: (a) the presence of chronic widespread pain (CWP; upper body, lower body, left side, right side, and axial) for a minimum of 3 months; in combination with (b) tenderness at 11 or more of 18 specific tender point sites 42.
CWP only group: This group consisted of 31 patients with CWP who consented to treatment at PRIDE. CWP only patients were defined as those with the presence of CWP and less than 11 tender point sites.
Chronic Low Back Pain (CLBP): This group consisted of 44 chronic regional lumbar pain patients, with no other specific pain complaints, who consented to treatment at PRIDE. It included CDOMD patients with fractures, sprains/strains, disc disorders, stenosis or spondylolisthesis.
Normative population (NP): This group consisted of 40 students from The University of Texas at Arlington, who were recruited from the university-wide subject pool. No subjects in this group were known to be engaged in any current medical intervention for chronic pain.
Design and Statistical Analyses
The CSI, which was developed in STUDY 1, was used in this validation study. The present study was designed to evaluate mean score differences of the CSI among the four subgroups. Chi-square and univariate analyses of variance (ANOVAs) were employed for demographic analyses. Statistical analyses of group mean differences were conducted using analysis of covariance (ANCOVA). A p value of .05 was set as the critical value. Post hoc analyses, using the Bonferroni criterion, were conducted to investigate which groups were considered statistically different. The effect size ω2 was also calculated for the group differences found by these analyses.
Results
Study 1
An item-analysis was conducted using a test-retest reliability assessment. The test-retest correlation for the total score was 0.817, indicating a high level of reliability between administrations. The means, standard deviations and test-retest correlations for each item are shown in Table 2. All item-analysis correlations were found to be statistically significant. To determine the internal consistency of the items on the measure, Cronbach's alpha was calculated to be 0.879. This indicates that the items within the scale are indeed measuring a similar construct.
Table 2. Central Sensitization inventory (CSI).
| Question | Mean (1) | SD (1) | Mean (2) | SD (2) | Pearson Correlation (r) | |
|---|---|---|---|---|---|---|
| 1 | Unrefreshed in morning | 1.95 | 1.04 | 1.72 | 1.00 | 0.388 |
| 2 | Muscles stiff/achy | 1.78 | 0.93 | 1.60 | 0.92 | 0.643 |
| 3 | Anxiety attacks | 0.93 | 0.98 | 0.80 | 0.91 | 0.788 |
| 4 | Grind/clench teeth | 1.04 | 1.14 | 0.92 | 1.07 | 0.855 |
| 5 | Diarrhea/Constipation | 1.12 | 0.98 | 0.99 | 0.91 | 0.781 |
| 6 | Need help daily activity | 0.48 | 0.69 | 0.43 | 0.65 | 0.544 |
| 7 | Sensitive to bright lights | 1.17 | 1.04 | 0.99 | 1.01 | 0.682 |
| 8 | Easily tired w/ physical activity | 1.69 | 1.00 | 1.52 | 0.92 | 0.566 |
| 9 | Pain all over body | 0.85 | 0.82 | 0.79 | 0.77 | 0.640 |
| 10 | Headaches | 1.78 | 0.96 | 1.74 | 1.02 | 0.764 |
| 11 | Bladder/urination pain | 0.32 | 0.58 | 0.34 | 0.57 | 0.576 |
| 12 | Do not sleep well | 1.67 | 0.95 | 1.66 | 0.98 | 0.714 |
| 13 | Difficulty concentrating | 1.85 | 0.91 | 1.70 | 0.88 | 0.729 |
| 14 | Skin problems | 1.17 | 1.12 | 1.19 | 1.13 | 0.790 |
| 15 | Stress makes symptoms worse | 1.67 | 1.13 | 1.58 | 1.14 | 0.688 |
| 16 | Sad or depressed | 1.33 | 0.95 | 1.16 | 0.90 | 0.721 |
| 17 | Low energy | 1.75 | 0.98 | 1.67 | 0.98 | 0.749 |
| 18 | Tension neck and shoulder | 1.75 | 1.11 | 1.58 | 1.09 | 0.711 |
| 19 | Pain in jaw | 0.72 | 1.03 | 0.60 | 0.92 | 0.679 |
| 20 | Certain smells make dizzy | 0.99 | 1.05 | 0.97 | 1.06 | 0.759 |
| 21 | Urinate frequently | 1.16 | 0.98 | 1.09 | 0.97 | 0.762 |
| 22 | Restless legs | 1.03 | 1.13 | 0.95 | 1.08 | 0.822 |
| 23 | Poor memory | 1.61 | 0.94 | 1.58 | 0.88 | 0.670 |
| 24 | Trauma as child | 0.35 | 0.75 | 0.27 | 0.64 | 0.810 |
| 25 | Pelvic Pain | 0.39 | 0.65 | 0.34 | 0.59 | 0.574 |
| Overall Total | 30.42 | 12.29 | 27.90 | 12.31 | 0.817 |
Table 3 shows the loadings for the Pattern Matrix for the factor analysis, including the specific CSI question items that contributed to the factors. The factor analysis yielded 4 factors that accounted for 53.4% of the variance in the dataset. The factors were labeled for meaningfulness, and the variance is provided below:
Table 3. Factor Analysis Breakdown of the CSI with the specific CSI Question Items that Contributed to Each Factor.
| Item | Question | Factor1 Physical Symptoms | Factor 2 Emotional Distress | Factor 3 Headache/Jaw Symptoms | Factor 4 Urological Symptoms | Items not loading on Factors |
|---|---|---|---|---|---|---|
| 1 | Unrefreshed in morning | X | ||||
| 2 | Muscles stiff/achy | 0.91 | ||||
| 3 | Anxiety attacks | 0.55 | ||||
| 4 | Grind/clench teeth | 0.73 | ||||
| 5 | Diarrhea/Constipation | X | ||||
| 6 | Need help daily activity | 0.76 | ||||
| 7 | Sensitive to bright lights | 0.56 | ||||
| 8 | Easily tired w/ physical activity | 0.65 | ||||
| 9 | Pain all over body | 0.64 | ||||
| 10 | Headaches | 0.55 | ||||
| 11 | Bladder/urination pain | 0.71 | ||||
| 12 | Do not sleep well | 0.77 | ||||
| 13 | Difficulty concentrating | 0.82 | ||||
| 14 | Skin problems | X | ||||
| 15 | Stress makes symptoms worse | 0.48 | ||||
| 16 | Sad or depressed | 0.67 | ||||
| 17 | Low energy | 0.48 | 0.47 | |||
| 18 | Tension neck and shoulder | 0.52 | ||||
| 19 | Pain in jaw | 0.68 | ||||
| 20 | Certain smells make dizzy | 0.69 | ||||
| 21 | Urinate frequently | 0.62 | ||||
| 22 | Restless legs | 0.61 | ||||
| 23 | Poor memory | 0.79 | ||||
| 24 | Trauma as child | 0.52 | ||||
| 25 | Pelvic Pain | 0.41 |
Factor 1 – Physical Symptoms (30.9%)
Factor 2 – Emotional Distress (7.2%)
Factor 3 – Headache/Jaw Symptoms (10.1%)
Factor 4 – Urological Symptoms (5.2%)
Study 2
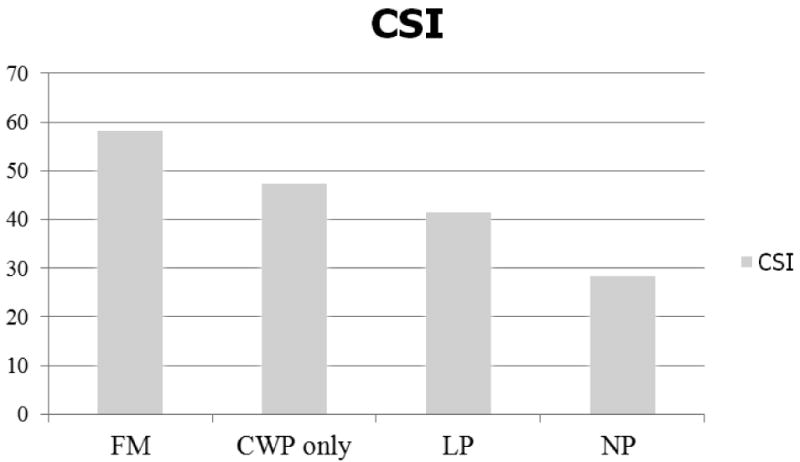
The basic demographic variables and total CSI scores for the four subsamples are presented in Table 4. There were statistically significant differences among the four groups regarding age and gender, F (3, 141) = 76.42, p <.001; χ2 (3) = 36.89, p <.001, respectively. In order to obtain the purer effect of group differences on the CSI scores, gender and age were controlled for by using an ANCOVA. There was a significant effect of clinical groups, after controlling for the effect of age and gender, F (3, 139) = 9.22, p <.05, with an effect size, ω2 = .01. The Bonferroni post-hoc test indicated that FM patients reported the highest CSI scores (x̄)= 58.2, range = 34-78)., and the normative control subjects reported the lowest CSI scores (x̄= 28.4, range = 4-55). There was no statistical difference between the CWP only and CLBP groups. Analyses were also conducted to evaluate the prevalence of the 10 disorders in Part B of the CSI among the four comparison groups. As can be seen in Table 4, there were significantly different rates of these disorders across the groups, F (3, 114) = 13.96, p <.001, with an effect size, ω2 = .15. Again, the FM group (x̄= 3.4 diagnoses) was significantly different from the other two chronic pain groups and the normative group (x̄= 0.5 diagnoses).
Table 4. Demographic Variables and CSI Scores for Validation.
| Variable | FM Group | CWP only Group | CLBP Group | Normative group | Significant test 1 | Effect size 2 |
|---|---|---|---|---|---|---|
| N | 30 | 31 | 44 | 40 | ||
| Age (yr) [mean [SD]] | 46.7 (7.6)a | 45.9 (11.5)a | 42.8 (10.0)a | 21.33 (13.6)b | F= 74.42** | |
| Gender (% female) | 73 | 26 | 25 | 77 | χ2 = 36.89** | |
| CSI Part A Total Score | ||||||
| [mean[SD]] [Range] |
58.2 (10.5)a [34-78] |
47.5 (14.9)b [20-74] |
41.6 (14.8)b [13-77] |
28.9 (13.5)c [4-55] |
F= 9.22** | ω2 =.01 |
| CSI Part B # of diagnoses | ||||||
| [mean[SD]] | 3.4 (2.7)a | 1.9 (1.6)b | 1.2 (1.6)b,c | 0.5c(1.1) | F= 13.96** | ω2 =.15 |
Significant test
<.05
<.001
Effect size ω2: .01 = small, .06 = medium, .14 = large
Between- group differences (mean scores with the same letter are not significant different).
Discussion
A growing body of evidence is demonstrating that Central Sensitization represents a common pathophysiological mechanism for the overlapping clinical features of CSS such as FM, chronic fatigue, IBS, and TMJ 16. Within this emerging model, one can view symptoms of CSS not as individual disorders, but as different manifestations of a common etiology. The purpose of the present investigation was to develop a comprehensive inventory that broadly assesses overlapping dimensions of CSS and quantifies the degree of CSS symptomology. This measure was developed by a multi- and inter-disciplinary team that specializes in assessing and treating patients with chronic pain conditions.
As shown in APPENDIX A, the CSI consists of two sections, Parts A and B. Part A contains 25 items with a range for the total score from 0-100. The item pool is intended to provide an overview of presenting symptoms that are common to CSS, with higher scores associated with a higher degree of symptomology. Part B identifies if one has been diagnosed by a physician with specific disorders within the CSS family, as well as related disorders, including anxiety and depression. Because co-occurrence of these disorders in patients diagnosed with CSS has been relatively well established 43, clinicians should consider the presence of a CSS in patients who endorse one or more disorders on this section (particularly when accompanied by a high CSI score from Part A).
In Study 1, focusing on Part A of the instrument, a “normal” population demonstrated a high degree of test-retest reliability, as well as a high Cronbach's alpha. Thus, the reliability of this assessment is psychometrically sound. This study also demonstrated that 4 factors accounted for 53.4% of the variance in the dataset (Physical Symptoms, Emotional Distress, Headache/Jaw, and Urological Symptoms Factors). Each of these factors related in some way to symptoms common in the highly heterogeneous domain of CSS. In Study 2, comparisons of the four sub-populations were conducted. FM was presumed to have the highest level of CSS symptomology, and CWP without FM, CLBP, and normative control subjects were presumed to have progressively less. In evaluating Part A of the CSI the FM group was statistically distinguishable from the CWP and CLBP groups, and all 3 chronic pain groups were statistically distinguishable from the normative control group. The high CSS (FM) group scored the highest on the total CSI scores, nearly double that of the normative group. It is also noteworthy in Study 2 that normative subjects have occasional symptoms, with the mean scores indicating that the symptoms of the 25-item CSI occur “sometimes” in most individuals (as can be seen in Figure 1). In evaluating Part B of the CSI, the FM group averaged 3.4 diagnoses, double that of the two other pain groups, while normative subjects averaged 0.5 diagnosis.
Figure 1. Mean CSI score differences among four samples.
It is the intent that this CSI will be a useful tool, in both general and specialty medical settings, to help identify patients whose presenting medical issues may be co-morbid symptoms of CSS. This identification can then help guide the practitioner's treatment approach. Patients with regional and specific symptoms, that appear unrelated to CS and other comorbid indications of CSS, can be guided through appropriate diagnostic testing and symptom-specific treatments and medications. For patients who report a wide range of co-morbid symptoms within the CSS family, the clinician may choose to pursue fewer expensive diagnostic tests and, instead, gear treatment towards biopsychosocial symptom management strategies and towards more appropriate and effective medications. For example, dual re-uptake inhibitors have been shown to target descending central pathways by enhancing serotonin and norepinephrine levels, resulting in decreased CS-related pain 44. For those patients who present with regional pain, especially in the back or neck, a high CSI score may be predictive of future development of CSS. Longitudinal studies have found that about one quarter of patients with chronic neck or back pain will develop CWP 25. The results of the CSI may also lead clinicians to consider other co-existing diseases with identifiable physical findings besides the presenting problem 22. A treatable associated condition should not be overlooked. Finally, it is the intent that the CSI will be a useful tool for assessing pre- to post-intervention responsiveness to biopsychosocial treatments for these syndromes.
In conclusion, this manuscript demonstrates the psychometric strength, the clinical utility, and the initial construct validity of the CSI in evaluating CS-related symptoms. The CSI is meant to give clinicians a “heads up” for considering the possibility of Central Sensitization serving as a starting point for more pragmatic therapeutic solutions 17. By overlooking the possibility of a CSS diagnosis, time, effort and resources may be wasted on inappropriate diagnostic testing and treatment, or in the application of invasive procedures with poor results when less expensive, and more effective, alternative interventions may be available. A limitation of the present study was the limited number of CSS comparison groups that were evaluated. Further research will be required to compare CSI scores of additional populations within the CSS family (such as chronic fatigue, headache, TMJ, and IBS), in order to identify associations between the individual factors on the CSI and clinical CSS symptom manifestations, and to establish clinically meaningful cut-off points.
Acknowledgments
This paper has in part been possible via a grant from the National Institutes of Health (1UO1 DEO10713-12A2). This manuscript was prepared without other financial support, and with no support of any kind that may represent a possible conflict of interest.
Appendix A
Central Sensitization Inventory: Part A.
| Please circle the best response to the right of each statement. | ||||||
|---|---|---|---|---|---|---|
| 1 | I feel unrefreshed when I wake up in the morning. | Never | Rarely | Sometimes | Often | Always |
| 2 | My muscles feel stiff and achy. | Never | Rarely | Sometimes | Often | Always |
| 3 | I have anxiety attacks. | Never | Rarely | Sometimes | Often | Always |
| 4 | I grind or clench my teeth. | Never | Rarely | Sometimes | Often | Always |
| 5 | I have problems with diarrhea and/or constipation. | Never | Rarely | Sometimes | Often | Always |
| 6 | I need help in performing my daily activities. | Never | Rarely | Sometimes | Often | Always |
| 7 | I am sensitive to bright lights. | Never | Rarely | Sometimes | Often | Always |
| 8 | I get tired very easily when I am physically active. | Never | Rarely | Sometimes | Often | Always |
| 9 | I feel pain all over my body. | Never | Rarely | Sometimes | Often | Always |
| 10 | I have headaches. | Never | Rarely | Sometimes | Often | Always |
| 11 | I feel discomfort in my bladder and/or burning when I urinate. | Never | Rarely | Sometimes | Often | Always |
| 12 | I do not sleep well. | Never | Rarely | Sometimes | Often | Always |
| 13 | I have difficulty concentrating. | Never | Rarely | Sometimes | Often | Always |
| 14 | I have skin problems such as dryness, itchiness or rashes. | Never | Rarely | Sometimes | Often | Always |
| 15 | Stress makes my physical symptoms get worse. | Never | Rarely | Sometimes | Often | Always |
| 16 | I feel sad or depressed. | Never | Rarely | Sometimes | Often | Always |
| 17 | I have low energy. | Never | Rarely | Sometimes | Often | Always |
| 18 | I have muscle tension in my neck and shoulders. | Never | Rarely | Sometimes | Often | Always |
| 19 | I have pain in my jaw. | Never | Rarely | Sometimes | Often | Always |
| 20 | Certain smells, such as perfumes, make me feel dizzy and nauseated. | Never | Rarely | Sometimes | Often | Always |
| 21 | I have to urinate frequently. | Never | Rarely | Sometimes | Often | Always |
| 22 | My legs feel uncomfortable and restless when I am trying to go to sleep at night. | Never | Rarely | Sometimes | Often | Always |
| 23 | I have difficulty remembering things. | Never | Rarely | Sometimes | Often | Always |
| 24 | I suffered trauma as a child. | Never | Rarely | Sometimes | Often | Always |
| 25 | I have pain in my pelvic area. | Never | Rarely | Sometimes | Often | Always |
| Total= | ||||||
Central Sensitization Inventory: Part B.
| Have you been diagnosed by a doctor with any of the following disorders? Please check the box to the right for each diagnosis and write the year of the diagnosis. | ||||
|---|---|---|---|---|
| NO | YES | Year Diagnosed | ||
| 1 | Restless Leg Syndrome | |||
| 2 | Chronic Fatigue Syndrome | |||
| 3 | Fibromyalgia | |||
| 4 | Temporomandibular Joint Disorder (TMJ) | |||
| 5 | Migraine or tension headaches | |||
| 6 | Irritable Bowel Syndrome | |||
| 7 | Multiple Chemical Sensitivities | |||
| 8 | Neck Injury (including whiplash) | |||
| 9 | Anxiety or Panic Attacks | |||
| 10 | Depression | |||
References
- 1.Reif W, Hessel A, Braehler E. Somatization symptoms and hypochondriacal features in the general population. Psychosomatic Medicine. 2001;63:595–602. doi: 10.1097/00006842-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Sperber AD, Dekel R. Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. Journal Neurogastroenterol Motil. 2010;16(2):113–119. doi: 10.5056/jnm.2010.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lydiard RB, Fossey MD, Marsh W, Ballenger JC. Prevalence of psychiatric disorders in patients with irritable bowel syndrome. Psychosomatics. 1993;34(3):229–234. doi: 10.1016/S0033-3182(93)71884-8. [DOI] [PubMed] [Google Scholar]
- 4.Ahles TA, Khan SA, Yunus MB, Spiegel DA, Masi AT. Psychiatric status of patients with primary fibromyalgia, patients with rheumatoid arthritis, and subjects without pain: A blind comparison of DSM-III diagnoses. American Journal of Psychiatry. 1991;148(12):1721–1726. doi: 10.1176/ajp.148.12.1721. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke K, Mangelsdorff A. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Annual Journal of Medicine. 1989;86(3):262–266. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 6.Lipkin M. Functional or organic? A pointless question. Annals of Internal Medicine. 1969;71(5):1013–1017. doi: 10.7326/0003-4819-71-5-1013. [DOI] [PubMed] [Google Scholar]
- 7.Barsky AJ, Borus JF. Functional somatic syndromes. Annals of Internal Medicine. 1999;130(11):910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. American Family Physician. 2002;66(6):1001–1008. [PubMed] [Google Scholar]
- 9.Schur E, Afari N, Furberg H, Olarte M, Goldberg J, Sullivan P. Feeling bad in more ways than one: Comorbidity patterns of medically unexplained and psychiatric conditions. Journal of General Internal Medicine. 2007;22(6):818–821. doi: 10.1007/s11606-007-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink P, Schröder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. Journal of Psychosomatic Research. 2010;68(5):415–426. doi: 10.1016/j.jpsychores.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Seminars in Arthritis Rheumatology. 2007;36(6):339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Yunus MB. Central sensitivity syndromes: A unified concept for fibromyalgia and other similar maladies. Journal of Indian Rheumatology Association. 2000;8:27–33. [Google Scholar]
- 13.Smart K, Blake C, Staines A, Doody C. The discriminative validity of “nociceptive,” “peripheral neurophathic,” and “central sensitization” as mechanism-based classifications of musculoskeletal pain. The Clinical Journal of Pain. 2011 doi: 10.1097/AJP.0b013e318215f16a. In Press. Epub April 5. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran M, Zheng Y, Timbol C, Merck S, Baraniuk J. Migraine headaches in chronic fatigue syndrom (CFS): Comparison of two prospective cross-sectional studies. BMC Neurol. 2011;11(30) doi: 10.1186/1471-2377-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf C. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yunus MB. Central sensitivity syndromes: A new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Seminars in Arthritis and Rheumatology. 2008;37(6):339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Van Houdenhove B, Luyten P. Central sensitivity syndromes: Stress system failure may explain the whole picture. Seminars in Arthritis and Rheumatology. 2009;39(1):218–219. doi: 10.1016/j.semarthrit.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Ang D, Chakr R, France C, et al. Association of nociceptive responsivity with clinical and the moderating effect of depression. Journal of Pain. 2011;12:384–389. doi: 10.1016/j.jpain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-de-Las-Penas C, Ortega-Santiago R, Cuadrado M, Lopez-de-Silanes C, Pareja A. Bilateral widespread mechanical pain hypersensitivity as sign of central sensitization in patients with cluster headache. Headache. 2011;51:384–391. doi: 10.1111/j.1526-4610.2010.01791.x. [DOI] [PubMed] [Google Scholar]
- 20.Bezov D, Ashina S, Jensen R, Bendtsen L. Pain perception studies in tension-type headache. Headache. 2011;51:262–271. doi: 10.1111/j.1526-4610.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersel D, Dror V, Cheung R. Central amplicfication and fibromylagia: Disorder of pain processing. Journal of Neuroscience Research. 2011;89:29–34. doi: 10.1002/jnr.22512. [DOI] [PubMed] [Google Scholar]
- 22.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Practices in Research and Clinical Rheumatology. 2007;21(3):481–491. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Robinson M, Craggs J, Price D, Perlstein M, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. Journal of Pain. 2011;12:436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kindler LL, Bennett RM, Jones KD. Central sensitivity syndromes: Mounting pathophysiologic evidence to link fibromyaglia with other common chronic pain disorders. Pain Management Nursing. 2011;12(1):15–24. doi: 10.1016/j.pmn.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindler LL, Jones KD, Perrin N, Bennett RM. Risk factors predicting the development of widespread pain from chronic back or neck pain. Journal of Pain. 2010;11(12):1320–1328. doi: 10.1016/j.jpain.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaffi F, Sarzi-Puttini P, Girolimetti R, Gasparini S, Atzeni F, Grassi W. Development and validation of the self-administered Fibromyalgia Assessment Status: a disease-specific composite measure for evaluating treatment effect. Arthritis Research & Therapy. 2009;11(4):R125. doi: 10.1186/ar2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. The Journal of Rheumatology. 2006;33(11):2291–2299. [PubMed] [Google Scholar]
- 28.Wolfe F, Hawley DJ, Goldenberg DL, Russell IJ, Buskila D, Neumann L. The assessment of functional impairment in fibromyalgia (FM): Rasch analyses of 5 functional scales and the development of the FM Health Assessment Questionnaire. The Journal of Rheumatology. 2000;27(8):1989–1999. [PubMed] [Google Scholar]
- 29.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. The Journal of Rheumatology. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 30.Han C, Pae CU, Patkar AA, et al. Psychometric Properties of the Patient Health Questionnaire-15 (PHQ-15) for Measuring the Somatic Symptoms of Psychiatric Outpatients. Psychosomatics. 2009 November 1;50(6):580–585. doi: 10.1176/appi.psy.50.6.580. [DOI] [PubMed] [Google Scholar]
- 31.Schat ACH, Kelloway EK, Desmarais S. The Physical Health Questionnaire (PHQ): Construct Validation of a Self-Report Scale of Somatic Symptoms. Journal of Occupational Health Psychology. 2005;10(4):363–381. doi: 10.1037/1076-8998.10.4.363. [DOI] [PubMed] [Google Scholar]
- 32.Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 33.Mumford DB, Bavington JT, Bhatnagar KS, Hussain Y, Mirza S, Naraghi MM. The Bradford Somatic Inventory. A multi-ethnic inventory of somatic symptoms reported by anxious and depressed patients in Britain and the Indo-Pakistan subcontinent. The British Journal of Psychiatry. 1991;158:379–386. doi: 10.1192/bjp.158.3.379. [DOI] [PubMed] [Google Scholar]
- 34.Wilke WS. New developments in the diagnosis of fibromyalgia syndrome: say goodbye to tender points? Cleveland Clinic Journal of Medicine. 2009;76(6):345–352. doi: 10.3949/ccjm.76a.08062. [DOI] [PubMed] [Google Scholar]
- 35.Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. Journal of Fatigue Syndrome. 2006;14(4):41–66. [Google Scholar]
- 36.Nijs J, Van de Velde B, De Meirleir K. Pain in patients with chronic fatigue syndrome: Does nitric oxide trigger central sensitization? Medical Hypotheses. 2005;64(3):558–562. doi: 10.1016/j.mehy.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Schmulson M, Lee OY, Chang L, Naliboff B, Mayer EA. Symptom differences in moderate to severe IBS patients based on predominant bowel habit. American Journal of Gastroenterology. 1995;94(10):2929–2935. doi: 10.1111/j.1572-0241.1999.01440.x. [DOI] [PubMed] [Google Scholar]
- 38.Reme SE, Darnley S, Kennedy T, Chalder T. The development of the irritable bowel syndrome-behavioral responses questionnaire. Journal of Psychosomatic Research. 2010;69(3):319–325. doi: 10.1016/j.jpsychores.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Shinozaki M, Kanazawa M, Palsson OS, et al. Validation of the Japanese version of comorbid conditions questionnaire (CCQ-J) and recent physical symptoms questionnaire (RPSQ-J) Internal Medicine. 2011;50:375–380. doi: 10.2169/internalmedicine.50.4589. [DOI] [PubMed] [Google Scholar]
- 40.Sanga O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism. 2003;15(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 41.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical report? Medical Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ. The epidemiology of chronic syndromes that are frequently unexplained: Do they have common associated factors? International Journal of Epidemiology. 2006;35(2):468–478. doi: 10.1093/ije/dyi265. [DOI] [PubMed] [Google Scholar]
- 44.Winfield JB. Fibromyalgia and related central sensitivity syndromes: Twenty-five years of progress. Seminars of Arthritis and Rheumatology. 2007;36(6):335–338. doi: 10.1016/j.semarthrit.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Bentley A, Bonnet M, Fahs J, et al. Does effective management of sleep disorders improve pain symptoms? Drugs. 2009;69(2):5–11. doi: 10.2165/11531260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Schur E, Afari N, Furberg H, et al. Feeling Bad in More Ways than One: Comorbidity Patterns of Medically Unexplained and Psychiatric Conditions. Journal of General Internal Medicine. 2007;22(6):818–821. doi: 10.1007/s11606-007-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yunus MB, Aldag JC. Restless legs syndrome and leg cramps in fibromyalgia syndrome: a controlled study. BMJ. 1996;312(7042):1339. doi: 10.1136/bmj.312.7042.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White K, Speechley M, Harth M, Òstbye T. Co-existence of chronic fatigue syndrome with fibromyalgia syndrome in the general population: A controlled study. Scandinavian Journal of Rheumatology. 2000;29(1):44–51. doi: 10.1080/030097400750001798. [DOI] [PubMed] [Google Scholar]
- 49.Reitblat T, Zamir D, Polishchuck I, Novochatko G, Malnick S, Kalichman L. Patients treated by tegaserod for irritable bowel syndrome with constipation showed significant improvement in fibromyalgia symptoms. A pilot study. Clinical Rheumatology. 2009;28(9):1079–1082. doi: 10.1007/s10067-009-1194-z. [DOI] [PubMed] [Google Scholar]
- 50.Mathieu N. Comorbidités somatiques dans le Syndrome de l'Intestin Irritable : fibromyalgie, syndrome de fatigue chronique et cystite interstitielle/syndrome de la vessie douloureuse. Gastroentérologie Clinique et Biologique. 2009;33(Supplement 1):S17–S25. doi: 10.1016/S0399-8320(09)71521-0. [DOI] [PubMed] [Google Scholar]
- 51.Kurland J, Coyle W, Winkler A, Zable E. Prevalence of Irritable Bowel Syndrome and Depression in Fibromyalgia. Digestive Diseases and Sciences. 2006;51(3):454–460. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- 52.Frissora C, Koch K. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Current Gastroenterology Reports. 2005;7(4):264–271. doi: 10.1007/s11894-005-0018-9. [DOI] [PubMed] [Google Scholar]
- 53.Stormorken H, Brosstad F. Frequent urination--an important diagnostic marker in fibromyalgia. Tidsskr Nor Laegeforen. 2005;125(1):17–19. [PubMed] [Google Scholar]
- 54.Amital D, Herskovitz C, Fostick L, et al. The Premenstrual Syndrome and Fibromyalgia—Similarities and Common Features. Clinical Reviews in Allergy and Immunology. 2010;38(2):107–115. doi: 10.1007/s12016-009-8143-0. [DOI] [PubMed] [Google Scholar]
- 55.Balasubramaniam R, de Leeuw R, Zhu H, Nickerson RB, Okeson JP, Carlson CR. Prevalence of temporomandibular disorders in fibromyalgia and failed back syndrome patients: A blinded prospective comparison study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2007;104(2):204–216. doi: 10.1016/j.tripleo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Fricton JR. The relationship of temporomandibular disorders and fibromyalgia: implications for diagnosis and treatment. Current Pain Headache Reports. 2004;8(5):355–363. doi: 10.1007/s11916-996-0008-0. [DOI] [PubMed] [Google Scholar]
- 57.Manchikanti L, Fellows B, Singh V. Understanding psychological aspects of chronic pain in interventional pain management. Pain Physician. 2002;5(1):57–82. [PubMed] [Google Scholar]
- 58.Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: Prevalence and symptom severity. Journal of Rheumatology. 1996;23(11):1948–1952. [PubMed] [Google Scholar]
- 59.Häuser W, Bernardy K, Üçeyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin - A meta-analysis of randomized controlled trials. Pain. 2009;145(1-2):69–81. doi: 10.1016/j.pain.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Weir PT, Harlan GA, Nkoy FL, et al. The Incidence of Fibromyalgia and Its Associated Comorbidities: A Population-Based Retrospective Cohort Study Based on International Classification of Diseases, 9th Revision Codes. Journal of Clinical Rheumatology. 2006;12(3):124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- 61.Marcus D, Bernstein C, Rudy T. Fibromyalgia and headache: an epidemiological study supporting migraine as part of the fibromyalgia syndrome. Clinical Rheumatology. 2005;24(6):595–601. doi: 10.1007/s10067-005-1121-x. [DOI] [PubMed] [Google Scholar]
- 62.Wenzel H, Mykletun A, Nilsen T. Symptom profile of persons self-reporting whiplash: a Norwegian population-based study (HUNT 2) European Spine Journal. 2009;18(9):1363–1370. doi: 10.1007/s00586-009-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heffez DS, Ross RE, Shade-Zeldow Y, et al. Clinical evidence for cervical myelopathy due to Chiari malformation and spinal stenosis in a non-randomized group of patients with the diagnosis of fibromyalgia. European Spine Journal. 2004;13(6):516–523. doi: 10.1007/s00586-004-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buskila D, Neumann L, Vaisberg G, Alkalay D, Wolfe F. Increased rates of fibromyalgia following cervical spine injury. A controlled study of 161 cases of traumatic injury. Arthritis and Rheumatism. 1997;40(3):446–452. doi: 10.1002/art.1780400310. [DOI] [PubMed] [Google Scholar]
- 65.Tishler M, Levy O, Amit-Vazina M. Can fibromyalgia be associated with whiplash injury? A 3-year follow-up study. Rheumatology International. 2010:1–5. doi: 10.1007/s00296-010-1412-7. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt-Wilcke T, Wood P, Lürding R. Schmerz und Aufmerksamkeit. Der Schmerz. 2010;24(1):46–53. doi: 10.1007/s00482-009-0872-8. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Andreu J, Ibanez-Bosch R, Portero-Vazquez A, Masramon X, Rejas J, Galvez R. Cognitive impairment in patients with fibromyalgia syndrome as assessed by the mini-mental state examination. BMC Musculoskeletal Disorders. 2009;10(162) doi: 10.1186/1471-2474-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aaron LA, Buchwald D. Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Practice & Research Clinical Rheumatology. 2003;17(4):563–574. doi: 10.1016/s1521-6942(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 69.Torresani C, Bellafire S, De Panfilis G. Chronic Urticaria is Usually Associated with Fibromyalgia Syndrome. Acta Dermato-Venereologica. 2009;89(4):389–392. doi: 10.2340/00015555-0653. [DOI] [PubMed] [Google Scholar]
- 70.Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis & Rheumatism. 2003;48(10):2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 71.Howard KJ, Mayer TG, Neblett R, Perez Y, Chohen H, Gatchel RJ. Fibromyalgia syndrome in chronic disabling occupational musculoskeletal disorders: Prevalence, risk factors and post-treatment outcomes. Submitted for publication. 2010 doi: 10.1097/JOM.0b013e3181fc838d. [DOI] [PubMed] [Google Scholar]
- 72.Kassam A, Patten S. Major depression, fibromyalgia and labour force participation: A population-based cross-sectional study. BMC Musculoskeletal Disorders. 2006;7(1):4. doi: 10.1186/1471-2474-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker E, Keegan D, Gardner G, Sullivan M, Katon W, Bernstein D. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: I. Psychiatric diagnoses and functional disability. Psychosom Med. 1997 November 1;59(6):565–571. doi: 10.1097/00006842-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Roy-Byrne P, Smith WR, Goldberg J, Afari N, Buchwald D. Post-traumatic stress disorder among patients with chronic pain and chronic fatigue. Psychological Medicine. 2004;34(2):363–368. doi: 10.1017/s0033291703008894. [DOI] [PubMed] [Google Scholar]
- 75.Wilson DR. Health Consequences of Childhood Sexual Abuse. Perspectives in Psychiatric Care. 2010;46(1):56–64. doi: 10.1111/j.1744-6163.2009.00238.x. [DOI] [PubMed] [Google Scholar]
- 76.Stisi S, Cazzola M, Buskila D, et al. Etiopathogenetic mechanisms of fibromyalgia syndrome. Reumatismo. 2008;60(1):25–35. [PubMed] [Google Scholar]
- 77.Aaron LA, Bradley LA, Alarcon GS, et al. Perceived physical and emotional trauma as precipitating events in fibromyalgia. Arthritis & Rheumatism. 1997;40(3):453–460. doi: 10.1002/art.1780400311. [DOI] [PubMed] [Google Scholar]


